Abstract
Modic changes (MC) are a common phenomenon on magnetic resonance imaging (MRI) in spinal degenerative diseases and strongly linked with low back pain (LBP). Histology, radiology, potential mechanisms, natural history and clinical studies of MC has formed the foundation on which our understanding of spinal degenerative diseases is built. The objective of this study was to provide a review of recent important advances in the study of MC and their clinical significance. This review article summarizes these studies, by delineating the possible mechanisms, and raising doubts and new questions. The related aspects such as discography and differential diagnosis with spinal infection and tumor on MRI are also discussed. Although most of researchers believe that MC are common findings in patients with spinal degenerative diseases and have an association with discogenic LBP, different results between studies may be produced from the differences in study design, inclusion criteria, and sample size. How the present knowledge of MC affects the management of spinal degenerative diseases remains unclear. Further studies of MC will explore therapeutic possibilities for future treatments of spinal degenerative diseases.
Keywords: Modic changes, Low back pain, Vertebral endplates, Magnetic resonance imaging, Discography
Introduction
Signal intensity changes of vertebral endplates and subchondral bone are often observed in magnetic resonance imaging (MRI) in the patients with spinal degenerative diseases. In 1988, Modic et al. [46, 47] summarized these changes and classified them into three types, and then modic changes (MC), as a medical term, were used in the studies on spinal degenerative diseases. With further research, MC was found to be one of parameters of the morphological changes in spinal degenerative diseases on MRI. Although the etiology of MC remains poorly understood, some progress has been achieved in basic research during the past two decades, including studies of the prevalence and some clinical significance.
Materials and methods
Peer-reviewed, full-text articles were identified using a Pubmed (http://www.ncbi.nlm.nih.gov/sites/entrez/) search strategy with the keywords of Modic change(s), vertebral endplate(s), low back pain, signal abnormality, magnetic resonance imaging and discography. The search was limited to studies on humans, published in English and in the period from January 1987 up to February 2008.
To be included in the study, it was strictly necessary for every article to provide information about the epidemiology, natural history, clinical significance of MC. More than 300 articles were identified in the search, and 207 abstracts and 68 full-text papers were read. Some of these articles recorded other information like differential diagnosis, mechanism, prevalence and distribution, relationship to discography. A total of 165 articles without adequate information regarding these issues were eliminated. Important studies were summarized in Tables 1 and 2 for further analysis.
Table 1.
The prevalence and conversion of MC
| Authors | Study population | Sex (F/M) | Age | P | N | Prevalence (%) | Follow-up | Conversion (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All type | Type I | Type II | Type III | 0 → I | 0 → II | I → II | II → I | I → 0 | |||||||
| Albert et al. [5] | Patients with sciatica | 18–65 | L | 181 | 25a | 10c | 14 | 1 | 14 months | 24c | |||||
| Chung et al. [16] | Asymptomatic subjects | 31/28 | 20–75 | L | 59 | 8b | 2 | 6 | |||||||
| Girard et al. [21] | Adult patients | 28/12 | 23–80 | T | 40 | 2.3b | 2.3 | 4–149 weeks | 0.8 | 0.8 | |||||
| Karchevsky et al. [28] | Consecutive patients | 62/38 | 15–88 | L | 100 | 58a | 24 | 33 | 1 | ||||||
| Kleinstuck et al. [33] | With chronic LBP | 26/27 | <65 | L | 53 | 62a | |||||||||
| Kjaer et al. [32] | General population | 234/205 | 12–14 | L | 439 | 0.5a | 0.5 | ||||||||
| Kjaer et al. [31] | The general population aged 40 | 213/199 | 40 | L | 412 | 22a | 15 | 7 | |||||||
| Kuisma et al. [36] | Unoperated sciatica patients |
21/39 | 23–76 | L | 60 | 23b | 2c | 21 | 3 years | 3c | 1 | 0.7 | 2.6c | ||
| Kuisma et al. [37] | Patients with LBP | 0/228 | 36–56 | L | 228 | 16b | 5 | 11 | |||||||
| Mitra et al. [43] | Patients with LBP or sciatica | 18–70 | L | 670 | 18a | 18 | 1–6 years | 52 | |||||||
| Modic et al. [47] | Patients with LBP and/or sciatica | L | 474 | 20a | 4 | 16 | 1–3 years | 83 | |||||||
| Peterson et al. [51] | Patients with neck pain | 71/47 | 14–94 | C | 118 | 16a | 11 | 2.5 | 4.2 | ||||||
| Toyone et al. [60] | Patients with LBP | L | 500 | 19a | |||||||||||
| Vital et al. [62] | Operated and with MC I | 10/7 | 35–62 | L | 17 | 100a | 100 | 6 months | 76 | 24 | |||||
| Weishaupt et al. [65] | LBP patients | 23/27 | 28–50 | L | 50 | 22b | 14 | 8 | |||||||
| Weishaupt et al. [64] | Asymptomatic subjects | 30/30 | 20–50 | L | 60 | 11a | 2 | 7 | 2 | ||||||
aThe percentage of MC in study population
bThe percentage of MC at per disc
cIncluding mixed type I and II
L lumbar, T thoracic, C cervical, P position, N number, MC Modic changes, LBP low back pain
Table 2.
The relationship between MC and LBP
| Author and location | Study population | Number | Association between MC and LBP | Other findings |
|---|---|---|---|---|
| Albert et al. [5] 2007 Denmark | Patients with sciatica | 181 | MC type I is more strongly associated with lumbar pain than MC type II | Disc herniation is a strong risk factor for developing MC (especially type I) |
| Carragee et al. [14] 2005 USA | Subjects with LBP or (non-lumbar) pain syndrome | 100 | MC are weakly associated with an adverse outcome | Psychosocial variables strongly predicted long- and short-term LBP problems |
| Jarvik et al. [25] 2005 USA | Veterans Affairs out-patients without LBP | 148 | No association between new LBP and MC type I | Depression is an important predictor of new LBP |
| Kjaer et al. [31] 2006 Denmark | 40-year-old Danes | 412 | MC are strongly associated with LBP | People with LBP and MC may deserve to be diagnosed as having specific LBP |
| Kjaer et al. [32] 2005 Denmark | 13-year old children | 439 | MC are strongly associated with LBP | |
| Kuisma et al. [37] 2007 Finland | Middle-aged male workers (159 train engineers and 69 sedentary controls) | 228 | MC show significant association with pain symptoms and increased frequency of LBP | MC at L5-S1 and MC type I are more likely to be associated with LBP than other types of MC or MC located at other lumbar levels |
| Schenk et al. [57] 2006 Switzerland | Female subjectswith persistent LBP | 109 | MC are found to be significantrisk factors for LBP | |
| Toyone et al. [60] 1994 Japan | Chronic LBP patients | 500 | MC type I are correlated with LBP |
MC Modic changes, LBP low back pain
Definition and classification
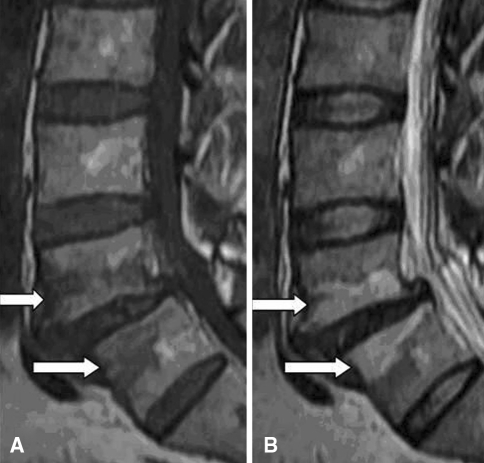
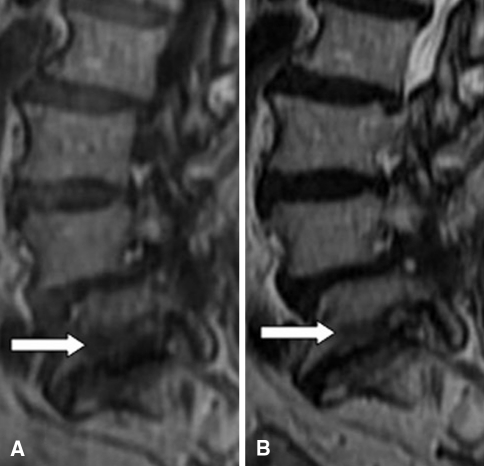
MC are bone marrow and endplate lesions visible on MRI. Bone marrow signal changes in the vertebral bodies were first reported by de Roos et al. [19]. Modic et al. [47] were credited with the classification of these signal intensity changes. Among 474 lumbar MRI performed in patients with LBP or sciatica, they observed MC type I [hypointense signal in T1-weighted imaging (T1WI) and hyperintense signal in T2-weighted imaging (T2WI), Fig. 1] corresponding to vertebral body edema and hyper-vascularity in 20 patients (4%) and MC type II (hyperintense signal in T1WI and hyperintense signal in T2WI, Fig. 2) reflecting fatty replacements of the red bone marrow in 77 patients (16%) in 1988. Afterwards, MC type III (hypointense signal in T1WI and hypointense signal in T2WI, Fig. 3) consisting of subchondral bone sclerosis were described by Modic et al. [46] at the same year and this type was much rarer.
Fig. 1.
MC type I (arrows): hypointense on T1WI (a) and hyperintense on T2WI (b)
Fig. 2.
MC type II (arrows): hyperintense on T1WI (a) and isointense or hyperintense on T2WI (b)
Fig. 3.
MC type III (arrows): hypointense on T1WI (a) and hypointense on T2WI (b)
In order to correspond to the Modic classification system, MC was divided into four grades by Miller [42] in 1990, namely grade 0: normal, no degeneration; grade one: equivalent to MC type I; grade two: equivalent to MC type II; grade three: equivalent to MC type III. Then, Weishaupt et al. [65], according to the vertebral height involved by endplate abnormalities on the midsagittal image, divided MC into four degrees: normal, no anomaly in T1, T2WI; mild, the scope of signal intensity changes equal to or less than 25% of vertebral height; moderate, the scope of signal intensity changes between 25 and 50% of vertebral height; and severe, the scope of signal intensity changes equal to or more than 50% of vertebral height.
Differential diagnosis from spinal infection and tumor on MRI
Although spinal infection and tumor may manifest like MC on MRI [9], the correct diagnosis is achieved usually by distinguishing their unique characteristics. With surrounding paravertebral soft-tissue edema or epidural mass effect, spondylodiscitis present as lesions with typically hyperintense signal on T2WI, compared to normal or hypointense signal on T2WI in degenerated disc, and with confluent hypointense signal on T1WI from the vertebral bodies and intervertebral disc space [9, 45]. In addition, the erosion of vertebral body and endplates are always observed in intervertebral disk space infection, whereas MC may be focal or diffuse along the endplates but tend to be linear and always parallel to the endplates [36]. In addition, the possibility of Schmorl’s node must always be kept in mind. Schmorl’s nodes are characterized by a localized defect (hypointense on T1WI and hyperintense on T2WI) in endplates with a well-defined herniation pit and a surrounding wall of hypointense signal (on T1WI and T2WI) in the vertebral body [66]. Although bone marrow edema and sclerosis on MRI may also be identified in patients with spondyloarthropathy [24], systematic symptoms and plain radiographs may help differentiate them from MC.
In fact, metastasis is the most common type of neoplastic lesion found in the spinal column. Rare instances of metastatic involvement of the disc have been noted [54]. Therefore, metastatic disease is readily distinguished from MC by the absence of disc space involvement.
Reliability of the classification system
In a special study on the reliability of the Modic classification [27], the authors found that the individual intraobserver agreement was substantial or excellent with kappa values ranging from 0.71 to 1.00 and the overall interobserver agreement was excellent with a kappa value of 0.85. Subsequently, Peterson et al. [50] reported the results of their study on the reliability of identifying and categorizing MC in clinical practice, showing that good intra- and interobserver agreement, especially at the L4–L5 level. In addition, other studies [16, 28, 36] with the kappa value at the range of 0.64–0.87 further supported intra- and interobserver reliability of the Modic classification system. Recently, Jensen et al. [26] confirmed that the “Nordic Modic Classification”, as a detailed evaluation protocol of vertebral endplate signal changes, was reproducible and helpful to future studies.
These results suggest that the Modic classification system is reliable, simple and easy to apply for observers with various clinical experiences. With confirmation of the reliability of Modic classification, the results of previous studies using the Modic classification system are more credible and the comparability among these studies regarding MC further is strengthened. Therefore, it was suggested the use of this system in future studies for evaluating the relationship between patient symptoms and treatment outcomes.
Prevalence and distribution
MC may be detected in the lumbar, cervical and thoracic spine of human being (Table 1) and in the spine of some animals [8]. The prevalence of MC varies from 18 to 62% in the patients with LBP, with different ratio for each type [5, 28, 33, 43, 47, 60]. According to the results of previous studies, type I and type II are the most common patterns in the lumbar spine. However, it is disputed whether type II is more frequent than type I, whereas two studies have shown that type I may be more common [31, 65]. Meanwhile, Kjaer et al. [32] identified the prevalence of MC was only 0.5% in a study of 439 thirteen-year-old children. The inconsistent results between studies might be produced from the differences in study design, inclusion criteria, and sample size. Furthermore, MC are associated with increasing age, weight and male gender [28, 39].
Two studies showed that the prevalence of MC in asymptomatic persons was lower than in the patients with LBP. Chung et al. [16] investigated the frequency and distribution of MC in 59 asymptomatic subjects and found 11 MC type I and 38 MC type II in 590 lumbar vertebral endplates. In other study of 60 asymptomatic subjects by Weishaupt et al. [64], one reader identified that the prevalence of MC on MRI only was 11% in the study population (type I, 2%; type II, 7% and type III, 2%).
Three distribution characteristics of MC in lumbar spine should be noted. First, Modic et al. [47] observed that the distribution of MC at L4–L5 or L5–S1 were most common. These observations were confirmed by Kuisma et al. [37] Moreover, the depth and extent of MC were greatest at L4–L5 and L5–S1 [36]. Second, for the location of the signal changes, the distribution of type I and II was more in anterior 1/3 of vertebra than in posterior 2/3 of vertebra and the distribution of type II was predominant in the superior endplate versus in the inferior endplate [16]. MC are also characterized with being parallel on both sides of the disc and with vertical depth varied between 3 and 30 mm [36]. Third, the location according to Chung et al. [16] for asymptomatic MC is at a higher level (superior endplates of L3 and L4) and anteriorly, whereas the symptomatic ones which apparently are at a lower level (surrounding the L4–5, L5–S1 discs) [36, 37, 47].
In contrast with plenty studies of MC in the lumbar spine, Peterson et al. [51] reported the prevalence and distribution of MC in the cervical spine in 118 patients with neck pain. In this study, MC was observed in 19 patients (16%) and type I and type III were far more common in the cervical spine with the C5–6 level being the most commonly involved. Girard et al. [21] found that MC was an uncommon manifestation of thoracic disc disease. The incidence of MC in thoracic vertebrae and endplates was 2.3% (11/480) and the new incidence was 1.6% (8/480) in 4–149 week follow-up, with the findings predominating in the lower intervertebral levels from T6 to T10.
Potential mechanisms
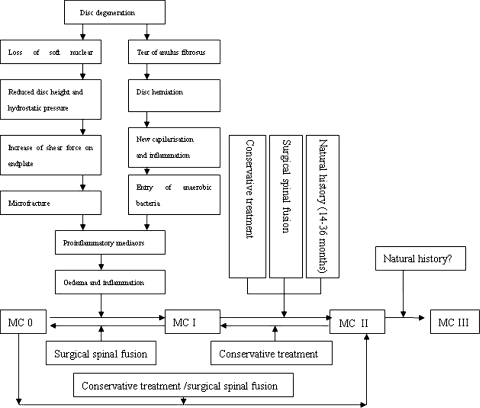
MC in subchondral bone and vertebra are not specific to degenerative disease [62] and can be detected on MRI in different conditions such as infectious, degenerative and immunological diseases. However, the pathogenetic mechanisms causing MC are not very clear. According to the current literature, there are two possibilities: biomechanical and biochemical causes (Fig. 4).
Fig. 4.
Mechanisms and conversion of MC and effects of clinical intervention (adapted from Vital et al. [62])
Biomechanics
Endplates play a very important role in the biomechanical functions of the spine. During human disc degeneration, the endplates undergo calcification with aging and replacement by bone, and exhibit altered structure, microfailure [22]. Such changes may lead to an uneven distribution of loads across the entire disc and thus may contribute to endplate fissures [47]. The endplates and vertebral body are ‘weak link’ of the spine, because microfractures and healing trabeculae are frequently observed in most cadaveric vertebral bodies [1]. In addition, the evidence provided by Adams et al. [1–3] showed that the loss of the nucleus pulposus, for the reason of bulging into the adjacent vertebra, herniation or desiccation and dehydration for severe degeneration, could increase the shear forces on the endplates and the trabeculae and result in the occurrence of the microfractures.
The microfractures and fissures in the endplates through the biomechanical mechanism may be a major source of MC. Modic et al. [47] demonstrated that MC type I was disruption and fissures of the endplates. If the microfractures take place recently, they will show the hypointense signal on T1WI and hyperintense signal on T2WI, equivalent to MC type I [23]. Hence, this phenomenon might intimately reflect edema and vascularisation following cumulative trauma and an inflammatory response after microfracture in the endplates. The study by Schmid et al. [58] showed that hyaline cartilage in the extruded disc material from the patients with MC was more than those in patients without MC. The notion that MC may be the expression of altered mechanical stress was further supported by the observation that the conversion of MC type I to type II for the fusion and instrumented stabilization [62].
Although the exact causes of MC are not clear, their occurrence may be closely related to some mechanical stress [44]. The abnormal load and stress will affect vertebral endplates and the microenvironment of adjacent vertebral bone marrow, resulting in histological changes, which exhibit signal intensity change on MRI, namely MC [47].
Biochemical mechanism
Compared with the study of biomechanical mechanism, more studies have been carried out for the biochemical mechanism of MC. Crock et al. [17, 18] suggested that upregulation of inflammatory mediators in the nucleus pulpous could result in a local inflammation associated with LBP. Therefore, MC possibly resulted from the inflammatory reaction by the toxic substances from degenerative disc [10]. Notably, the theory introduced by Albert et al. [4] claims that a disc herniation is the entry point of the bacteria and that MC likely are the result of entry of anaerobic bacteria resulting in oedema and inflammation surrounding the extruded nuclear material. However, this hypothesis has not been proved up to now.
Numerous studies have reported chemical anomalies involved in the disc herniation. The higher levels of proinflammatory mediator such as interleukin-6 (IL-6), interleukin-8 (IL-8), and prostaglandin E2 (PGE2) were observed by Burke et al. [11] in the discs of patients with LBP than in those with sciatica. Using the immunohistochemical method, Ohtori et al. [49] found that protein gene product 9.5 (PGP)-immunoreactive nerve fibers and tumor necrosis factor (TNF)-immunoreactive cells in the endplates from patients with MC was significantly more than in normal endplates on MRI. Moreover, the number of TNF-immunoreactive cells in endplates with MC type I was higher than in endplates with MC type II. The authors suggested that inflammation mediators and nerve ingrowth into vertebral endplates might be a cause of discogenic LBP and that MC type I was more likely resulted from inflammation mediators, whereas MC type II/III appeared to represent a more stable state. Additionally, it was recently confirmed by Rannou et al. [53] that the level of high-sensitivity C-reactive protein (hsCRP) in patients with chronic LBP and MC type I was higher than in the patients with MC 0 and MC type II. These studies indicated that the upregulation of proinflammatory mediators within the degenerative disc may be the major origin of discogenic LBP. This is further supported by the study of Masaryk et al. [41], who produced MC in adjacent endplates by injecting chymopapain into the discs of patients with disc herniation. The authors believed that the endplate changes were inflammatory. The biochemical mechanism of MC is also supported by the fact that patients with chronic LBP and predominantly MC type I had better short-term efficacy following intradiscal steroid injection than those with predominantly MC type II [20].
Natural history and conversion between patterns
In order to clarify the differences among MC types, the conversion and natural history of MC have been assessed with a long- or short-term follow-up (Table 1). Modic et al. [46, 47] firstly found that MC type I in five of six patients converted to a type II pattern in 14 months to 3 years without any intervention and MC type II in ten patients remained stable over a 2 to 3-year period. Subsequently, in a follow-up study for 12–72 months of 48 disc levels with type I, Mitra et al. [43] identified 18 (37.5%) converting fully to type II, 7 (14.6%) partially converting to type II, 19 (39.6%) becoming worse (more extensive type I) and 4 (8.3%) showing no change. In another 3-year study, 10 of 70 discs (14%) with MC at baseline displayed another type at final follow-up [36]. According to the current literature, the interconversion among type 0, I and II possibly occur. The conversion of type I to II is most common and the time span of different conversion is at least 1 year.
Most of conversions are often localized at L5–S1 and colocalized with a symptomatic disc herniation [36]. However, the new MC are more remarkable phenomenon. Kuisma et al. [36] reported the incidence of new MC during the 3-year follow-up was 6% (13 of 230). Most of new MC were found also at L4–L5 or L5–S1, and colocalized with a symptomatic disc herniation. These findings were confirmed by Albert et al. [5], who found that the incidence of new MC type I was closely related to a previous disc herniation and higher in patients who had undergone surgery for lumbar disc herniation.
MC type I appear to be more fluid and variable and will become seriously or convert into type II in most cases, whereas MC type II appear to be a more stable state. However, MC type II is not unchangeable and may convert into MC type I in unstable conditions. Given the current evidence, the incidence of type I converting to II, type I converting to 0 (normal), and type II converting to I degraded in order. Consequently, MC type I represent the major transition point with normality and it is the intermediate process from the early degeneration induced instability to restabilization at later stage.
Nevertheless, Marshman et al. [40] recently detected the unstable and symptomatic MC type II in two cases. Although MC type II transformed reversely into MC type I in two female patients, the severity of chronic LBP did not change. Therefore, the authors thought that MC type II was not stable and quiescent as originally believed. Furthermore, mixed MC (I/II and II/III) have also been identified, suggesting that all MC perhaps are interchangeable from one type to another and that they all present different stages of the same pathologic process [10, 36]. It is possible that direct conversion among MC types should be justified in the future study.
Relationship to LBP
It is frequently stated that only a small proportion (approximately 20%) of patients with LBP can certainly be diagnosed based on a pathoanatomical entity [63]. To clarify the relations between MC and LBP, a series of prospective studies have been conducted (Table 2). The study of Albert and Manniche [5] showed that the prevalence of MC type I increased from 9% at baseline to 29% during the follow-up in patients with LBP, indicating a strong association between MC (especially type I) and nonspecific LBP. Similar results have been described by Kjaer et al. [30, 31] in the study of 412 40-year-old Danes. Afterwards, Kuisma et al. [37] showed that MC at L5–S1 and MC type I are more possible to be related with LBP than other levels and types of MC. Isolating the subgroup of the patients with MC and LBP may deserve with diagnostic and therapeutic interest. Nevertheless, a 5-year follow-up study by Carragee et al. [14] showed that moderate or severe MC were weakly associated with poor outcome in the patients with persistent LBP. Meanwhile, Jarvi et al. [25] showed that MC type I was not a risk factor of new LBP in a study of 148 outpatients without LBP, indicating that the appearance of LBP and the occurrence of MC perhaps did not synchronize.
Most of the results aforementioned confirmed that MC are strongly linked with discogenic LBP, but there exist controversies regarding the relationship between MC and the outcomes following clinical intervention or new LBP. The controversies may come from either the differences of inclusion criteria, study methods and sample size, or the differences of the compliance, social and psychological factors in selected patients and the experience of the researchers.
How can MC cause the pain? Pain may be generated from all types of tissue where free nerve endings are present, these have been demonstrated as they are present in most types of tissue in the spinal column [7, 15]. Pathological changes of these structures will stimulate inevitably the corresponding pain receptors, thus resulting in pain. Other than mechanical problems of the spine, a large number of inflammatory and signaling substances, including different cytokines such as TNF, IL-6, IL-8 and PGE2, have been suggested to play a role in LBP [11, 49]. In a randomized trial, Korhonen et al. [35] evaluated the long-term efficacy of infliximab (a monoclonal antibody against TNF-α) in patients with acute/subacute sciatica secondary to disc herniation. The patients in the infliximab group appeared to be treated with better efficacy in cases of a L4–L5 (or L3–L4) disc herniation with the concurrence of MC, although similar efficacy was noted between infliximab and placebo group. Therefore, there may be an association between TNF-α and LBP induced by MC. Accordingly, the obvious variation of inflammation mediators in the endplates with MC would be one of the causes of LBP. Then, the reason that MC type I is more associated with LBP perhaps is that MC type I represents the earlier stage of inflammation and produces more proinflammatory chemical mediators.
Some authors investigated the MC among different occupational groups. An MRI study [57] was performed in 109 female subjects selected from nursing and administrative professions with LBP. Only nerve root compromise and endplate changes in the lower lumbar spine were significant risk factors for LBP. However, there is the similar incidence of MC in nurses and administrative workers with LBP (30.4 and 29.4%, respectively). Kuisma et al. [37] studied 228 middle-aged male workers (159 train engineers and 69 sedentary). The prevalence of MC was similar in both occupational groups. The two results may provide the evidence that the type of occupation will not influence on the incidence of MC.
Furthermore, a MRI study [59] of 125 patients showed an incidence of 4.8% for retrolisthesis combined with MC at L5–S1 level and the coexistence of retrolisthesis and MC were more often in the smokers and those without insurance. The relationship between heavy smoking and MC also was showed by Leboeuf-Yde et al. [39]. Additionally, MC in the pedicles adjacent to spondylolytic defects on MRI were noted by Ulmer et al. [61] in 24 (40%) of the 60 patients with lumbar spondylolysis. These findings may provide assistance in diagnosing correctly spondylolysis on MRI.
Relationship to discography
At present, it is disputed that discography is an effective means to diagnose discogenic LBP. In a discography study [10], the presence of MC showed a highly significant association with pain reproduction at discography. Toyone et al. [60] reported that when the signal intensity changes in the endplates and decreased signal intensity in degenerative lumbar discs were combined, the specificity of using MRI to diagnose disc pain disease would increase from 79 to 97%. The signal intensity changes in endplates indicate a high degree of specificity, but the lack of sensitivity in discogenic LBP. Buttermann et al. [12] also confirmed that MC have high pain sensitivity on discography (severe concordant pain at low intradiscal pressures).
MC are of important value in the diagnosis of discogenic LBP, but MRI does not completely replace the discography due to the lack of the sensitivity. MRI was performed immediately before and within 2 h after uncomplicated lumbar discography in a study of 20 consecutive patients [55], and 7 of them were reassessed average 72 days after discography. The results showed that the uncomplicated lumbar discography would not lead to the occurrence of new MC. However, in other studies [34, 56], pain reproduction at discography were not evidently linked with the presence or absence of the lumbar MC. Therefore, further studies would be required to determine the association between discography and MC.
Clinical intervention and prognosis
As different types of MC may have different clinical significances, the influence of clinical intervention on MC has been evaluated regarding surgical and conservative treatment (Fig. 4).
Arthrodesis is a common surgical method for the patients with spinal instability. In the study of Lang et al. [38], it was suggested that the occurrence of MC type I are the signs of the pseudarthrosis formation by evaluating segmental spinal instability in 33 patients after spinal fusion. This result was consistent with the observation of Buttermann et al. [13] that nonfusion was associated predominantly with the persistence of MC type I. Toyone et al. [60] found that patients with MC type I tended to show hypermobility, when compared with those with MC type II, and require fusion surgery. Additionally, MC also has an impact on surgical results. Kim et al. [29] reported that the presence of MC was a risk factor for recurrence after successful percutaneous endoscopic lumbar discectomy. Similar result by Barth et al. [6] was shown that MC was a factor of the etiology of unfavorable clinical outcome after surgery for disc herniations.
It has been found that most MC type I naturally tend to convert to type II in 18–24 months [47, 62]. Nevertheless, the clinical intervention may effect on the course of MC. Vital et al. [62] examined 17 patients with MC type I using MRI 6 month after posterior instrumented posterolateral fusion and found the conversion from type I to type 0 in 4 patients and from type I to type II in the remaining 13 patients. It appears that posterior fusion accelerates the course of MC type I, probably by correcting mechanical instability. Putzier et al. [52] compared sole nucleotomy with nucleotomy and additional dynamic stabilization in the treatment of symptomatic disc prolapse in 119 patients with MC type I. At 3 months follow-up, accelerated segmental degeneration existed in the solely nucleotomized group but no progression of disc degeneration was noted in the dynamically stabilized group. It is thus evident that the changes of mechanical conditions in spinal segments would slow the proceeding of MC.
Up to now, there are not unanimous conclusions on minimally invasive surgery for treating degenerative disc diseases with MC. The evidence [12] showed that spinal steroid injections were more effective for treating lumbar degenerative disc diseases with MC. However, there were no changes of MC and disc height after the intradiscal electrothermal therapy procedure in the patients with symptomatic intervertebral disc [48].
According to the information aforementioned, there are intimate relations between MC and the outcomes following clinical intervention. Good efficacy of clinical intervention on MC type I have been shown, even if those studies have the limitations of small sample size and short-term follow-up. The studies regarding MC type II and III are still scant. Although stabilization or fusion procedure has been suggested for the patients with symptomatic MC type I, this recommendation seems somewhat premature according to our current understanding of MC. Further studies are required to delineate the natural history of MC and to determine the relationship to biomechanical factors and symptoms. In a logical sense, an animal model would be necessary to prove these hypotheses. Unfortunately, this is not the case [44].
Summary and perspective
MC are a common phenomenon on MRI of spinal degenerative diseases. Two hypotheses, the biomechanical and biochemical causes, are possibly potential mechanisms underlining MC. To date, however, the evidences supporting the two hypotheses are not sufficient and how they play a synergistic role in the process of MC in the human body is not clear. Furthermore, the conclusion that patients with MC are a specific subgroup within LBP patients perhaps be premature, although MC have been strongly associated with LBP according to current evidence. The natural history and conversion of MC can be affected by some measures of clinical intervention, which do not aim directly and specially at treating MC but other diseases or symptoms.
Areas of future research should mainly include the mechanisms, natural history and relationship to symptoms. The substantial mechanisms of MC are very important for the patients with LBP because they possibly lead to a correct diagnosis. Identification of a precise mechanism for the factors involved in the progression of MC may be helpful for clinical treatment. Hence, two problems are urgently needed to be solved that the clarification of the determinants of resulting in LBP in patients with MC, and the interpretation that how these potential mechanisms synergize with together. Mechanisms of spontaneous conversion are of great importance for efforts to repair or step down the development of MC through clinical intervention. Therefore, further studies with longer follow-up periods, more frequent MRI and larger sample size will be required to reveal what factors contribute to the conversion of MC [40]. Clinically, how the knowledge of MC we understand currently can affect the management remains unclear. More prospective randomized clinical trial shall be carried out to reveal the relationship between MC and clinical symptoms, although clinical intervention, such as conservative and surgical treatment, is efficient to the patients with LBP and MC. The outcome of such research will explore the novel possibilities for future treatments of spinal degenerative diseases.
Under ideal circumstances, we should have a rodent model to solve these problems. The establishment of animal models is possible because signal intensity changes on MRI have been identified in thoracolumbar endplates in dogs [8]. However, there are certainly many difficulties in establishing the models for reasons that the long duration of MC and the necessity of frequent MRI.
Footnotes
This study was supported by National Natural Science Foundation of China (No. 3880785 and No. 30700851).
Contributor Information
Xiao-Dong Chen, Email: chenxdmd@yahoo.com.
Li-Yang Dai, FAX: +86-21-65795173, Email: chinaspine@163.com.
References
- 1.Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178–188. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs: the effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620X78B6.1287. [DOI] [PubMed] [Google Scholar]
- 4.Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C. Modic changes: possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16:977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth M, Diepers M, Weiss C, Thome C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy. part 2: radiographic evaluation and correlation with clinical outcome. Spine. 2008;33:273–279. doi: 10.1097/BRS.0b013e31816201a6. [DOI] [PubMed] [Google Scholar]
- 7.Beaman DN, Graziano GP, Glover RA, Wojtys EM, Chang V. Substance P innervation of lumbar spine facet joints. Spine. 1993;18:1044–1049. doi: 10.1097/00007632-199306150-00014. [DOI] [PubMed] [Google Scholar]
- 8.Besalti O, Pekcan Z, Sirin YS, Erbas G. Magnetic resonance imaging findings in dogs with thoracolumbar intervertebral disk disease: 69 cases (1997–2005) J Am Vet Med Assoc. 2006;228:902–908. doi: 10.2460/javma.228.6.902. [DOI] [PubMed] [Google Scholar]
- 9.Boden SD, Davis DO, Dina TS, Sunner JL, Wiesel SW. Postoperative diskitis: distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184:765–771. doi: 10.1148/radiology.184.3.1509065. [DOI] [PubMed] [Google Scholar]
- 10.Braithwaite I, White J, Saifuddin A, Renton P, Taylor BA. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363–368. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620X.84B2.12511. [DOI] [PubMed] [Google Scholar]
- 12.Buttermann GR. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4:495–505. doi: 10.1016/j.spinee.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Buttermann GR, Heithoff KB, Ogilvie JW, Transfeldt EE, Cohen M. Vertebral body MRI related to lumbar fusion results. Eur Spine J. 1997;6:115–120. doi: 10.1007/BF01358743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carragee EJ, Alamin TF, Miller JL, Carragee JM. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24–35. doi: 10.1016/j.spinee.2004.05.250. [DOI] [PubMed] [Google Scholar]
- 15.Cavanaugh JM, Ozaktay AC, Yamashita T, Avramov A, Getchell TV, King AI (1997) Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop Relat Res 166–180 [PubMed]
- 16.Chung CB, Berg BC, Tavernier T, Cotten A, Laredo JD, Vallee C, et al. End plate marrow changes in the asymptomatic lumbosacral spine: frequency, distribution and correlation with age and degenerative changes. Skeletal Radiol. 2004;33:399–404. doi: 10.1007/s00256-004-0780-z. [DOI] [PubMed] [Google Scholar]
- 17.Crock HV. A reappraisal of intervertebral disc lesions. Med J Aust. 1970;1:983–989. [PubMed] [Google Scholar]
- 18.Crock HV. Internal disc disruption: a challenge to disc prolapse fifty years on. Spine. 1986;11:650–653. doi: 10.1097/00007632-198607000-00028. [DOI] [PubMed] [Google Scholar]
- 19.Roos A, Kressel H, Spritzer C, Dalinka M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. AJR Am J Roentgenol. 1987;149:531–534. doi: 10.2214/ajr.149.3.531. [DOI] [PubMed] [Google Scholar]
- 20.Fayad F, Lefevre-Colau MM, Rannou F, Quintero N, Nys A, Mace Y, et al. Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J. 2007;16:925–931. doi: 10.1007/s00586-006-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girard CJ, Schweitzer ME, Morrison WB, Parellada JA, Carrino JA. Thoracic spine disc-related abnormalities: longitudinal MR imaging assessment. Skeletal Radiol. 2004;33:216–222. doi: 10.1007/s00256-003-0736-8. [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE, Hanley EN., Jr Recent advances in disc cell biology. Spine. 2003;28:186–193. doi: 10.1097/00007632-200301150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Hansson T, Roos B. Microcalluses of the trabeculae in lumbar vertebrae and their relation to the bone mineral content. Spine. 1981;6:375–380. doi: 10.1097/00007632-198107000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Hoenen-Clavert V, Rat AC, Loeuille D, Bettembourg-Brault I, Michel-Batot C, Blum A, et al. Inflammatory and structural evaluation in spondyloarthritis: magnetic resonance imaging analysis of axial and peripheral involvement. J Rheumatol. 2007;34:762–768. [PubMed] [Google Scholar]
- 25.Jarvik JG, Hollingworth W, Heagerty PJ, Haynor DR, Boyko EJ, Deyo RA. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine. 2005;30:1541–1549. doi: 10.1097/01.brs.0000167536.60002.87. [DOI] [PubMed] [Google Scholar]
- 26.Jensen TS, Sorensen JS, Kjaer P. Intra- and interobserver reproducibility of vertebral endplate signal (Modic) changes in the lumbar spine: the Nordic Modic Consensus Group classification. Acta Radiol. 2007;48:748–754. doi: 10.1080/02841850701422112. [DOI] [PubMed] [Google Scholar]
- 27.Jones A, Clarke A, Freeman BJ, Lam KS, Grevitt MP. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30:1867–1869. doi: 10.1097/01.brs.0000173898.47585.7d. [DOI] [PubMed] [Google Scholar]
- 28.Karchevsky M, Schweitzer ME, Carrino JA, Zoga A, Montgomery D, Parker L. Reactive endplate marrow changes: a systematic morphologic and epidemiologic evaluation. Skeletal Radiol. 2005;34:125–129. doi: 10.1007/s00256-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Lee SH, Ahn Y, Yoon DH, Lee CD, Lim ST. Recurrence after successful percutaneous endoscopic lumbar discectomy. Minim Invasive Neurosurg. 2007;50:82–85. doi: 10.1055/s-2007-982504. [DOI] [PubMed] [Google Scholar]
- 30.Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine. 2005;30:1173–1180. doi: 10.1097/01.brs.0000162396.97739.76. [DOI] [PubMed] [Google Scholar]
- 32.Kjaer P, Leboeuf-Yde C, Sorensen JS, Bendix T. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine. 2005;30:798–806. doi: 10.1097/01.brs.0000157424.72598.ec. [DOI] [PubMed] [Google Scholar]
- 33.Kleinstuck F, Dvorak J, Mannion AF. Are “structural abnormalities” on magnetic resonance imaging a contraindication to the successful conservative treatment of chronic nonspecific low back pain? Spine. 2006;31:2250–2257. doi: 10.1097/01.brs.0000232802.95773.89. [DOI] [PubMed] [Google Scholar]
- 34.Kokkonen SM, Kurunlahti M, Tervonen O, Ilkko E, Vanharanta H. Endplate degeneration observed on magnetic resonance imaging of the lumbar spine: correlation with pain provocation and disc changes observed on computed tomography diskography. Spine. 2002;27:2274–2278. doi: 10.1097/00007632-200210150-00017. [DOI] [PubMed] [Google Scholar]
- 35.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine. 2006;31:2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 36.Kuisma M, Karppinen J, Niinimaki J, Kurunlahti M, Haapea M, Vanharanta H, et al. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine. 2006;31:1714–1718. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 37.Kuisma M, Karppinen J, Niinimaki J, Ojala R, Haapea M, Heliovaara M, et al. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32:1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 38.Lang P, Chafetz N, Genant HK, Morris JM. Lumbar spinal fusion. Assessment of functional stability with magnetic resonance imaging. Spine. 1990;15:581–588. doi: 10.1097/00007632-199006000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Leboeuf-Yde C, Kjaer P, Bendix T, Manniche C. Self-reported hard physical work combined with heavy smoking or overweight may result in so-called Modic changes. BMC Musculoskelet Disord. 2008;9:5. doi: 10.1186/1471-2474-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshman LA, Trewhella M, Friesem T, Bhatia CK, Krishna M. Reverse transformation of Modic type 2 changes to Modic type 1 changes during sustained chronic low-back pain severity Report of two cases and review of the literature. J Neurosurg Spine. 2007;6:152–155. doi: 10.3171/spi.2007.6.2.152. [DOI] [PubMed] [Google Scholar]
- 41.Masaryk TJ, Boumphrey F, Modic MT, Tamborrello C, Ross JS, Brown MD. Effects of chemonucleolysis demonstrated by MR imaging. J Comput Assist Tomogr. 1986;10:917–923. doi: 10.1097/00004728-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Miller G. The spine. In: Berquist T, editor. MRI of the musculoskeletal system, 2nd edn. New York: Raven; 1990. [Google Scholar]
- 43.Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004;14:1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 44.Modic MT. Modic type 1 and type 2 changes. J Neurosurg Spine. 2007;6:150–151. doi: 10.3171/spi.2007.6.2.150. [DOI] [PubMed] [Google Scholar]
- 45.Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–166. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 46.Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 47.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 48.Narvani AA, Tsiridis E, Wilson LF. High-intensity zone, intradiscal electrothermal therapy, and magnetic resonance imaging. J Spinal Disord Tech. 2003;16:130–136. doi: 10.1097/00024720-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31:1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. [DOI] [PubMed] [Google Scholar]
- 50.Peterson CK, Gatterman B, Carter JC, Humphreys BK, Weibel A. Inter- and intraexaminer reliability in identifying and classifying degenerative marrow (Modic) changes on lumbar spine magnetic resonance scans. J Manipulative Physiol Ther. 2007;30:85–90. doi: 10.1016/j.jmpt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Peterson CK, Humphreys BK, Pringle TC. Prevalence of Modic degenerative marrow changes in the cervical spine. J Manipulative Physiol Ther. 2007;30:5–10. doi: 10.1016/j.jmpt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Putzier M, Schneider SV, Funk JF, Tohtz SW, Perka C. The surgical treatment of the lumbar disc prolapse: nucleotomy with additional transpedicular dynamic stabilization versus nucleotomy alone. Spine. 2005;30:E109–E114. doi: 10.1097/01.brs.0000154630.79887.ef. [DOI] [PubMed] [Google Scholar]
- 53.Rannou F, Ouanes W, Boutron I, Lovisi B, Fayad F, Mace Y, et al. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 2007;57:1311–1315. doi: 10.1002/art.22985. [DOI] [PubMed] [Google Scholar]
- 54.Resnick D, Niwayama G. Intervertebral disc abnormalities associated with vertebral metastasis: observations in patients and cadavers with prostatic cancer. Invest Radiol. 1978;13:182–190. doi: 10.1097/00004424-197805000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Saifuddin A, Renton P, Taylor BA. Effects on the vertebral end-plate of uncomplicated lumbar discography: an MRI study. Eur Spine J. 1998;7:36–39. doi: 10.1007/s005860050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandhu HS, Sanchez-Caso LP, Parvataneni HK, Cammisa FP, Jr, Girardi FP, Ghelman B. Association between findings of provocative discography and vertebral endplate signal changes as seen on MRI. J Spinal Disord. 2000;13:438–443. doi: 10.1097/00002517-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Schenk P, Laubli T, Hodler J, Klipstein A. Magnetic resonance imaging of the lumbar spine: findings in female subjects from administrative and nursing professions. Spine. 2006;31:2701–2706. doi: 10.1097/01.brs.0000244570.36954.17. [DOI] [PubMed] [Google Scholar]
- 58.Schmid G, Witteler A, Willburger R, Kuhnen C, Jergas M, Koester O. Lumbar disk herniation: correlation of histologic findings with marrow signal intensity changes in vertebral endplates at MR imaging. Radiology. 2004;231:352–358. doi: 10.1148/radiol.2312021708. [DOI] [PubMed] [Google Scholar]
- 59.Shen M, Razi A, Lurie JD, Hanscom B, Weinstein J. Retrolisthesis and lumbar disc herniation: a preoperative assessment of patient function. Spine J. 2007;7:406–413. doi: 10.1016/j.spinee.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Vertebral bone-marrow changes in degenerative lumbar disc disease: an MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994;76:757–764. [PubMed] [Google Scholar]
- 61.Ulmer JL, Elster AD, Mathews VP, Allen AM. Lumbar spondylolysis: reactive marrow changes seen in adjacent pedicles on MR images. AJR Am J Roentgenol. 1995;164:429–433. doi: 10.2214/ajr.164.2.7839983. [DOI] [PubMed] [Google Scholar]
- 62.Vital JM, Gille O, Pointillart V, Pedram M, Bacon P, Razanabola F, et al. Course of Modic 1 six months after lumbar posterior osteosynthesis. Spine. 2003;28:715–721. doi: 10.1097/00007632-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 63.Waddell G. 1987 Volvo award in clinical sciences: a new clinical model for the treatment of low-back pain. Spine. 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661–666. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- 65.Weishaupt D, Zanetti M, Hodler J, Min K, Fuchs B, Pfirrmann CW, et al. Painful lumbar disk derangement: relevance of endplate abnormalities at MR imaging. Radiology. 2001;218:420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 66.Williams FM, Manek NJ, Sambrook PN, Spector TD, Macgregor AJ. Schmorl’s nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007;57:855–860. doi: 10.1002/art.22789. [DOI] [PubMed] [Google Scholar]