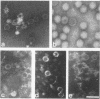
Abstract
The nucleocapsid of the enveloped double-stranded RNA bacteriophage phi 6 was isolated by extraction with the nonionic detergent Triton X-114 and subjected to disruption analysis with chelating and protein-denaturing agents. The subnucleocapsid particles were separated in rate-zonal sucrose gradients, and their ultrastructure and protein composition were analyzed. The role of divalent cations in the nucleocapsid structure was studied by using a precipitation assay of the isolated nucleocapsid proteins. The phi 6 nucleocapsid had a cagelike skeleton consisting of a single polypeptide species (P1). Two other proteins (P2 and P4) were associated with the P1 cage. These three early proteins were previously known to be involved in the RNA synthesis machinery of the virus. The stability of the nucleocapsid surface lattice consisting of protein P8 was dependent on Ca2+ ions.
Full text
PDF
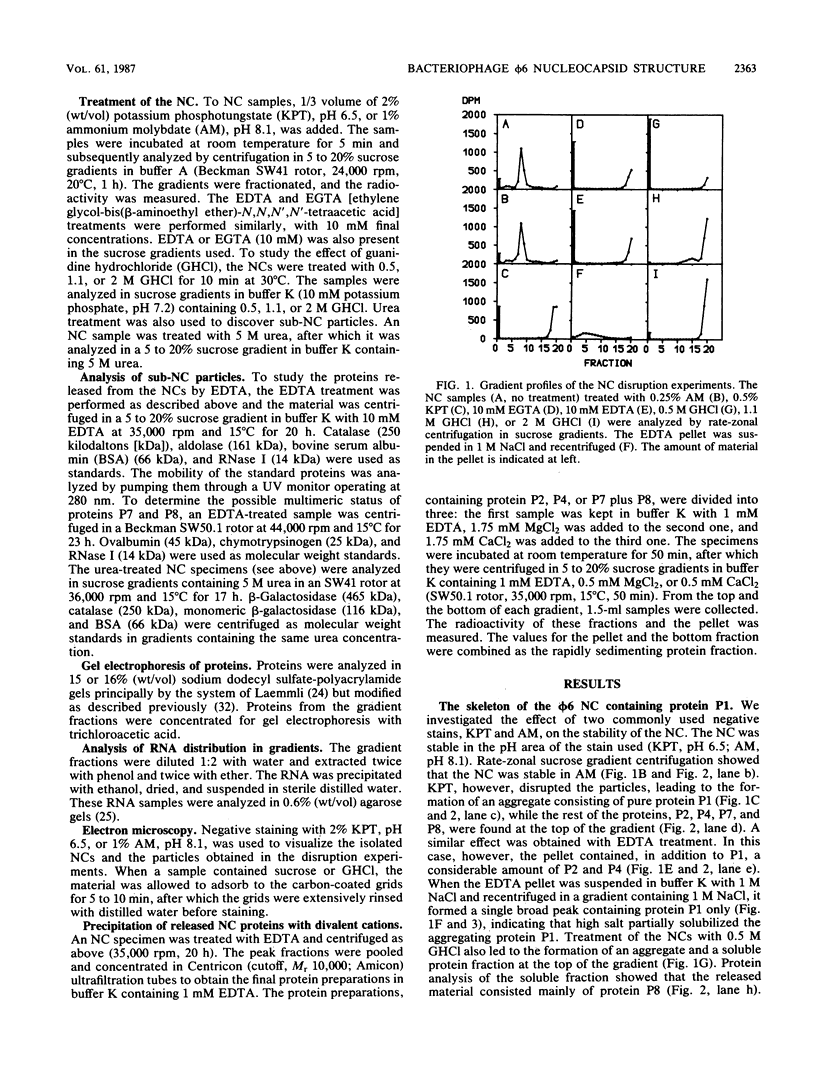
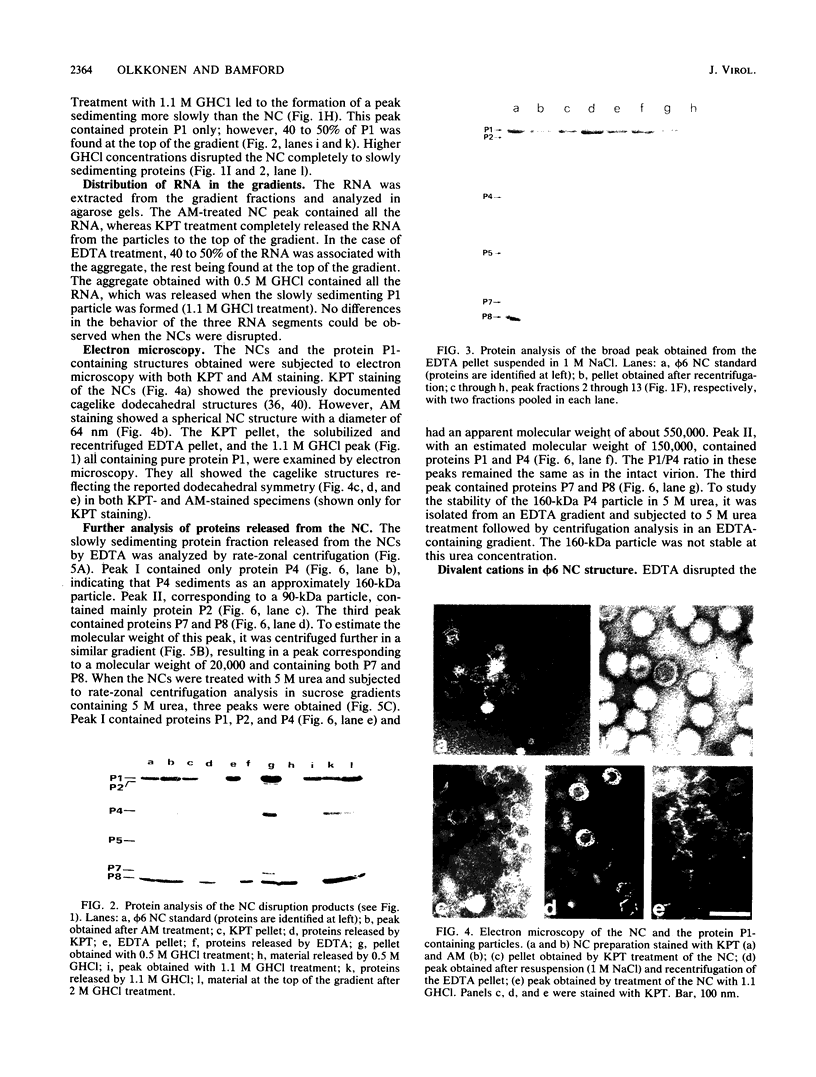
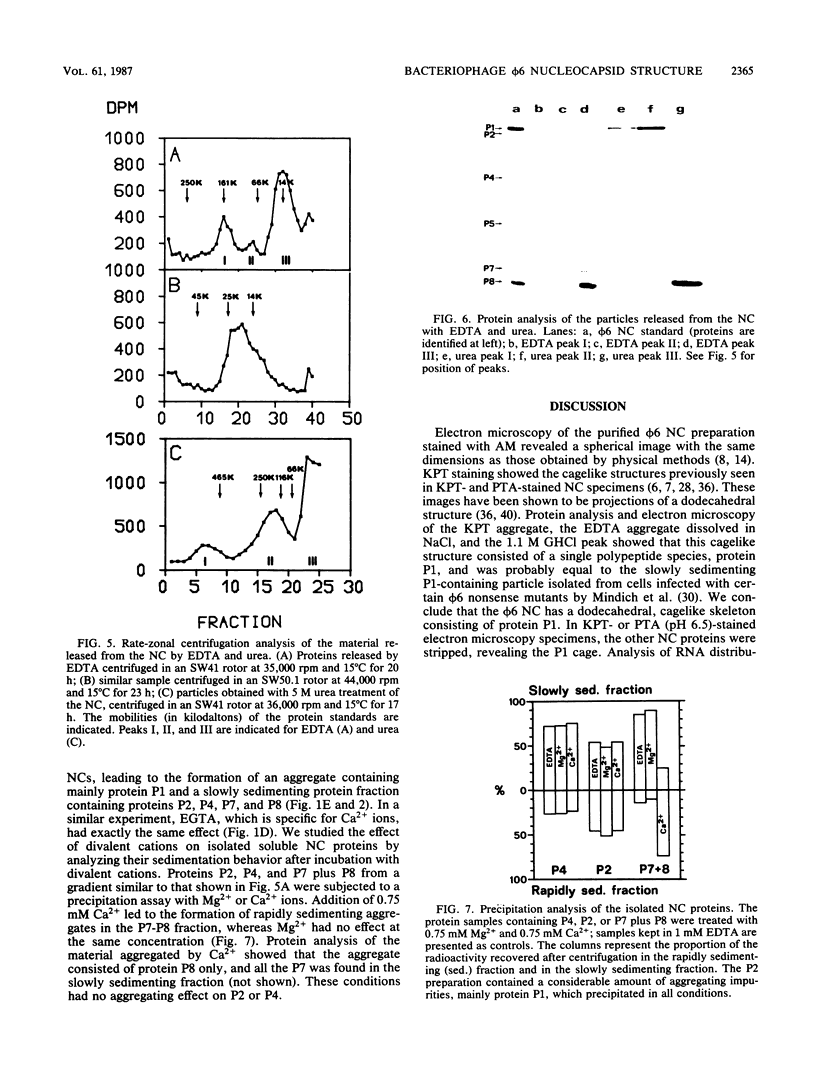


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Mindich L. Electron microscopy of cells infected with nonsense mutants of bacteriophage phi 6. Virology. 1980 Nov;107(1):222–228. doi: 10.1016/0042-6822(80)90287-1. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Palva E. T., Lounatmaa K. Ultrastructure and life cycle of the lipid-containing bacteriophage phi 6. J Gen Virol. 1976 Aug;32(2):249–259. doi: 10.1099/0022-1317-32-2-249. [DOI] [PubMed] [Google Scholar]
- Bamford D. H., Palva E. T. Structure of the lipid-containing bacteriophage phi 6. Disruption by Triton X-100 treatment. Biochim Biophys Acta. 1980 Sep 18;601(2):245–259. doi: 10.1016/0005-2736(80)90530-1. [DOI] [PubMed] [Google Scholar]
- Bamford D. H. lipid-containing bacterial viruses: disruption studies on phi 6. Prog Clin Biol Res. 1981;64:477–489. [PubMed] [Google Scholar]
- Berger H., Kennedy K. Physical measurements on the lipid-containing bacteriophage phi 6. Biochim Biophys Acta. 1980 Nov 17;633(1):68–76. doi: 10.1016/0304-4165(80)90038-0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Etten J. V., Lane L., Gonzalez C., Partridge J., Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976 May;18(2):652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Protein interfaces and intersubunit bonding. The case of tomato bushy stunt virus. Biophys J. 1980 Oct;32(1):139–153. doi: 10.1016/S0006-3495(80)84930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N., Finch J. T. Structure and symmetry of B. stearothermophilus pyruvate dehydrogenase multienzyme complex and implications for eucaryote evolution. Cell. 1979 May;17(1):85–93. doi: 10.1016/0092-8674(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Sehgal O. P., Pickett E. E. Stabilizing effect of divalent metal ions on virions of southern bean mosaic virus. Virology. 1976 Feb;69(2):587–595. doi: 10.1016/0042-6822(76)90487-6. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. Structure and function of the reovirus genome. Microbiol Rev. 1981 Dec;45(4):483–501. doi: 10.1128/mr.45.4.483-501.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Koike K. Structure, assembly and function of mammalian alpha-keto acid dehydrogenase complexes. Adv Biophys. 1976:187–227. [PubMed] [Google Scholar]
- Ktistakis N. T., Lang D. The dodecahedral framework of the bacteriophage phi 6 nucleocapsid is composed of protein P1. J Virol. 1987 Aug;61(8):2621–2623. doi: 10.1128/jvi.61.8.2621-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGraw T., Mindich L., Frangione B. Nucleotide sequence of the small double-stranded RNA segment of bacteriophage phi 6: novel mechanism of natural translational control. J Virol. 1986 Apr;58(1):142–151. doi: 10.1128/jvi.58.1.142-151.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Cohen J., Weisburd M. Isolation of nonsense suppressor mutants in Pseudomonas. J Bacteriol. 1976 Apr;126(1):177–182. doi: 10.1128/jb.126.1.177-182.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Davidoff-Abelson R. The characterization of a 120 S particle formed during phi 6 infection. Virology. 1980 Jun;103(2):386–391. doi: 10.1016/0042-6822(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., Lehman J. Cell wall lysin as a component of the bacteriophage phi 6 virion. J Virol. 1979 May;30(2):489–496. doi: 10.1128/jvi.30.2.489-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Sinclair J. F., Cohen J. The morphogenesis of bacteriophage phi6: particles formed by nonsense mutants. Virology. 1976 Nov;75(1):224–231. doi: 10.1016/0042-6822(76)90021-0. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Ewen M. E., Brusslan J., Pagratis N. Generation of cDNA clones of the bacteriophage phi 6 segmented dsRNA genome: characterization and expression of L segment clones. Virology. 1986 Dec;155(2):402–417. doi: 10.1016/0042-6822(86)90203-5. [DOI] [PubMed] [Google Scholar]
- Romantschuk M., Bamford D. H. Function of pili in bacteriophage phi 6 penetration. J Gen Virol. 1985 Nov;66(Pt 11):2461–2469. doi: 10.1099/0022-1317-66-11-2461. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Shirley J. A., Beards G. M., Thouless M. E., Flewett T. H. The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Mindich L. RNA synthesis during infection with bacteriophage phi6. Virology. 1976 Nov;75(1):209–217. doi: 10.1016/0042-6822(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Steely H. T., Jr, Lang D. Electron microscopy of bacteriophage phi 6 nucleocapsid: two-dimensional image analysis. J Virol. 1984 Aug;51(2):479–483. doi: 10.1128/jvi.51.2.479-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lang D. Electron microscopy of bacteriophage phi 6 nucleocapsid: three-dimensional image analysis. J Virol. 1984 Aug;51(2):484–488. doi: 10.1128/jvi.51.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]