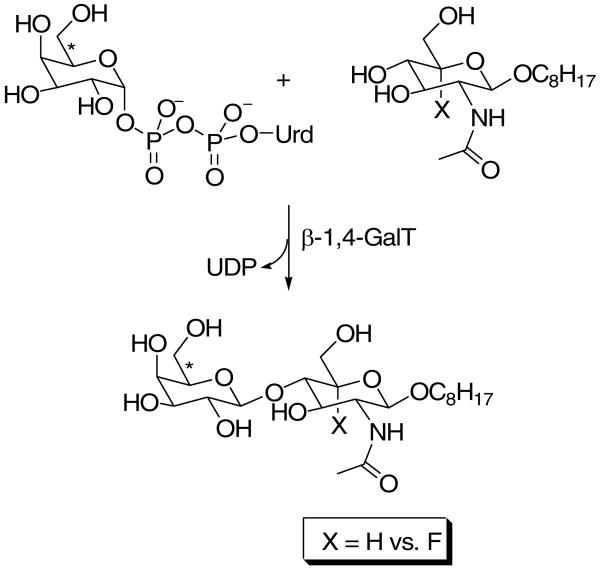
Abstract
In glycosyltransferase-catalyzed reactions a new carbohydrate-carbohydrate bond is formed between a carbohydrate acceptor and the carbohydrate moiety of either a sugar nucleotide or lipid-linked saccharide donor. It is currently believed that most glycosyltransferase-catalyzed reactions occur via an electrophilic activation mechanism with the formation of an oxocarbenium ion-like transition state, a hypothesis that makes clear predictions regarding the charge development on the donor (strong positive charge) and acceptor (minimal negative charge) substrates. To better understand the mechanism of these enzyme-catalyzed reactions, we have introduced a strongly electron-withdrawing group (fluorine) at C-5 of both donor and acceptor substrates in order to explore its effect on catalysis. In particular, we have investigated the effects of the 5-fluoro analogs on the kinetics of two glycosyltransferase-catalyzed reactions mediated by UDP-GlcNAc:GlcNAc-P-P-Dol N-acetylglucosaminyltransferase (chitobiosyl-P-P-lipid synthase, CLS) and β–N-acetylglucosaminyl-β-1,4 galactosyltransferase (GalT). The 5-fluoro group has a marked effect on catalysis when inserted into the UDP-GlcNAc donor, with the UDP(5-F)-GlcNAc serving as a competitive inhibitor of CLS rather than a substrate. The (5-F)-GlcNAc β-octyl glycoside acceptor; however, is an excellent substrate for GalT. Both of these results support a weakly associative transition state for glycosyltransferase-catalyzed reactions that proceed with inversion of configuration.
Glycosyltransferases are involved in a wide variety of biological processes ranging from the processing of N-linked glycan structures (1) to signal transduction (2) to bacterial cell wall (3) and natural product synthesis (4). A thorough understanding of these enzyme-catalyzed reactions will be important for the design of molecular therapeutics targeting these enzymes. Based on sequence homology, glycosyltransferases have been classified into an expanding list of families (5) (89 as of April 2007; available at http://afmb.cnrs-mrs.fr/CAZY. Despite this sequence diversity, glycosyltransferases can be classified into three superfamilies based on current crystal structures (6). Although there are likely to be some subtle differences between the superfamilies, the mechanisms of glycosyltransferase-catalyzed reactions fall into two categories: retaining, where the product glycoside has the same stereochemistry as the activated leaving group, and inverting, where the reaction proceeds with inversion of stereochemistry at C-1.
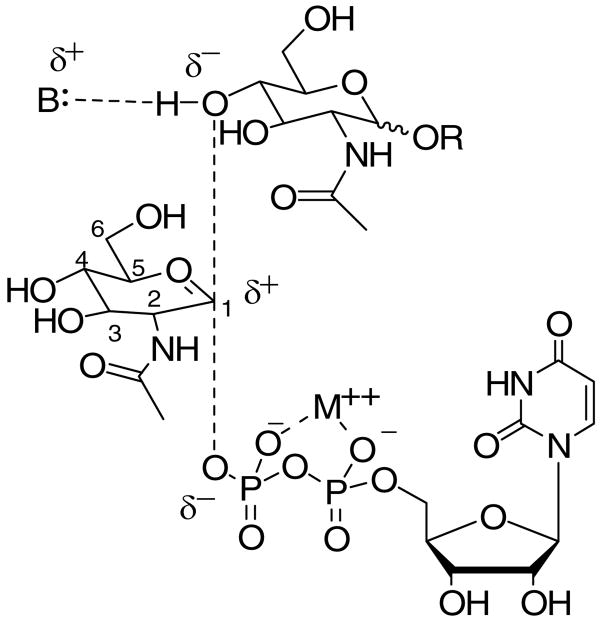
A number of studies using substrate analogs (7-9) have suggested that inverting glycosyltransferase-catalyzed reactions, like those of glycosidases, proceed via a oxocarbenium ion-like (“exploded SN2” (10)) transition state (11). This hypothesis suggests a reaction coordinate in which the bond between C-1 of the glycosyl donor and the pyrophosphate leaving group is broken in the transition state, yet little bond formation between the glycosyl acceptor and the oxocarbenium center has occurred (Figure 1).
Figure 1.
Proposed weakly associative transition state for glycosyltransferases involving a UDP-GlcNAc donor and various GlcNAc-containing acceptor substrates.
One clear implication of this hypothesis is that during catalysis the glycosyl donor substrate develops a partial positive charge at C-1 and O-5 due to the formation of the oxocarbenium ion. The amount of negative charge development on the glycosyl acceptor, on the other hand, would be expected to be small, since little deprotonation or bond formation (and thereby stabilization of the developing oxocarbenium ion) occurs in the transition state.
We rationalized that both of these tenets could be investigated through the introduction of a charge-altering 5-fluoro group that would exert an electronic effect on both the developing oxocarbenium ion, if incorporated into the donor, and the C-4 or C-6 hydroxyl group, if incorporated into the glycosyl acceptor. In the realm of carbohydrate enzymology, 5-fluoro glycosyl fluorides have been used extensively for the investigation of glycosidase mechanisms (12), but difficulty in preparing the requisite 5-fluoro substrates has prevented the extension of this approach to the use of 5-fluoro glycosides and glycosylphosphates in the studies of glycosyltransferases.
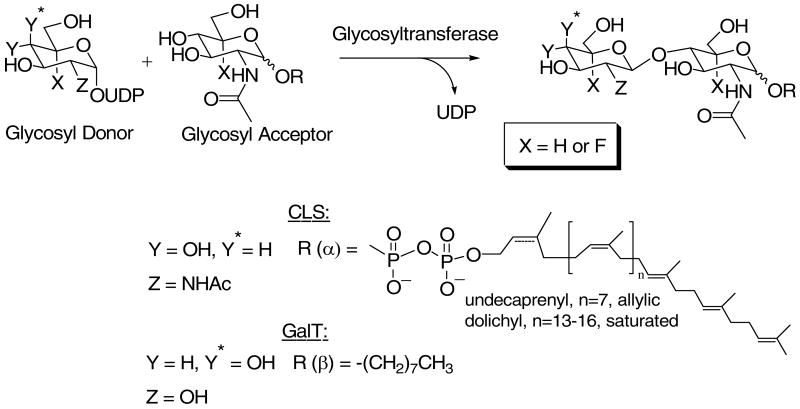
An efficient route for the synthesis of potential glycosyltransferase acceptors and donors containing 5-fluoro N-acetylglucosamine ((5-F)-GlcNAc) has now been developed in our laboratory (13). Because of our ongoing interest in N-linked glycosylation (14-16) and the biosynthesis of the oligosaccharyl donor, Glc3Man9GlcNAc2-P-P-dolichol (17), we chose UDP-GlcNAc:GlcNAc-P-P-Dol N-acetylglucosaminyl transferase (chitobiosyl-P-P-lipid synthase, CLS, EC 2.4.1.141) as an initial target for our mechanistic investigations (Scheme 1). CLS has been shown recently to exist as an heterodimeric complex in yeast consisting of Alg13p and Alg14p in which Alg14p functions as an ER binding partner for the catalytic subunit Alg13p (18-20). It remains to be established conclusively if the mammalian enzyme exists as a similar dimeric complex. In this regard, Gao et al. have reported that although the human ALG13 and ALG14 orthologues apparently do not pair with their respective yeast counterparts, they are able to functionally complement for the loss of either ALG13 or ALG14 when co-expressed in yeast (19). This multi-subunit structure is reminiscent of dolichylphosphomannose synthase (DPM) which has three subunits, DPM1, DPM2 and DPM3 (21). In this mannosyltransferase, the catalytic subunit, DPM1, is associated with the ER via its interaction with DPM3.
Scheme 1.
The mammalian CLS has the added advantage that both the donor and acceptor contain GlcNAc, allowing the investigation of charge buildup in both substrates for a single enzyme-catalyzed reaction. However, only limited quantities of the CLS acceptor substrates, GlcNAc-P-P-Dol (and 5-fluoro GlcNAc-P-P-Dol), are available via biosynthesis. Therefore, to corroborate the tentative conclusions reached with the biosynthetic CLS acceptor substrates, we also chose to study β-1,4-galactosyltransferase (GalT, EC 2.4.1.38, Family GT7) which utilizes synthetically accessible GlcNAc-containing acceptors. The effects of the 5-fluoro substrate analogs on these enzyme-catalyzed reactions support a weakly associative transition state for inverting glycosyltransferase-catalyzed reactions.
Experimental Procedures
Materials
Unless otherwise indicated, all chemicals were purchased from Aldrich or Sigma and were used without further purification. Pyridine and CH3OH were distilled from CaH2 prior to use. [3H]Ac2O (100 mCi/mmol, lot #011126) and UDP-[6-3H]galactose ([3H]UDP-Gal, 40-60 Ci/mmol) were purchased from American Radiolabeled Chemicals Inc (St. Louis, MO). GalT from bovine milk (10 I.U., MW 44,000), was purchased from Sigma, dissolved in water and divided so as to provide aliquots containing 0.5 units. These aliquots were lyophilized and stored at -80 °C prior to use. 5-Fluoro-glucosamine 1-phosphate, UDP-(5-F)-GlcNAc, and octyl 5-fluoro-2-deoxy-2-acetamido-β-D-glucopyranoside ((5-F)-GlcNAc β-octyl glycoside) were synthesized as previously described (13). GlcNAc β-octyl glycoside was synthesized as described in the literature (22). Pyridinium acetate solutions were prepared by adding the requisite amount of acetic acid followed by addition of pyridine to pH 4.5 and dilution with water to provide the desired concentration. Baker Si250 thin layer plates were from VWR Scientific Products (Willard, OH). Analytical HPLC was carried out on a Waters 996 photodiode array system running Millenium32 software. Econo-safe™ biodegradable scintillation counting mixture is a product of Research Products International (Mount Prospect, IL). EDTA-free protease inhibitor cocktail was purchased from Roche. Reactive Blue 4 immobilized on cross-linked 4% agarose was from Sigma Chemical Co. (St. Louis, MO). Tn-10 CHO cells were generously provided by Dr. Mark Lehrman (UT-Southwestern, Dallas, TX).
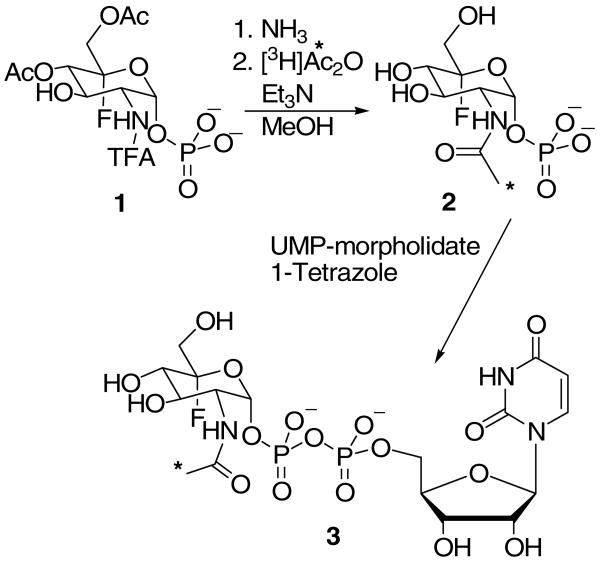
Synthesis of UDP-(5-F)-[3H]GlcNAc
Et3N (11 μL, 0.080 mmol) was added to a stirred suspension of crude 5-fluoro glucosamine 1-phosphate (0.010 mmol) in dry CH3OH (100 μl) in a sealable glass tube. The supplied vial with [3H]Ac2O (5 mCi, 100 mCi/mmol) was cooled to −78°C to prevent any escape of volatile [3H]Ac2O, and 50 μL methanol was added. This solution was added to the 5-fluoro glucosamine 1-phosphate containing tube to initiate the reaction; quantitative transfer was ensured by rinsing the vial with [3H]Ac2O with an additional 50 μL of methanol. The tube was sealed and stirred at room temperature for 4 h. The reaction vessel was opened and the mixture was evaporated under high vacuum via a line that contained a series of four traps to prevent radioactive contamination (dry ice/acetone (2), liquid N2 (1), and K2CO3 (1)). The resulting brown oil was dissolved in CH3OH (1 mL) and cooled to −10°C. NH3 was bubbled through the solution and the flow out of the flask was passed through a check valve (to prevent reverse flow) and two aqueous NaHCO3 traps. After 1 h, the solvent was removed under a stream of N2 (passing through the same traps). The resulting brown residue was dissolved in water (∼10 mL) and passed through a Bio-Rad AG 1X8 anion exchange column (1 × 6 cm, acetate form). The column was washed with water (20 mL) followed by elution of the desired product with 500 mM pyridinium acetate (40 mL). The pyridinium acetate effluent was collected as a single fraction and evaporated using a Speed-Vac™ vacuum centrifuge to provide (5-F)-[3H]GlcNAc 1-phosphate pyridinium salt. The resulting oil was passed through a cation exchange column (Dowex 50WX8-200, Et3NH+ form), eluting with several column volumes of CH3OH. The fractions with significant radioactivity were pooled and evaporated leaving (5-F)-[3H]GlcNAc 1-phosphate triethylammonium salt as an oil (240 μCi). To this oil was added UMP-morpholidate (4.9 mg, 0.0072 mmol) and pyridine (200 μL). The resulting yellow solution was evaporated and then co-evaporated with pyridine (2 × 200 μL). To this yellow oil were added 1H-tetrazole (0.33 mg, 0.0047 mmol) and pyridine (200 μL). The reaction was monitored by TLC analysis (3:1 CH3CN:H2O). After 8 days the reaction was quenched by addition of 50 mM aqueous pyridinium acetate (100 μL) and evaporated. The resulting yellow oil was purified by chromatography on Bio Gel P-2 (80 cm × 1.5 cm) with 50 mM pyridinium acetate as the eluant. Fractions (100 drops) were collected and analyzed by TLC (UV absorbance). Fractions that contained the desired product were pooled, giving the title compound contaminated with [3H]acetate. The acetate was removed by HPLC purification in several portions on a C18 Varian Microsorb-MV 300Å column (4 mm × 25 cm) eluting with 400 mM aqueous hexafluoro-2-propanol adjusted to pH 7.8 with Et3N at 1 mL/min. The fractions containing the desired product were pooled and evaporated on a Speed-Vac vacuum centrifuge yielding 5 μCi of UDP-(5-F)-[3H]GlcNAc.
Each reaction in the synthesis of UDP-(5-F)-[3H]GlcNAc (Scheme 2) was first examined on an identical small scale using unlabeled precursors. The purity and chemical identification of each intermediate was ascertained using standard methods (TLC, NMR, MS). The final product, UDP-(5-F)-[3H]GlcNAc was chromatographically identical to unlabeled UDP-(5-F)-GlcNAc by both size exclusion chromatography and HPLC. In addition, as described below, this product was shown to be a WecA substrate in the biosynthesis of (5-F)-[3H]GlcNAc-P-P-Undec which, in turn is a CLS substrate (vide infra).
Scheme 2.
Biosynthesis of [3H] GlcNAc-P-P-Undec and (5-F)-[3H] GlcNAc-P-P-Undec for Use as Chitobiosyl-P-P-lipid Synthase (CLS) Acceptor Substrates
Each reaction solution contained E. coli membranes (8 μg total protein) prepared as described by Rush et al. (23), UDP-(5-F)-[3H]GlcNAc (215,000 cpm, 97 cpm/pmol) or UDP-[3H]GlcNAc (858,000 cpm, 58,500 cpm/pmol), 52 mM Tris-HCl (pH 8), 11 mM sucrose, 7.4 mM 2-mercaptoethanol, 0.5 mM EDTA, 40 mM MgCl2 and 0.58 % CHAPS in a total volume of 50 μL. The reactions were incubated for 30 min at 37°C. After extraction with CHCl3:CH3OH (2:1), the organic layer was dried by a stream of N2 and the radiolabeled glycolipid substrate was dispersed in 1% Triton X-100 (200 μL for [3H]GlcNAc-P-P-Undec or 22 μL for (5-F)-[3H]GlcNAc)-P-P-Undec to be tested as substrates in CLS reactions.
Biosynthesis of [3H]GlcNAc-P-P-Dol
[3H]GlcNAc-P-P-Dol was synthesized enzymatically and purified as described elsewhere (24) except microsomes from Tn-10 CHO cells overexpressing GPT (25) were used.
Solubilization and Partial Purification of UDP-GlcNAc:GlcNAc-P-P-Dol N-Acetylglucosaminyltransferase (Chitobiosyl-P-P-Lipid Synthase, CLS) from CHO Cells
CHO cells were grown to confluence in F-12 media with 9% bovine serum and suspended in 0.9% NaCl, 3 mM EDTA (pH 8). The suspended cells were homogenized by sonication (15 sec), and a crude particulate fraction was obtained by centrifugation (3000 rpm, 4°C, 15 min). Crude microsomes were sedimented from the low-speed supernatant fluid by centrifugation (60,000 rpm, 4°C, 40 min) in a Beckman TL-100 Ultracentrifuge. The membranous pellet was washed and resuspended in 1 mL sonication buffer. CLS activity was solubilized by extraction of the membrane fraction (2 mg/mL protein) in 10% glycerol, 25 mM Tris-HCl (pH 8.0), 0.65% Triton X-100, 5 mM β-mercaptoethanol, and 1× proteinase inhibitor cocktail (EDTA-free) for 60 min on ice with stirring. The solubilized CLS activity was recovered in the supernatant fluid after centrifugation of the detergent-extract (46,000 g, 40 min, 4°C).
The solubilized CLS activity (specific activity = 5,400 cpm/min/mg) was then partially purified by ion-exchange chromatography on a TSK-Gel Toyopearl DEAE-650M column (40-90 microns) washed by 15 vol of 25 mM Tris-HCl (pH8.0) and equilibrated with 5 vol of 20% glycerol, 25 mM Tris-HCl (pH 8.0), 0.05% Triton X-100, and 5 mM β-mercaptoethanol. Approximately 6 mg membrane protein was applied to a 1 mL column at a rate of 30 mL/hr. The column was then washed with 2 vol of 20% glycerol, 25 mM Tris-HCl (pH 8.0), 0.05% Triton X-100, 0.2mM PMSF, 2 μg/mL leupeptin, 1× proteinase cocktail for mammalian tissues (Sigma) and 5 mM β-mercaptoethanol. The TSK-Gel Toyopearl DEAE-650M column was eluted with a linear NaCl gradient (0-0.3 M NaCl in the equilibration buffer), eluting at a rate of 35 mL/hr. The fractions from a single peak of CLS activity were pooled and then desalted and concentrated using an Amicon filtration cell fitted with a YM100 membrane. The desalted fraction was applied to a butyl-Sepharose column and eluted with a decreasing linear salt gradient (1.0 M –0.0 M NaCl). Fractions corresponding to the peak of CLS activity were pooled and applied to a second TSK-Gel Toyopearl DEAE-650M column. The column was eluted with a linear NaEDTA gradient (0-5 mM). The fractions containing CLS activity were pooled and concentrated as above, applied to a Reactive Blue 4-agarose column, and eluted with a linear NaCl gradient (0-0.8 M). Although it was estimated that this fraction was 730-fold purified relative to the crude microsomes, 10 polypeptide bands were still visualized by SDS-PAGE. CLS activity is based on the conversion of exogenous acceptor substrate, [3H]GlcNAc-P-P-Dol, to [3H](GlcNAc-β-(1-4)GlcNAc)-P-P-Dol (cpm/mg protein) in the standard assay described below. It is not possible to determine the specific activity of biosynthetic [3H]GlcNAc-P-P-Dol because of the presence of an unknown amount of endogenous GlcNAc-P-P-Dol in the substrate preparation. The highly purified fractions from the Reactive Blue 4-agarose column were used for the enzymatic studies described in the Results Section.
Enzyme Assays
Chitobiosyl-P-P-lipid Synthase
For routine assay during purification of CLS, each reaction mixture contained partially purified fractions of CLS (3.6 μg protein), 55 mM Tris-HCl (pH 8), 0.25 mM sucrose, 4% glycerol, 1 mM 2-mercaptoethanol, 8 mM NaCl, 5 mM EDTA, 5 mM AMP, 0.5% Triton X-100, 0.1 mM UDP-GlcNAc and [3H]GlcNAc-P-P-Dol (4,000 cpm) in a total volume of 100 μL. This assay method is a slight modification of that described previously (24). After incubation for 20 min at 37°C unless indicated otherwise, the reactions were terminated by the addition of 20 vol of CHCl3:CH3OH (2:1) and washed with 0.9% NaCl (0.4 mL). The lower phase was washed once with an equal volume of CHCl3:CH3OH:0.9% NaCl, (3:48:47), and the lower organic layer was removed. Brain phosphatidylcholine (75 μg) was added and the organic solvent was evaporated under a stream of N2 at ambient temperature. The resulting residue was redissolved in CHCl3:CH3OH (2:1) (12 μL) and loaded onto a Baker Si250 TLC plate eluting with CHCl3:CH3OH:H2O (65:25:4). The radioactive zones corresponding to 3H-labeled GlcNAc-P-P-Dol substrate and GlcNAc-β-(1,4)-GlcNAc-P-P-Dol product were located using a BioScan System 200 Imaging Scanner (1 h scan/lane), and the percentage of the glycolipid substrate converted to the chitobiosyl-P-P- lipid product was determined.
To compare the ability of CLS to catalyze the reaction of fluorinated vs. non-fluorinated substrates, partially purified CLS (600 ng protein) was assayed with either 0.5 mM UDP-GlcNAc or UDP-(5-F)-GlcNAc as the nucleotide sugar donor substrate and [3H]GlcNAc-P-P-Undec (7,000 cpm, 58,500 cpm/pmol) or (5-F)-[3H]GlcNAc-P-P-Undec (7,000 cpm, 97 cpm/pmol) as the glycolipid acceptor substrate in a total volume of 25 μL. Reaction conditions and product analysis were carried out as described above.
β-1,4-Galactosyltransferase – General Procedure
For assays, a concentrated (162.5×) stock solution (25 munits/μL) of GalT containing 20 mM Tris-HCl, 2 mM BME, 2 mM EDTA, 0.5 mg/mL BSA, pH 7.5 was prepared and stored at −20 °C in 4 μL aliquots. In this form the enzyme was stable for several months. GlcNAc β-octyl glycoside and (5-F)-GlcNAc β-octyl glycoside solutions were prepared in DMSO and stored at −20 °C. (5-F)-GlcNAc β-octyl glycoside solutions decomposed over several weeks at rt in DMSO and were prepared fresh weekly. UDP-Gal (Sigma) was prepared as a 10 mM stock solution (determined gravimetrically) and stored at −20 °C. UDP-[3H]Gal was purchased as a 1 mCi/mL solution in 70% aq. EtOH and was diluted 40-fold with water to prepare a 20× solution for assays. This solution was stored at −20 °C.
Each enzyme assay (100 μL) contained 100 mM Na cacodylate (pH 7.5), 10 mM MnCl2, 0.5 mg/mL BSA, GlcNAc β-octyl glycoside (various concentrations in DMSO), 100 μM UDP-Gal, UDP-[3H]Gal (ca. 0.4 μCi), and β-GalT from bovine milk (11.3 ng), in an aqueous solution containing 5% DMSO. The assay solutions were preincubated for 9 min, at 37 °C prior to addition of the enzyme (5 μL in a stock solution of 20 mM Tris-HCl, 2 mM BME, 2 mM EDTA, 0.5 mg/mL BSA, pH 7.5). After 5 min. incubation at 37 °C, the reaction was stopped by dilution with ice-cold deionized water (500 μL) or ice-cold 100 mM EDTA (500 μL). The assay solution was passed through a Sep-Pak cartridge either immediately (water quench) or after storage on ice (EDTA quench). The assay tube was rinsed with water (2 × 500 μL), and each aqueous rinse was applied to the same Sep-Pak cartridge. The Sep-Pak was washed with 20 mL H2O followed by 4 × 2.5 mL MeOH. The MeOH eluant was analyzed via liquid scintillation counting. Background values were determined by addition of DMSO without any acceptor.
The assays for (5-F)-GlcNAc-β-octyl glycoside were completed in the identical manner as above, except (5-F)-GlcNAc-β-octyl glycoside was added after the preincubation step, 30 s prior to the addition of enzyme. The assay solutions were incubated for 9 min at 37 °C, and were passed through the Sep-Pak immediately after quenching to minimize decomposition of the disaccharide product (26).
Gal T- Steady-state kinetic analysis
The assays followed the general procedure with UDP-Gal (19, 24, and 33 μM) and GlcNAc β-octyl glycoside (0, 17, 21, 30, 50, and 75 μM). The reactions were performed on the same day and were quenched with ice-cold 100 mM EDTA. For (5-F)-GlcNAc-β-octyl glycoside, the assays followed the general procedure with UDP-Gal (27.3, 33.3, 42.8, 60, 100, and 300 μM) and GlcNAc-β-octyl glycoside (0, 100, 129, 180, 300, and 900 μM). The 100 μM UDP-Gal assay utilized a 2× enzyme stock, and the 300 μM UDP-Gal assay utilized a 5× enzyme stock prepared from a fresh enzyme aliquot (see General Procedure).
Data Collection and Analyses
Unless indicated otherwise each assay was performed in duplicate and the error bars signify the range of the data collected (varied time or concentration). The kinetic constants were determined by least squares fitting to either the Michaelis-Menten equation using Kaleidagraph (CLS) (Synergy Software) or eq. 1 using the Leanora program (27), and the errors reported are standard deviations. The standard errors for kcat and Km were not propagated in the kcat/Km calculations.
Results
Synthesis of UDP-(5-F)-[3H]GlcNAc
To facilitate biochemical assays with UDP-(5-F)-GlcNAc, a tritium label (represented by *) was incorporated in the penultimate acetylation step (Scheme 2). Precursor 1 was prepared as previously described (13). The acetate and trifluoroacetyl (TFA) groups were removed with methanolic ammonia, and the radiolabel was then introduced using [3H]Ac2O. Following nucleotide sugar formation with UMP-morpholidate (28), purification by HPLC provided the desired product 3 in radiochemically pure form.
Synthesis of (5-F)-GlcNAc-P-P-Lipid
Initially, synthesis of a (5-F)-GlcNAc-containing acceptor substrate for CLS was attempted using methods described in the literature for the synthesis of substituted pyrophosphates. Two routes were investigated involving reacting either 1 or 2 with a lipid morpholidate (28, 29) or imidazolide (30) intermediate. The latter method has been used in our lab (14, 31) and by others (32-35) for the synthesis of (GlcNAc–β–(1-4)GlcNAc)-P-P-Dol and lipid analogs. Unfortunately, neither method was effective in yielding the (5-F)-GlcNAc-containing analog (36). Given these difficulties, a biosynthetic route to (5-F)-GlcNAc-P-P-lipid was pursued using UDP-GlcNAc:Dol-P-GlcNAc 1-phosphate transferase (GPT). Enzymatic synthesis of the 5-fluoro substrate, (5-F)-GlcNAc-P-P-Dol, containing the lipid found in mammalian cells, is not possible because UDP-(5-F)-GlcNAc is not a substrate for the mammalian GPTs (data not shown). Thus, an alternate strategy using a truncated, unsaturated lipid, undecaprenol, to afford (5-F)-GlcNAc-P-P-Undec was undertaken. This strategy appeared promising because CLS had been shown previously to accept a completely unsaturated GlcNAc-P-P-lipid substrate (37), and it was determined that UDP-(5-F)-GlcNAc is a substrate for a bacterial GPT, WecA (data not shown). Fortunately, this approach allowed for the synthesis of [3H]GlcNAc-P-P-Undec and (5-F)-[3H]GlcNAc-P-P-Undec by WecA-catalyzed N-acetylglucosaminylphospho transfer. Each product was isolated and then tested as a CLS acceptor substrate.
Solubilization, Partial Purification and Properties of CLS Associated with CHO Microsomes
To resolve CLS from other membrane-associated GlcNAc-transferases, the enzyme was solubilized and partially purified from CHO membranes. A preliminary screen of detergents revealed that stable fractions could be effectively solubilized by extraction with Triton X-100. Approximately 56% of the membrane protein and 80% of the enzyme activity were solubilized by extraction of CHO microsomes with 0.5% Triton X-100. The activity was also fairly stable in Nonidet P-40 extracts, but Tween 20, Tween 80, CHAPS, sodium taurocholate and sodium deoxycholate markedly inhibited the activity. The effects of the detergents on CLS activity were virtually the same for microsomal fractions from both CHO cells and bovine brain.
A highly purified fraction of CLS (730-fold relative to CHO microsomes) was obtained by a combination of chromatographic steps using TSK-Gel Toyopearl-DEAE 650M, butyl-agarose and Reactive Blue 4-agarose as the chromatographic supports (Table 1). Although the CLS activity was enriched 730-fold relative to CHO microsomes, the pooled fractions still contained approximately 10 polypeptides as visualized by Coomassie blue staining of SDS-PAGE gels. Additional studies will clearly be required to identify the polypeptide(s) that constitute the active enzyme (18-20). The partially purified preparation of CLS exhibited a broad pH optimum between 8.0-8.5. Partially purified CLS also does not appear to have a divalent cation requirement, atypical of most N-acetylglucosaminyl transferases, and was actually stimulated by 10 mM EDTA. The partially purified fractions from the Reactive Blue 4-agarose column were used for the enzymology studies described below. It is not known if these partially purified fractions contained the mammalian homologues of both Alg13 and Alg14 (18-20).
Table 1.
Partial purification of CLS activity from CHO microsomes
| Purification Step | Protein Recovered (mg) | Enzyme Activity (×10-5) | Specific Activitya | Fold Purification |
|---|---|---|---|---|
| Microsomes | 192 | 4.6 | 2,396 | 1 |
| Detergent Extracts | 120 | 4.3 | 3,583 | 1.5 |
| Toyo-DEAE-I | 9.1 | 4.0 | 43,956 | 18.3 |
| Butyl-agarose | 2.0 | 3.9 | 195,000 | 81.4 |
| Toyo-DEAE-II | 1.2 | 3.85 | 320,833 | 134 |
| Reactive Blue 4-agarose | 0.12 | 2.1 | 1,750,000 | 730.4 |
Specific activity is based on the amount of [3H]GlcNAc-P-P-Dol converted to [3H]GlcNAc2-P-P-Dol (cpm/mg protein) as described in the Experimental Section.
Analysis of UDP-(5-F)-GlcNAc as a donor substrate for CLS
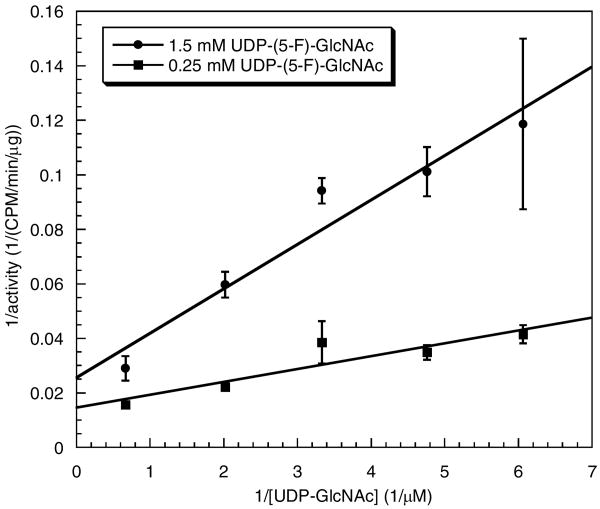
The first enzyme of interest, CLS (38), was partially purified from CHO cells as described above and used to evaluate the effect of a 5-F substituent in either the nucleotide sugar donor or the GlcNAc-lipid acceptor substrate. Using the natural acceptor substrate, [3H]GlcNAc-P-P-Dol, typical saturation kinetics data were obtained with the natural donor substrate, UDP-GlcNAc to provide a Km of 392 ± 83 μM and a Vmax of 27.5 ± 1.9 cpm/min/μg protein (Figure S1, Supporting Information). In contrast, UDP-(5-F)-GlcNAc was completely inactive as a glycosyl donor for CLS even under extended incubation conditions that led to complete conversion of [3H]GlcNAc-PP-Dol to a disaccharide product using UDP-GlcNAc as the donor substrate (data not shown). Moreover, UDP-(5-F)-GlcNAc apparently bound to the enzyme based on its ability to inhibit catalysis. Increasing the concentration of UDP-(5-F)-GlcNAc at a constant concentration of UDP-GlcNAc (300 μM, ∼Km) led to a marked reduction in rate with an IC50 of approximately 1.25 mM for UDP-(5-F)-GlcNAc (data not shown). As shown in Figure 2, inhibition at two different concentrations of UDP-(5-F)-GlcNAc could be overcome by increasing the amount of UDP-GlcNAc, thus indicating that UDP-(5-F)-GlcNAc acts as a competitive or mixed inhibitor of the CLS-catalyzed reaction. Limited availability of biosynthetic [3H]GlcNAc-P-P-Dol acceptor substrate prevented more extensive kinetic analysis of the inhibition of CLS by UDP-(5-F)-GlcNAc and a precise determination of the concentration of the glycolipid substrate used in these studies.
Figure 2.
Inhibition of CLS by UDP-(5-F)-GlcNAc. Each assay was carried out for 5 min as described in Materials and Methods using partially purified CLS (3.6 μg), UDP-(5-F)-GlcNAc (0.25 mM or 1 mM), and UDP-GlcNAc (165 μM-1.5 mM).
5-Fluoro GlcNAc lipid pyrophosphate as a CLS acceptor substrate
CLS-catalyzed glycosylation of (5-F)-GlcNAc-P-P-Undec (2.8 μM) acceptor substrate by UDP-GlcNAc for a period of 20 min led to acid-labile products (characteristic of the polyisoprenol phosphate linkage), which were chromatographically identical to (GlcNAc-β-(1,4)-GlcNAc)-P-P-lipids (data not shown). Thus, the presence of a 5-fluoro substituent does not interfere with the ability of (5-F)-GlcNAc-P-P-Undec to act as a glycosyl acceptor. Unfortunately, limited availability of the radiolabeled acceptors, [3H]GlcNAc-P-P-Undec and (5-F)-[3H]GlcNAc-P-P-Undec, available only via biosynthesis, prevented further kinetic analysis. UDP-(5-F)-GlcNAc was also tested as a glycosyl donor with the acceptor, (5-F)-[3H]GlcNAc-P-P-Undec. As expected, UDP-(5-F)-GlcNAc did not serve as a glycosyl donor under these conditions (data not shown).
5-Fluoro GlcNAc β-octyl glycoside as a GalT acceptor substrate
Because [3H]GlcNAc-P-P-Undec and (5-F)-[3H]GlcNAc-P-P-Undec were available in only small quantities, full kinetic analysis of (5-F)-GlcNAc-P-P-Undec as a CLS acceptor was not feasible. Thus, another enzyme, β-1,4-galactosyltansferase (GalT, EC 2.4.1.38) (39-41), that catalyzes a mechanistically similar reaction with readily available substrates, was investigated. To measure rates of the GalT-catalyzed reaction, a Sep-Pak assay (42) was used with both GlcNAc β-octyl glycoside (22) and (5-F)-GlcNAc β-octyl glycoside as glycosyl acceptors (Scheme 3). A preliminary comparison of octyl GlcNAc and octyl (5-F)-GlcNAc at near saturating concentrations of UDP-Gal (100 μM) was reported in a previous publication from this laboratory (13). These data indicated that the Km for the octyl (5-F)-GlcNAc was increased, whereas the kcat slightly decreased, thus leading to a ca. 8-fold decrease in kcat/Km. Here we report a full kinetic analysis of the GalT-catalyzed reactions with octyl GlcNAc and octyl (5-F)-GlcNAc as acceptor substrates.
Scheme 3.
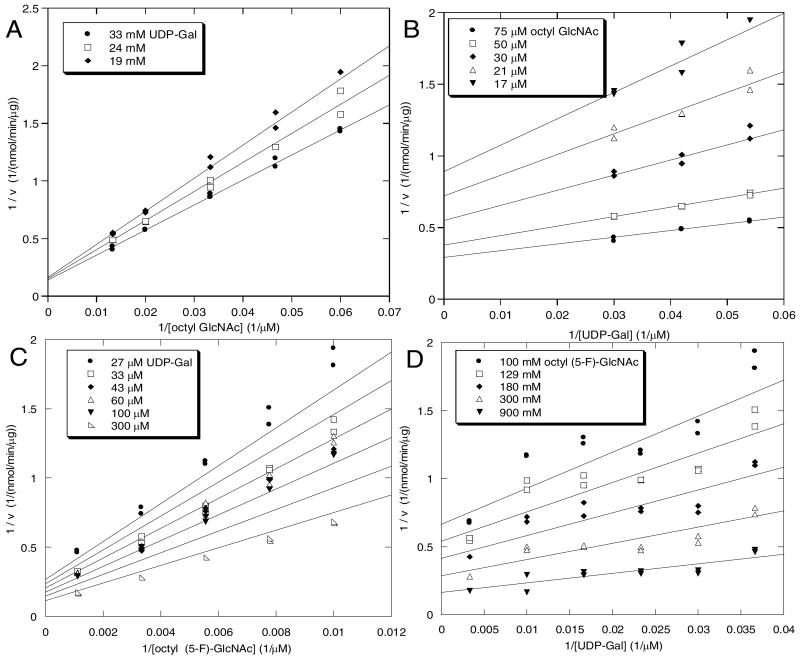
Saturation with both octyl-GlcNAc and octyl (5-F)-GlcNAc could not be achieved due to pronounced substrate inhibition. Double reciprocal plots at constant UDP-Gal concentrations (71.4 μM for octyl GlcNAc and 41 μM for octyl (5-F)-GlcNAc) show upward curvature at high substrate concentrations (See Supporting Information for details). Therefore, for determination of the kinetic parameters, both acceptor and donor substrates were varied in a non-inhibitory range. The non-fluorinated glycoside, octyl GlcNAc was evaluated as a GalT acceptor substrate with UDP-Gal as the donor, and double reciprocal plots of the resulting data are shown in Figure 3A and 3B. In a similar way, the fluorinated glycoside, octyl (5-F)-GlcNAc was evaluated with UDP-Gal as the donor, and double reciprocal plots of the resulting data are shown in Figure 3C and 3D. Using these data, kinetic constants were calculated for both acceptors based on least-squares fitting to eq 1, the rate equation for a steady-state ordered bi bi mechanism that has been demonstrated for a number of glycosyltransferases, including GalT (27, 39). The parameters Vmax, KiAKB, KB, and KA, derived from this fit, were inserted into the equation eq 1 and the predicted double reciprocal linear transforms were obtained (Figure 3 A-D, solid lines).
Figure 3.
Double reciprocal plots of GalT-catalyzed glycosylation of GlcNAc-β-octyl glycoside (3A, 3B) and (5-F)-GlcNAc-β-octyl glycoside (3C, 3D). The data were fit to eq. 1 (see text for details), and the lines shown are derived from inserting the fitted parameters into eq. 1.
| (1) |
Visual inspection indicates that the data obtained for GlcNAc-β-octyl glycoside as the acceptor substrate result in a better fit to the equation than data for the 5-fluoro analog. This is presumably due to an apparent slight instability of the fluorinated acceptor substrate and/or product (See Experimental Procedures). Based on analysis of these data, the value of kcat/KB for octyl (5-F)-GlcNAc is 4.3-fold less than the value for octyl GlcNAc (Table 2), due primarily to a significant effect on KB (5-fold increase) rather than kcat (∼10% increase). Within error, the kcat values for these two substrates are the same, signifying that the 5-fluoro group has little effect on the chemical step of the GalT reaction.
Table 2.
GalT kinetics data: GlcNAc-β-octyl glycoside vs. (5-F)-GlcNAc-β-octyl glycoside
| Acceptor Substrate | KiAKB (μM)2 |
KA (μM)
(Donor) |
KB (μM)
(Acceptor) |
kcat (s-1) |
kcat/KB (104 M-1s-1) |
|---|---|---|---|---|---|
| Octyl GlcNAc | 2500 ± 290 | 6.5 ± 6.6 | 110 ± 34 | 6.1 ± 1.3 | 5.7 ± 0.6 |
| Octyl (5-F)-GlcNAc | 22000 ± 3600 | 46 ± 20 | 560 ± 156 | 7.3 ± 1.5 | 1.31 ± 0.13 |
Discussion
In order to investigate the effect of fluorine substitution in glycosyltransferase donor and acceptor substrates, an isotopically labeled donor, UDP-(5-F)-[3H]GlcNAc, has been synthesized via acetylation of 5-fluoro glucosamine-1-phosphate with [3H]Ac2O to provide a radiolabeled 5-fluoro GlcNAc-1-phosphate, 2, which was converted to UDP-(5-F)-[3H]GlcNAc, 3, by standard methods available for the synthesis of nucleotide sugars. (5-F)-GlcNAc-β-octyl glycoside (13) was employed as the fluorinated acceptor substrate. The glycosyltransferases used in this study were UDP-GlcNAc:GlcNAc-P-P-Dol N-acetylglucosaminyl transferase (EC 2.4.1.141, chitobiosyl-P-P-lipid synthase, CLS), partially purified as described herein, and commercially available N-acetyllactosamine synthase (galactosyltransferase, GalT, EC 2.4.1.38). It is not clear if the partially purified fractions of CLS contained mammalian homologues to the Alg13 and Alg14 subunits of the heterodimeric complex of the yeast enzyme (18-20).
5-Fluorinated GlcNAc carbohydrates as donor substrates
Studies of CLS with UDP-(5-F)-GlcNAc as a donor clearly show that it serves as a competitive inhibitor of UDP-GlcNAc (Figure 2) rather than a substrate. Thus, a very strong inactivating effect is exerted simply by addition of the small but strongly electron-withdrawing 5-fluoro group. The simplest explanation of the magnitude of this effect is that the enzyme-catalyzed reaction proceeds through an oxocarbenium ion-like transition state which is destabilized by the presence of the electron-withdrawing fluorine at the neighboring 5-position. This type of mechanism has been supported by other studies of glycosyltransferases (7-9), but the experiments described herein provide the first evidence in support of an oxocarbenium ion-like transition state in a glycosyltransferase utilizing UDP-GlcNAc as a donor.
5-Fluorinated GlcNAc glycans as acceptor substrates
Substrate analogs with 5-fluoro substituents for both enzymes are competent acceptor substrates (Figure 3). Due to limitations in the quantity of biosynthetic substrates, GlcNAc-P-P-lipid or the 5-fluoro analog, a full kinetic study of the CLS-catalyzed reaction could not be undertaken. With GalT, however, octyl 5-fluoro GlcNAc is clearly a good substrate, with kcat/KB decreased by only 4.4-fold relative to the non-fluorinated substrate. Moreover, the modest reduction in catalytic efficiency results almost entirely from a higher KB; the values of kcat for the two substrates are within experimental error.
There are several possible explanations for why the values of kcat for the non-fluorinated and fluorinated substrates are nearly the same. First, if the bond-forming step of the GalT-catalyzed reaction is not rate limiting, the effect of the 5-fluoro group will be masked. Among many earlier studies on the kinetics of GalT-catalyzed glycosylation, a significant secondary isotope effect at C-1 was observed (DVmax/HVmax = 1.21) (43). A secondary isotope effect of this magnitude provides strong support for a reaction mechanism in which the chemical step of disaccharide formation, and not substrate binding or product release, is rate limiting.
A second possibility is that the 5-fluoro group is too distal to exert an electronic effect on the 4-OH. By analogy with the effect of a β-fluoro group on 2-fluoroethanol, we expect that the pKa of octyl-(5-F)-GlcNAc should be reduced by 1.6 units relative to octyl GlcNAc (44, 45). Electronic effects on general base-catalyzed-nucleophilic substitution reactions can be expressed using Hammett plots to give a correlation constant (βnuc) which estimates the amount of charge build up on the nucleophilic atom in the transition state of the reaction (46). Due to the complexity in synthesizing the required fluorinated substrates, it was not feasible to do a full analysis of βnuc for the GalT reaction to verify that the expected linear free energy relationship holds. Therefore, we cannot be certain that the transition state remains the same when the pKa of the acceptor is altered over a wide range. However, there is precedent that a linear free energy relationship holds for mechanistically similar enzyme-catalyzed reactions. For example, the deglycosylation of the galactosyl enzyme intermediate in β-galactosidase, a general base-catalyzed reaction with an oxocarbenium-like transition state, was shown to have a small and slightly negative (-0.06) β-nuc (47). Assuming the linear free energy relationship holds for GalT, even a reaction with a small nucleophilic correlation constant (βnuc) of 0.2, a change in pKa of -1.6 units would predict decrease in rate of 2-fold. This clearly was not observed in our experiments (Table 2). Our kinetics data are consistent with other studies suggesting that the GalT reaction proceeds via an oxocarbenium ion-like transition state consistent with a small βnuc. First, the significant secondary isotope effect observed for the GalT-catalyzed reaction (vide supra) led Raushel and coworkers to conclude that “substantial sp2 character develops on the anomeric carbon in the transition state.” Second, the fact that UDP-(2-F)-Gal acts as a competitive inhibitor of UDP-Gal and not an alternate substrate is consistent with an oxocarbenium ion-like transition state that is destabilized by the adjacent electron-withdrawing fluorine substituent at C-2 (48).
Taken together, the data for the 5-fluoro acceptors and donors support a transition state with significant bond breaking of the anomeric sugar-UDP bond yet little bond formation with the GlcNAc acceptor for the CLS and GalT-catalyzed reactions. Through the use of 5-fluoro glycosyltransferase donors and acceptors, the involvement of such a weakly associative transition state can be investigated for both substrates. The usefulness of nucleotide 5-fluoro sugars should extend well beyond the current study. In particular, other GlcNAc-transferases which catalyze reactions with retention of configuration, for which mechanistic data is fairly unclear, would be particularly amenable to this non-disruptive approach (11). 5-Fluoro nucleotide sugars could be used for many other carbohydrate processing enzymes that catalyze chemistry at or near the C-5 position including enzymes involved in nucleotide sugar epimerization and dehydration (13). Similarly, we envision 5-fluoro glycosides as useful tools for the investigation of glycosyltransferases that form bonds at the C-3 or C-6 OH positions.
Supplementary Material
Saturation curves for UDP-GlcNAc and GlcNAc-P-P-Dol with CLS, and evidence for uncompetitive substrate inhibition of GalT with octyl GlcNAc are shown in supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank Prof. Stephen Withers for stimulating discussions and encouragement.
Abbreviations
- BME
β-mercaptoethanol
- CHAPS
3-[(3-cholamidopropyl)dimethylamonio]-1-propanesulfonate
- CLS
UDP-GlcNAc:GlcNAc-P-P-Dol N-acetylglucosaminyltransferase (EC 2.4.1.141)
- Dol
dolichyl
- Dol-P
dolichyl phosphate
- GalT
β-N-acetylglucosaminyl-glycopeptide β-1,4-galactosyltransferase (EC 2.4.1.38)
- GlcNAc-P-P-Dol
GlcNAc-P-P-dolichol
- GlcNAc-P-P-Undec
GlcNAc-P-P-undecaprenol
- Glc3Man9GlcNAc2-P-P-Dol
Glc3Man9GlcNAc2-P-P-dolichol
- GPT
UDP-GlcNAc:Dol-P-GlcNAc 1-phosphate transferase (EC 2.7.8.15)
- octyl GlcNAc or GlcNAc β-octyl glycoside
octyl 2-deoxy-2-acetamido-β-D-glucopyranoside
- octyl (5-F)-GlcNAc or (5-F)-GlcNAc β-octyl glycoside
octyl 5-fluoro-2-deoxy-2-acetamido-β-D-glucopyranoside
- P-P
pyrophosphoryl linkage (-O-P(O)(OH)-O-P(O)(OH)-O-)
- Undec
undecaprenyl
- Undec-P
undecaprenyl phosphate
Footnotes
This research was supported in part by the Vahlteich Research Fund administered by the College of Pharmacy, University of Michigan (JKC) and by NIH grant GM36065 (CJW).
References
- 1.Sears P, Wong CH. Enzyme action in glycoprotein synthesis. Cell Mol Life Sci. 1998;54:223–252. doi: 10.1007/s000180050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signalling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 3.Hancock IC. Bacterial cell surface carbohydrates: structure and assembly. Biochem Soc Trans. 1997;25 doi: 10.1042/bst0250183. [DOI] [PubMed] [Google Scholar]
- 4.Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci. 2000;5:380–386. doi: 10.1016/s1360-1385(00)01720-9. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 6.Breton C, Snajdrova L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16:29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- 7.Murray BW, Takayama S, Schultz J, Wong CH. Mechanism of Human α-1,3-Fucosyltransferase V: Glycosidic Cleavage Occurs Prior to Nucleophilic Attack. Biochemistry. 1997;36:823–831. doi: 10.1021/bi962284z. [DOI] [PubMed] [Google Scholar]
- 8.Burkart MD, Vincent A, Duffels BW, Ley SV, Wong CH. Chemo-enzymatic synthesis of fluorinated sugar nucleotide: Useful mechanistic probes for glycosyltransferases. Bioorg Med Chem. 2000;8:1937–1946. doi: 10.1016/s0968-0896(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 9.Compain P, Martin OR. Design, Synthesis and Biological Evaluation of Iminosugar-Based Glycosyltransferase Inhibitors. Curr Top Med Chem. 2003;3:541–560. doi: 10.2174/1568026033452474. [DOI] [PubMed] [Google Scholar]
- 10.Banait NS, Jencks WP. Reactions of anionic nucleophiles with α-D-glucopyranosyl fluoride in aqueous solution through a concerted, ANDN (SN2) mechanism. J Am Chem Soc. 1991;113:7951–7958. [Google Scholar]
- 11.Lairson LL, Withers SG. Mechanistic analogies amongst carbohydrate modifying enzymes. Chem Comm. 2004:2243–2248. doi: 10.1039/b406490a. [DOI] [PubMed] [Google Scholar]
- 12.Williams SJ, Withers SG. Glysosyl fluorides in enzymatic reactions. Carbohydr Res. 2000;327:27–46. doi: 10.1016/s0008-6215(00)00041-0. [DOI] [PubMed] [Google Scholar]
- 13.Hartman M, Coward JK. Synthesis of 5-Fluoro N-Acetylglucosamine Glycosides and Pyrophosphates via Epoxide Fluoridolysis: Versatile Reagents for the Study of Glycoconjugate Biochemistry. J Am Chem Soc. 2002;124:10036–10053. doi: 10.1021/ja0127234. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Coward JK. Enzyme-catalyzed glycosylation of peptides using a synthetic lipid disaccharide substrate. J Org Chem. 1992;57:4126–4135. [Google Scholar]
- 15.Lee J, Coward JK. Oligosaccharyltransferase: Synthesis and use of deuterium-labeled peptide substrates as mechanistic probes. Biochemistry. 1993;32:6794–6801. doi: 10.1021/bi00077a034. [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Coward JK. 13C- and 15N-labeled peptide substrates as mechanistic probes of oligosaccharyltransferase. Biochemistry. 1997;36:14683–14689. doi: 10.1021/bi9719511. [DOI] [PubMed] [Google Scholar]
- 17.Schenk B, Fernandez F, Waechter CJ. The ins(ide) and outs(ide) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11:61R–70R. doi: 10.1093/glycob/11.5.61r. [DOI] [PubMed] [Google Scholar]
- 18.Chantret I, Dancourt J, Barbat A, Moore SEH. Two proteins homologous to the N- and C-terminal domains of the bacterial glycosyltransferase MurG are required for the second step of dolichyl-linked oligosaccharide synthesis in Saccharomyces cerevisiae. J Biol Chem. 2005;280:9236–9242. doi: 10.1074/jbc.M413941200. [DOI] [PubMed] [Google Scholar]
- 19.Bickel T, Lehle L, Schwarz M, Aebi M, Jakob CA. Biosynthesis of lipid-linked oligosaccharides in Saccharomyces cerevisiae - Alg13p AND Alg14p for a complex required for the formation of GlcNAc2-PP-dolichol. J Biol Chem. 2005;280:34500–34506. doi: 10.1074/jbc.M506358200. [DOI] [PubMed] [Google Scholar]
- 20.Gao XD, Tachikawa H, Sato T, Jigami Y, Dean N. Alg14 recruits Alg13 to the cytoplasmic face of the endoplasmic reticulum to form a novel bipartite UDP-N-acetylglucosamine transferase required for the second step of N-linked glycosylation. J Biol Chem. 2005;280:36254–36262. doi: 10.1074/jbc.M507569200. [DOI] [PubMed] [Google Scholar]
- 21.Ashida H, Maeda Y, Kinoshita T. DPM1, the catalytic subunit of dolichol-phosphate mannose synthase, is tethered to and stabilized on the endoplasmic reticulum membrane by DPM3. J Biol Chem. 2006;281:894–904. doi: 10.1074/jbc.M511311200. [DOI] [PubMed] [Google Scholar]
- 22.Boullanger P, Chevalier Y, Croizier MC, Lafont D, Sancho MR. Synthesis and surface-active properties of some alkyl 2-amino-2-deoxy-β-D-glucopyranoses. Carbohydr Res. 1995;278:91–101. [Google Scholar]
- 23.Rush JS, Rick PD, Waechter CJ. Polyisoprenyl phosphate specificity of UDP-GlcNAc:undecaprenylphosphate N-acetylglucosaminyl 1-P transferase from E. coli. Glycobiology. 1997;7:315–322. doi: 10.1093/glycob/7.2.315. [DOI] [PubMed] [Google Scholar]
- 24.Waechter CJ, Harford JB. A Dolichol-Linked Trisaccharide from Calf Brain: Biosynthesis and Structure. Arch Biochem Biophys. 1979;192:380–390. doi: 10.1016/0003-9861(79)90106-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Zeng Y, Lehrman M. Evidence that the Hamster Tunicamycin Resistance Gene Encodes UDP-GlcNac-Dolichol Phosphate N-Acetylglucosamine-1-Phosphate Transferase. J Biol Chem. 1992;267:8895–8902. [PubMed] [Google Scholar]
- 26.The experiment in which GlcNAc-β-octyl glycoside was used as the acceptor substrate was completed using a single stock solution of reagents for all of the experiments, and all of the assays were quenched in a single day. However, assays with (5-F)-GlcNAc-β-octyl glycoside were completed over several days from separate, freshly prepared reagent stock solutions. This precautionary method was employed due to concerns about the stability of (5-F)-GlcNAc-β-octyl glycoside, and perhaps the disaccharide product, in aqueous buffer.
- 27.Cornish-Bowden A. Analysis of Enzyme Kinetic Data. Oxford University Press; Oxford: 1995. [Google Scholar]
- 28.Wittmann V, Wong CH. 1H-Tetrazole as a Catalyst in Phosphomorpholidate Coupling Reactions: Efficient Synthesis of GDP-Fucose, GDP-Mannose, and UDP-Galactose. J Org Chem. 1997;62:2144–2147. doi: 10.1021/jo9620066. [DOI] [PubMed] [Google Scholar]
- 29.Moffatt JG, Khorana HG. Nucleoside Polyphosphates. X. The Synthesis and Some Reactions of Nucleoside-5′Phosphomorpholidates and Related Compounds. Improved Methods for the Preparation of Nucleoside-5′Polyphosphates. J Am Chem Soc. 1961;83:649–658. [Google Scholar]
- 30.Hoard D, Ott DG. Conversion of Mono- and Oligodeoxyribonucleotides to 5 -Triphosphates. J Am Chem Soc. 1965;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- 31.Fang X, Gibbs BS, Coward JK. Synthesis and Evaluation of Synthetic Analogues of Dolichyl-P-P-Chitobiose As Oligosaccharyltransferase Substrates. Bioorg Med Chem Lett. 1995;5:2701–2706. [Google Scholar]
- 32.Tai VWF, Imperiali B. Substrate specificity of the glycosyl donor for oligosaccharyl transferase. J Org Chem. 2001;66:6217–6228. doi: 10.1021/jo0100345. [DOI] [PubMed] [Google Scholar]
- 33.Tai VWF, O'Reilly MK, Imperiali B. Substrate specificity of N-acetylglucosaminyl(diphosphodolichol) N-acetylglucosaminyl transferase, a key enzyme in the dolichol pathway. Bioorg Med Chem. 2001;9:1133–1140. doi: 10.1016/s0968-0896(00)00334-5. [DOI] [PubMed] [Google Scholar]
- 34.Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. Better substrates for bacterial transglycosylases. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Fechter EJ, Wang TS, Barrett D, Walker S, Kahne DE. Synthesis of heptaprenyl-lipid IV to analyze peptidoglycan glycosyltransferases. J Am Chem Soc. 2007;129:3080–3081. doi: 10.1021/ja069060g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman MCT. PhD Thesis. University of Michigan; Ann Arbor: 2002. [Google Scholar]
- 37.McLachlan KR, Krag SS. Three enzymes involved in oligosaccharide-lipid assembly in Chinese hamster ovary cells differ in the lipid substrate preference. J Lipid Res. 1994;35:1861–1838. [PubMed] [Google Scholar]
- 38.Kean EL, Niu N. Kinetics of formation of GlcNAc-GlcNAc-P-P-dolichol by microsomes from the retina of the embryonic chick. Glyconjugate J. 1998;15:11–17. doi: 10.1023/a:1006931230848. [DOI] [PubMed] [Google Scholar]
- 39.Morrrison JF, Ebner KE. Studies of galactosyltransferase - kinetic investigations with N-acetylglucosamine as galatosyl group acceptor. J Biol Chem. 1971;246:3971–3984. [PubMed] [Google Scholar]
- 40.Bell JE, Beyer TA, Hill RL. The kinetic mechanism of bovine milk galactosyltransferase - role of lactalbumin. J Biol Chem. 1976;251:3003–3013. [PubMed] [Google Scholar]
- 41.Ramakrishnan B, Qasba PK. Crystal structure of lactose synthase reveals a large conformational change in its catalytic component, the β-1,4-galactosyltransferase-1. J Mol Biol. 2001;310:205–218. doi: 10.1006/jmbi.2001.4757. [DOI] [PubMed] [Google Scholar]
- 42.Palcic MM, Heerze LD, Pierce M, Hindsgaul O. The use of hydrophobic synthetic glycosides as acceptors in glycosyltransferase assays. Glycoconjugate J. 1988;5:49–53. [Google Scholar]
- 43.Kim SC, Singh AN, Raushel FM. Analysis of the galactosyltransferase reaction by positional isotope exchange and secondary deuterium isotope effects. Arch Biochem Biophys. 1988;267:54–59. doi: 10.1016/0003-9861(88)90007-0. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi S, Cohen LA, Miller HK, Peake EG. Calculation of the pKa values from sigma constants and from the carbonyl frequencies of their esters. J Org Chem. 1971;36:1205–1209. [Google Scholar]
- 45.Timperley CM, White WE. The steric and electronic effects of aliphatic fluoroalkyl groups. J Fluorine Chem. 2003;123:65–70. [Google Scholar]
- 46.Ta-Shma R, Jencks WP. How does a reaction change its mechanism? General base catalysis of the addition of alcohols to 1-phenylethyl carbocations. J Am Chem Soc. 1986;108:8040–8050. [Google Scholar]
- 47.Richard JP, Westerfeld JG, Lin S, Beard J. Structure-reactivity relationships for beta-galactosidase (Escherichia coli, lac Z). 2. Reactions of the galactosyl-enzyme intermediate with alcohols and azide ion. Biochemistry. 1995;34:11713–11724. doi: 10.1021/bi00037a008. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi T, Murray BW, Wang R, Wong CH. A chemoenzymatic synthesis of UDP-(2-deoxy-2-fluoro)galactose and evaluation of its interaction with galactosyltransferase. Bioorg Med Chem Lett. 1997;5:497–500. doi: 10.1016/s0968-0896(96)00263-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Saturation curves for UDP-GlcNAc and GlcNAc-P-P-Dol with CLS, and evidence for uncompetitive substrate inhibition of GalT with octyl GlcNAc are shown in supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.