Abstract
A considerable number of patients complain about pain after lumbar surgery. The spinal dura mater has been debated as a possible source of this pain. However, there is no information if laminectomy influences the nociceptive sensory innervation of the dura. Therefore, we quantitatively evaluated the density of SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis in an animal model of laminectomy. Twelve adult Lewis rats underwent laminectomy, in six of them the exposed dura was covered by an autologous fat graft. Further six animals without surgical treatment served as controls. Six weeks after surgery, the animals were perfused and the lumbar dura was processed immunohistochemically for the detection of CGRP- and SP-containing nerve fibers. In controls, the peptidergic nerve fibers were found predominantly in the ventral but rarely in the dorsal dura mater lumbalis. After laminectomy, the density of SP- and CGRP-immunopositive neurons significantly increased in ventral as well as in dorsal parts of the dura. Axonal spines could be observed in some cases at the site of laminectomy. The application of autologous fat grafts failed to inhibit the significant increase in the density of peptidergic afferents. Thus, we have provided the first evidence that laminectomies induce an increase in the density of putative nociceptive SP- and CGRP-immunopositive neurons in the lumbar dura mater ascribable to an axonal sprouting of fine nerve fibers. This effect was not prevented by using autologous fat grafts. It is conceivable that the neuronal outgrowth of nociceptive afferents is a cause of low back pain observed after lumbar surgery.
Keywords: Laminectomy, Pain, Fat graft, Dural innervation, SP-/CGRP-immunopositive nerve fibres
Introduction
Low back pain persists or recurs in up to 40% of patients who underwent one or more surgical procedures on the lumbo-sacral spine [49, 67]. It can result in chronic pain and disability with a poor clinical outcome. Postoperative ongoing pain is not only a physiological problem but also a complex with socioeconomic and psychosocial implications like depression, anxiety, sleeplessness or deconditioning [58]. Many patients are consigned to a life of long-term narcotic treatment with little chance of recovery.
The reasons for persisting pain in appropriate spinal surgery remain in many cases are unclear. Adhesive arachnoiditis and the formation of scar tissue referred to as epidural fibrosis are suspected as the causes [4, 8, 43]. Based on these complex physiological processes, it is conceivable that the spinal dura is a trigger of pain after surgery. Various authors have considered the dura mater not only as a structure protecting the spinal cord, but also as a possible source of low back pain [2, 7, 11, 18, 51, 57]. Blikra [6] reported that patients experienced relief from low back pain after removal of herniated lumbar disc material occupying the whole dural sac. McCarron et al. [45] suspected a nonmechanical irritation of the dural sac and nerve roots as one of the causes of pain. These authors postulated that tension applied to the dura might produce back pain if an inflammatory response is involved.
In recent studies, Sekiguchi et al. [59] demonstrated that in rats a large number of sensory nerves distribute to the dura mater of the spinal cord via the sinuvertebral nerve. This may provide an anatomical substrate for the understanding of back pain [26]. However, up to now, there is no information if and how the sensory innervation of the dura mater is influenced by lumbar spine surgery.
For this reason, the purpose of the current study was to investigate the potentially nociceptive innervation of the dura mater lumbalis of rats under normal conditions and after laminectomy of L4 by using substance P (SP) and calcitonin gene-related peptide (CGRP) antibodies as markers for fine sensory afferent nerve fibers.
In lumbar disc surgery, the transplantation of free fat grafts is frequently employed to improve the surgical outcome. However, the effectiveness of this procedure is under debate. To clarify this inconsistency, we further applied free autologous fat grafts on the exposed dura mater lumbalis to examine if the sensory innervation is affected by the adipose tissue.
Materials and methods
Eighteen adult male Lewis rats, each weighing 300–350 g, were used in this study. In six animals, no surgical treatment was performed (controls, group 1). Twelve animals were anaesthetized. Six of them underwent laminectomy of lamina 4 and exposure of the dura mater lumbalis (group 2) and in the remaining six animals the dura was covered by an autologous fat graft following laminectomy (group 3).
All animals were handled according to German laws governing animal experiments.
Surgical procedure
Anesthesia was induced and maintained with 1.5% isoflurane. During surgery, the eyes of the animals were protected using sterile lubricant ointment. The animals were positioned prone on the operating table. With the aid of a surgical microscope and using an aseptic technique laminectomy of L4 was performed. The dorsal skin and fascia were incised in the midline. After separation of the paraspinal muscles the proc. spin., the fourth lamina, the ligamentum flavum and epidural fat were removed. For the full extent of laminectomy, the dura was then cleaned and exposed (Fig. 1). The laminectomy cavity was irrigated using sterile saline solution. Additionally, in group 3, the dura was covered with an autologous fat graft which was taken from the animals’ belly fat. Haemostasis was obtained by applying gentle pressure with cotton.
Fig. 1.
Exposed and cleaned dura mater of lamina 4 after laminectomy
The surgical wound was then closed in layers with No. 4–0 nylon sutures. The animals were carefully returned to their cages. Water and food were given ad libidum. Complications did not occur in the animals. All wounds healed uneventfully.
Tissue preparation
Six weeks after surgery the animals were anaesthetized by CO2, killed by an overdose of 400 mg/kg sodium thiopental i.p., and perfused intracardially with 250 ml Tyrode solution (pH 7.4) followed by 350 ml of fixative consisting of 4% paraformaldehyde and 0,2% picric acid in 0.1 M phosphate-buffered saline (PBS; pH 7.4). With the aid of an operating microscope whole tissue samples of the lumbar spinal cord of L4 including the dura mater were carefully removed from the vertebral column. In a few cases, epidural scarring adherent to the dorsal aspect of the dura was seen. The dura was dissected free from this structures, incised along the ventral midline by using microdissection scissors, removed from the spinal cord, spread out, postfixed in fresh fixative for up to 4 h, and placed in 30% sucrose solution overnight at 4°C.
Then the specimen were stained immunohistochemically as a whole mount preparation either for CGRP- (four of each group) or for SP-containing nerve fibers (two of each group).
Immunohistochemistry
The durae matres were washed in PBS, incubated in 0.15% H202 for 30 min, rinsed in PBS and treated with graded ethanol (10, 25, 40, 25 and 10%; 5 min each) to facilitate antibody penetration. Then they were rinsed in three changes of PBS and subsequently preincubated in 5% normal goat serum (NGS) in PBS containing 0.5% Triton X-100 for 30 min. Afterwards, the durae were placed in primary antiserum raised in rabbits, either anti-SP (1:500; DiaSorin), or anti-CGRP (1:2,000; Peninsula) and incubated overnight at room temperature with gentle agitation. All antisera were diluted in PBS containing 1% NGS and 0.5% Triton X-100. The primary antibodies were visualized by the avidin–biotin complex (ABC) method (Vectastain Elite ABC Kit). The durae matres were rinsed in PBS (5 × 10 min), incubated in the secondary antibody (biotinylated goat anti-rabbit IgG, 1:200) for 1 h and then placed in AB-complex for 30 min. After rinsing with PBS (5 × 10 min) the peroxidase label was visualized with 0.03% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma), activated with 0.01% H202. The reaction was stopped with aqua dest. and excess reaction product was washed out with PBS. The durae were dehydrated in ethanol, cleared in xylene, and coverslipped in DePeX.
Specificity of immunostaining was tested either by omitting the primary antiserum or by preabsorbing the antibodies with 100 μg/ml of SP or with 50 μg/ml of synthetic rat CGRP overnight at 4°C. No staining was obtained in either of these cases.
Light microscopic examination and data analysis
The CGRP- and SP-immunoreactive (-ir) nerve fibers were localized and characterized in regard to their topography, distribution and innervation density with a Zeiss Axiophot microscope at a magnification of 200×. The density of nerve fibers in ventral and dorsal parts of the dura was determined by counting the number of immunopositive neurons that were longer than 15 μm per microscopical vision field (700 × 420 μm) and by fitting it into an analogue scale ranging from 0 (no fibers) to 10 (>91 fibers) (Table 1).
Table 1.
Density of immunopositive neurons per microscopical vision field (700 × 420 μm)
| Density | Number of fibers |
|---|---|
| 0 | No fibers found |
| 1 | 1–10 |
| 2 | 11–20 |
| 3 | 21–30 |
| 4 | 31–40 |
| 5 | 41–50 |
| 6 | 51–60 |
| 7 | 61–70 |
| 8 | 71–80 |
| 9 | 81–90 |
| 10 | >91 |
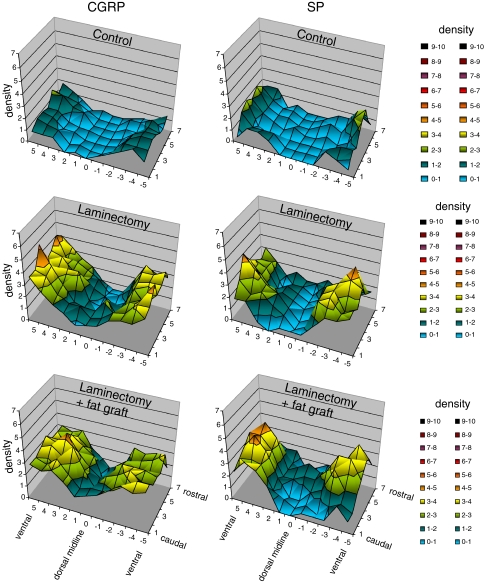
For each dura, 77 fields (11 rows × 7 columns; see Fig. 2) were analyzed, 42 for the ventral and 35 for the dorsal parts. To show the overall distribution of CGRP- and SP-positive nerve fibers the numbers of density of these fields were then averaged for each group. In order to compare the innervation density in the three experimental groups, the mean fiber densities were calculated for the dorsal (vision fields of rows 2, 1, 0, −1, −2; see Fig. 2) and the ventral (vision fields of rows 3, 4, 5 and −3, −4, −5; see Fig. 2) parts of the durae.
Fig. 2.
Three dimensional graphs showing the mean densities of CGRP- and SP-immunopositive nerve fibers per microscopical vision field in the dura of L4 of controls (group 1), laminectomized rats (group 2), and rats with laminectomy that received free autologous fat grafts (group 3). Six weeks post laminectomy the mean densities increased in ventral as well as in dorsal parts of the dura, independent of the use of fat grafts
The results were expressed as means and standard errors of the mean. The difference between groups was compared using the unpaired Student’s t test. All P values were compared to a α-value of 0.05 to determine significance.
Results
Distribution and density of CGRP- and SP-immunopositive nerve fibers in the lumbar dura mater of control animals (group 1)
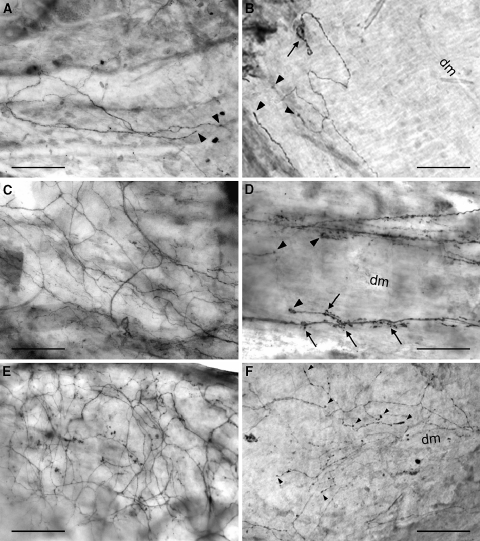
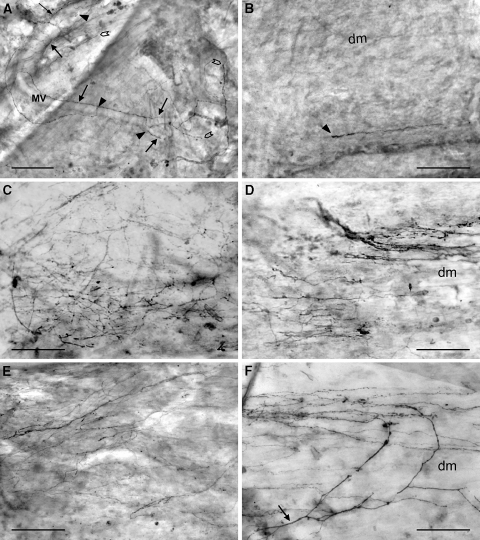
In the controls (group 1), CGRP- and SP-positive nerve fibers, many of which are thought to be nociceptors, were predominantly found in the ventral parts of the dura mater lumbalis, whereas they were rarely observed in dorsal areas (Fig. 2). No difference in the frequency of SP- and CGRP-ir fibers was seen. The stained neurons that were mostly very small in diameter were running either in thin bundle with varying numbers of fibers or as individuals (Figs. 3a, b, 4a, b). Some of them accompany blood vessels (Fig. 4a). In the ventral parts of the dura, smaller bundles and single immunopositive fibers branch off from “main” bundles that run longitudinally and they extend into the areas of connective tissue thereby forming a fiber network. In dorsal areas of the lumbar dura mater, most of the immunopositive neurons were found running singly or in very small bundles of two or three stained axons. Single immunopositive fibers showed multiple varicosities and appeared like strings of pearls. They either divided into branches and some of them formed coiled endings (Fig. 3b) or terminated in a single straight end branch that usually appeared thickened (Figs. 3a, b, 4a, b). Nerve fiber endings were rarely seen in close association with blood vessels (Fig. 4a). Most of them are terminated in the connective tissue between vessels (Figs. 3a, b, 4a, b).
Fig. 3.
CGRP-immunopositive nerve fibers in the ventral (a, c, e) and dorsal (b, d, f) dura mater lumbalis of controls (a, b), laminectomized rats (c, d), and animals that underwent laminectomy and application of fat grafts (e, f); light microscopic micrographs of whole-mount preparations. a Thin bundles of nerve fibers and single neurons of small diameter innervating the ventral dura. Some of them terminate with straight and thickened end branches in the connective tissue (arrowheads). b Nerve fibers running in parallel to the dorsal midline and showing a coiled (arrow) and straight thickened terminals (arrowheads) in the connective tissue of the dorsal dura. c Dense network of immunopositive nerve fibers in the ventral dura mater lumbalis. d Small bundles of stained varicose nerve fibers branching and terminating (arrowheads) in the dorsal dura. Note the axonal spine-like structures (arrows) at the end branches. e Prominent network of immunopositive nerve fibers in the ventral dura. f Network of stained varicose nerve fibers arborizing and terminating in the connective tissue along the dorsal midline. Note the thickened nerve terminals (arrowheads). dm dorsal midline. Bars 100 μm
Fig. 4.
SP-immunopositive nerve fibers in the ventral (a, c, e) and dorsal (b, d, f) dura mater lumbalis of controls (a, b), laminectomized rats (c, d), and animals that underwent laminectomy and application of free autologous fat grafts (e, f); light microscopic micrographs of whole-mount preparations. a Small bundles of nerve fibers and single neurons innervating the ventral dura. Some of them (arrows) accompany delicate meningeal blood vessels. A few nerve fibers terminate at or close to the blood vessels (arrowheads) others end up in the connective tissue (open arrowheads). b Single nerve fiber with a straight thickened terminal (arrowhead) in the connective tissue of the dorsal dura. c Dense network of immunopositive nerve fibers in the ventral dura mater lumbalis. Note the prominent varicosities. d Dense innervation of the dorsal dura mater lumbalis after laminectomy. Immunopositive nerve fibers are predominantly arranged parallel to the dorsal midline. e Widespread fiber network in the ventral dura. f Network of stained nerve fibers running in parallel to the dorsal midline. Note one fiber (arrow) that extends from the ventral dura to the dorsal parts and arborizes along the midline. dm dorsal midline, MV meningeal blood vessels. Bars 100 μm
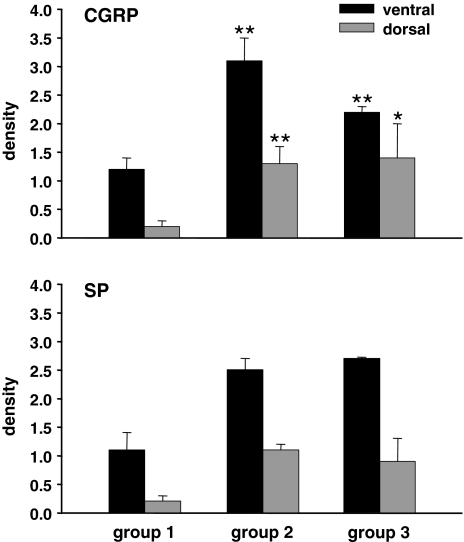
The mean densities of CGRP- and SP-positive nerve fibers per vision field are represented for the whole piece of dura in a three-dimensional graph (Fig. 2). For the dorsal midline and the adjacent dorsal areas the fiber density varied between 0 and 0.8 for CGRP and between 0 and 0.5 for SP. Toward the ventral parts the density increased up to a maximum of 2.3 in the case of CGRP and to 3.0 for SP. To facilitate the comparison between the experimental groups, mean fiber densities were calculated for the dorsal and for the ventral areas. For the controls (group 1) the density of CGRP-positive nerve fibers was 1.2 ± 0.2 (mean ± SD) in the ventral and 0.2 ± 0.1 in the dorsal dura mater and the density of SP-positive neurons was 1.1 ± 0.3 in ventral and 0.2 ± 0.1 in dorsal parts (Fig. 5).
Fig. 5.
Density of CGRP- and SP-immunopositive nerve fibers in the ventral and dorsal dura of controls (group 1), laminectomized rats (group 2), and animals that underwent laminectomy and application of free autologous fat grafts (group 3). At 6 weeks post surgery the density of CGRP-ir fibers increased significantly above control in the dorsal and ventral dura of the laminectomized animals. The application of fat grafts could not prevent the increase in the density of stained nerve fibers. For C GRP-ir comparisons were carried out with Student’s unpaired t test; n = 4. *P < 0.05, **P < 0.01
Changes in pattern and density of peptidergic nerve fibers in the lumbar dura mater following laminectomy of L4 (group 2) and additional application of fat grafts (group 3)
Six weeks post-laminectomy there was a clearly visible increase in the peptidergic sensory innervation of groups 2 and 3 in the ventral and, particularly, in the dorsal parts of the dura mater (Figs. 2, 3, 4). In the ventral dura a higher number of CGRP- (Fig. 3c, e) and SP-ir (Fig. 4c, e) small nerve fiber bundles and single fibers formed a widespread fiber network with branched endings terminating mainly in the connective tissue. In the affected durae, the nerve fibers extended to the dorsal parts and arborized along the dorsal midline (Fig. 4f). These immunopositive nerve fibers had a higher number of varicosities and the tips of the end branches usually appeared thicker (Fig. 3f) compared to controls. In a few cases, a sprouting of nerve fibers at the level of the terminal receptive fields could be seen in the dorsal dura. The outgrowth of neurons became visible by the formation of axonal spine-like structures (Fig. 3d). This phenomenon was observed after laminectomy as well as after application of autologous fat grafts following laminectomy.
In group 2, the density of immunopositive neurons increased significantly in the ventral areas: nearly threefold for CGRP (3.1 ± 0.4; P = 0.004) and more than twofold for SP (2.5 ± 0.2). In the dorsal parts of the affected dura, the increase was even more pronounced: more than sixfold for CGRP (1.3 ± 0.3; P = 0.006) and about fivefold for SP (1.1 ± 0.1) (Fig. 5).
Application of fat grafts (group 3) did not significantly reduce the sprouting of immunoreactive nerve fibers following laminectomy. The density of CGRP-positive neurons was still enhanced in ventral (2.2 ± 0.1; P = 0.004) as well as in dorsal (1.4 ± 0.6; P = 0.046) areas of the dura compared to control and that of SP-ir neurons was increased to 2.7 ± 0.02 in the ventral and to 0.9 ± 0.4 in the dorsal dura (Fig. 5).
Discussion
The current study provides the first evidence that laminectomy, regardless of the use of fat grafts, induces an increase in the density of potentially nociceptive CGRP- and SP-immunopositive nerve fibers in the dura mater lumbalis.
Innervation of the spinal dura mater lumbalis
The innervation of the spinal dura has been controversial for many years. It is generally accepted that the lumbar dura mater is innervated mainly through the fibers of the sinuvertebral nerve and its branches. The sinuvertebral nerve is composed of spinal and sympathetic nerve fibers emerging from the sympathetic trunk or the rami communicantes [52, 65]. Using acetyl cholinesterase immunohistochemistry Groen et al. [26] demonstrated a rich plexus of nerve fibers in the ventral spinal dura of the human fetus. The nerve supply to the dorsal aspects of the spinal dura is still under debate. Whereas some groups did not observe nerve fibers in the dorsal parts [17, 36, 52], others described a sparse innervation [26, 63]. The fibers in the dura were characterized as small in size and were shown to terminate in non-corpuscular, naked nerve endings [26]. Together with the finding that the neurons in the lumbar dura mater are capsaicin sensitive [35] and that at least a good portion of the cells are SP- and/or CGRP-immunoreactive provided evidence that these structures may contribute to nociception and might be a trigger of back pain.
The presence of sensory neuropeptidergic nerve fibers in the spinal dura was described by a few groups. Kumar et al. [39] reported a sparse innervation of CGRP- and SP-positive neurons in the ventral part of the dura mater lumbalis, but observed no fibers in the dorsal dura. In contrast to this finding, other authors demonstrated a network of CGRP- and SP-positive primary afferents in the ventral dura mater lumbalis and a less dense innervation in the dorsal aspects [1, 2, 30, 39]. The present study confirms the sensory peptidergic innervation of the ventral as well as the dorsal parts of the lumbar dura and adds quantitative data on their innervation density. Ventrally we observed a network of small immunopositive fibers crossing the dense connective tissue of the dura and terminating as free nerve endings. Only very few nerve terminals were associated with blood vessels. Towards the dorsal midline, the density of SP- and CGRP-positive nerve fibers decreased to about one-sixth of those determined for the ventral part. In the dorsal dura, mainly very small immunopositive nerve bundles and single nerve fibers with multiple varicosities and straight thickened end branches were seen.
Interestingly, the density of CGRP-positive nerve fibers is not different from that determined for SP-immunoreactive neurons in the lumbar dura. This is in contrast to observations in the dura mater encephali of rats and cats [47]. In this tissue, it was shown that CGRP-positive nerve fibers were much more abundant than SP-positive fibers. In the trigeminal ganglion of the rat, nearly twice as many CGRP-ir neurons as SP-positive cells were found [50]. Using retrograde tracing these authors showed that this difference was associated with a higher density of CGRP-positive afferents innervating intracranial vessels.
Influence of laminectomy on the sensory peptidergic innervation density
Six weeks post lesion, we demonstrated a considerable increase in the density of fine peptidergic primary afferents in the dura mater lumbalis. The increase was even more pronounced in the dorsal parts at the site of the laminectomy cavity. This sensory sprouting of potentially nociceptive CGRP- and SP-immunopositive nerve fibers became visible in some cases by the formation of axonal spine-like structures. The occurrence of axonal spines is well investigated in the central nervous system. Various authors have demonstrated that the formation of axonal spines leads to a sprouting of fine nerve fibers [56, 61, 66]. To our knowledge, the presence of such axonal spines in peripheral tissues has not been described in prior studies. However, further studies are required to prove whether the increased density of the neuropeptide containing nerve fibers is, in fact, caused through axonal sprouting or is due to an increased neuropeptide expression.
The phenomenon of axonal sprouting in peripheral tissues has been shown in different studies. Grelik et al. [25] demonstrated an increase in CGRP-immunopositive nerve fibers in the skin of the lower lip of rats following chronic constriction injury. Mosconi and Krüger [48] found increased numbers of unmyelinated sensory axons in sections of sciatic nerves after application of polyethylene cuff, and Yen et al. [68] observed a migration of CGRP-immunopositive nerve fibers into the upper dermis of the rat hind paw in a model of partial peripheral nerve injury. In these cases, axons were damaged and both regenerative and collateral sprouting may have taken place. Also, an outgrowth of nerve fibers was seen in the inflamed skin of the rat hind paw [41], in the mucosa of inflamed bladders [15], in inflamed skeletal muscle [55], and in biopsies of human contact eczema [33] and sprouting and rearrangement of nerve fibers have been demonstrated in the cornea [27].
Our observations further support the hypothesis of a plasticity of peripheral neuron terminals in the mammalian nervous system in response to various stimuli. The sprouting of fine sensory afferent neurons and the novel fiber arrangement may play an important role in the development and persistence of low back pain following spine surgery.
Possible mechanisms of sensory sprouting and pathophysiological relevance of increased fiber density
The sensory sprouting presented in the current study was a surprising observation since it was unlikely that the dura mater lumbalis was mechanically damaged by the laminectomy and that axonal injury was caused to the dural neurons. The outgrowth of neurites, therefore, requires specific factors either existing in the microenvironment of the nerve fibers or provided by systemic modulation.
An enhanced local synthesis of modulators like cytokines or different nerve growth factors could be a mechanism for the increased density of peptidergic nerve fibers. In a recent study, Matsuda et al. [44] demonstrated that the wound healing process, for example after surgery, induces increased levels of the nerve growth factor “NGF” in the tissue. This peptide is not only an important component of wound healing and tissue repair [31] but also an inductor of sprouting of sensory afferent nerve fibers [9, 54]. Diamond and his group [13, 14, 22, 23] stated that NGF mediates collateral sprouting in rats, but that regenerative sprouting is NGF independent. In fact, the sprouting of cutaneous nerves and an increase of NGF at the same time has been shown by Leslie et al. [41] in inflamed rat paws. In the allergic contact eczema, the changes in the skin innervation were accompanied by an increase in NGF content and in the number of GAP-43-positive nerve fibers, suggesting actively sprouting nerve endings [33]. NGF up-regulation was also shown to be necessary for collateral sprouting of sensory neurons in the thoracic skin of the rat [46]. Further evidence comes from studies using transgenic animals. The over expression of NGF in mouse airways increased the sensory innervation [28] and animals that over expressed NGF in the epidermis showed an increase of cutaneous nociceptors and sympathetic fibers [3, 12].
NGF can be produced by numerous cells including inflammatory cells, fibroblasts, mast cells, macrophages, astrocytes, and Schwann cells. Probable sources of NGF in our animal model are the fibroblasts that could be found in the laminectomy cavity. It is likely that NGF released from the surrounding (scar) tissue penetrates the dura mater lumbalis and acts as a neurotrophic molecule by stimulating the sprouting of nerve fibers.
Besides local modulation, systemic mechanisms can take place in triggering the reorganization of nerve fibers. The Lewis rat strain used in our study is known to have decreased blood corticosterone levels due to a reduced HPA-axis activity and to be more susceptible for e.g. inflammatory stimuli [37, 62]. Corticosteroids have been shown to depress NGF synthesis induced by pro-inflammatory cytokines in endoneural fibroblasts from the rat sciatic nerve [42] and they can inhibit the stimulatory effect of NGF on primary neurons of the superior cervical ganglion [19]. Moreover, corticosteroids are negative regulators of GAP-43 [19] that is expressed in actively sprouting nerve endings. It is, therefore, conceivable that the low corticosterone levels seen in Lewis rats lead to a disinhibition of NGF synthesis and GAP-43 expression following affecting stimuli. In humans, it could be shown that patients exhibiting a relative hypocortisolism along with enhanced blood IL-6 levels are at a higher risk for a poor outcome in disc surgery compared to patients with normal cortisol levels [21].
Until now there are no studies investigating whether peripheral sprouting as seen here after laminectomy can be a mechanism for triggering, maintaining, and exacerbating pain. However, it is very likely that the free nerve endings containing SP and CGRP found in a higher number in our study are nociceptors. Once a painful stimulus acts on tissues with increased nociceptor density it will excite more nociceptors and consequently result in enhanced pain. Moreover, there is good evidence that the unmyelinated nociceptive nerve fibers are not uniquely sensory in function. When stimulated, they release their peptides and start a local neurogenic inflammatory response leading to a further release of inflammatory mediators that in turn may sensitize the nociceptors. In this way, even normally innocuous (mechanical) stimuli can elicit pain. The process of neurogenic inflammation is discussed for the dura mater encephali as one possible mechanism explaining the pathogenesis of migraine and cluster headaches [32, 47].
It is quite possible that laminectomies in humans also result in sprouting of nociceptive peptidergic nerve fibers in the lumbar dura mater. This increase in nociceptor density, the expansion of the input region along with proinflammatory processes as part of the wound healing cascade that lead to a sensitization of nociceptors may contribute to the subjective symptoms associated with low back pain.
Are fat grafts an appropriate material to reduce fiber sprouting?
In human spine surgery, many materials have been used to enhance successful surgical outcome. Beside dextran [10], methyl metacrilate [38], fibrin glue [64], silastic membrane [16], sodium hyaluronate [60], ADCON-L [53], Tachocomp, and many others, autologous fat grafts [5, 20, 24, 29, 34, 40] were applied to reduce failed-back surgery syndrome. In previous clinical studies, contradictory results were found. Most of them suggested that the use of free fat grafts in lumbar disc surgery is clinically ineffective [5, 24, 29]. On the other hand, Gambardella et al. [20] found a positive effect in the reconstruction of epidural fat with autografts of adipose tissue to prevent postoperative failed-back syndrome.
In the present study, we have clearly demonstrated that the increase in the density of nociceptive CGRP- and SP-positive nerve fibers in the dura mater lumbalis following laminectomy can not be significantly reduced by the application of autologous fat grafts. In our opinion, the effectiveness of such adipose grafts remains still unclear.
Conclusion
We documented for the first time that laminectomies induce an increase in the density of putative nociceptive CGRP- and SP-immunopositive primary afferents in the lumbar dura mater of rats, which is ascribable to an axonal sprouting of the fine nerve fibers. Enhanced local synthesis and penetration of NGF and/or modulations by low corticosterone blood levels are discussed as possible mechanisms for the neuronal reorganization. We suggest that the increased density of nociceptive afferents is a cause of low back pain observed after lumbar surgery. Treatments decreasing dural sensory hyper-innervation might be, therefore, effective in reducing low back pain following spinal surgery. However, the use of autologous fat grafts was not effective in inhibiting peripheral sprouting of presumably nociceptive peptidergic nerve fibers in our animal model.
References
- 1.Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. SP- and CGRP-immunoreactive nerve fibers in the rat lumbar spine. Neuroorthopedics. 1991;12:19–28. [Google Scholar]
- 2.Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M, Neuropeptide Y. Tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine. 1993;18(2):268–273. doi: 10.1097/00007632-199302000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benner B, Ehni G. Spinal arachnoiditis. Spine. 1978;3:40–44. doi: 10.1097/00007632-197803000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bernsmann K, Krämer J, Ziozios I, Wehmeier J, Wiese M. Lumbar micro disc surgery with and without autologous fat graft. A prospective randomized trial evaluated with reference to clinical and social factors. Arch Orthop Trauma Surg. 2001;121(8):476–480. doi: 10.1007/s004020100277. [DOI] [PubMed] [Google Scholar]
- 6.Blikra G. Intradural herniated lumbar disc. J Neurosurg. 1969;31(6):676–679. doi: 10.3171/jns.1969.31.6.0676. [DOI] [PubMed] [Google Scholar]
- 7.Bogduk N. The sources of low back pain. In: Jayson IV, editor. The lumbar spine and back pain. Edinburgh: Churchill Livingston; 1992. pp. 61–88. [Google Scholar]
- 8.Burton CV. Lumbosacral arachnoiditis. Spine. 1978;3:24–30. doi: 10.1097/00007632-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 10.Ceviz A, Arslan A, Ak HE, Inalöz S. The effect of urokinase in preventing the formation of epidural fibrosis and/or leptomeningeal arachnoiditis. Surg Neurol. 1997;47(2):124–127. doi: 10.1016/S0090-3019(96)00038-9. [DOI] [PubMed] [Google Scholar]
- 11.Cyriax J. Dural pain. Lancet. 1978;1(8070):919–921. doi: 10.1016/S0140-6736(78)90693-1. [DOI] [PubMed] [Google Scholar]
- 12.Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387:489–506. doi: 10.1002/(SICI)1096-9861(19971103)387:4<489::AID-CNE2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Diamond J, Foerster A, Holmes M, Coughlin M. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J Neurosci. 1992;12:1467–1476. doi: 10.1523/JNEUROSCI.12-04-01467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A. Peptideregic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;139:671–685. doi: 10.1016/j.neuroscience.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 16.DiFazio FA, Nichols JB, Pope MH, Frymoyer JW. The use of expanded polytetrafluoroethylene as an interpositional membrane after lumbar laminectomy. Spine. 1995;20(9):986–991. doi: 10.1097/00007632-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Edgar MA, Nundy S. Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatry. 1966;29:530–534. [Google Scholar]
- 18.El-Mahdi MA, Abdel Latif FY, Janko M. The spinal nerve root “innervation”, and a new concept of the clinicopathological interrelations in back pain and sciatica. Neurochirurgia (Stuttg) 1981;24(4):137–141. doi: 10.1055/s-2008-1054051. [DOI] [PubMed] [Google Scholar]
- 19.Federoff HJ, Grabczyk E, Fishman MC. Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids. J Biol Chem. 1988;263(36):19290–19295. [PubMed] [Google Scholar]
- 20.Gambardella G, Gervasio O, Zaccone C, Puglisi E. Prevention of recurrent radicular pain after lumbar disc surgery: a prospective study. Acta Neurochir Suppl (Wien) 2005;92:151–154. doi: 10.1007/3-211-27458-8_33. [DOI] [PubMed] [Google Scholar]
- 21.Geis A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Gloster A, Diamond J. Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J Comp Neurol. 1992;326:363–374. doi: 10.1002/cne.903260305. [DOI] [PubMed] [Google Scholar]
- 23.Gloster A, Diamond J. NGF-dependent and NGF-independent recovery of sympathetic function after chemical sympathectomy with 6-hydroxydopamine. J Comp Neurol. 1995;359:586–594. doi: 10.1002/cne.903590406. [DOI] [PubMed] [Google Scholar]
- 24.Gorgulu A, Simsek O, Cobanoglu S, Imer M, Parsak T. The effect of epidural free fat graft on the outcome of lumbar disc surgery. Neurosurg Rev. 2004;27:181–184. doi: 10.1007/s10143-003-0310-9. [DOI] [PubMed] [Google Scholar]
- 25.Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci. 2005;21(9):2475–2487. doi: 10.1111/j.1460-9568.2005.04089.x. [DOI] [PubMed] [Google Scholar]
- 26.Groen GJ, Baljet B, Drukker J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien) 1988;92(1–4):39–46. doi: 10.1007/BF01401971. [DOI] [PubMed] [Google Scholar]
- 27.Harris LW, Purves D. Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci. 1989;9(6):2210–2214. doi: 10.1523/JNEUROSCI.09-06-02210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18(2):149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- 29.Jensen TT, Asmussen K, Berg-Hansen EM, Laurutzen B, Manniche C, Vinterberg H, et al. First-time operation for lumbar disc herniation with or without free fat transplantation Prospective triple-blind randomized study with reference to clinical factors and enhanced computed tomographic scan 1 year after operation. Spine. 1996;21(9):1072–1076. doi: 10.1097/00007632-199605010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Kallakuri S, Cavanaugh J, Blagoev D. An immunohistochemical study of innervation of lumbar spinal dorsal dura and longitudinal ligaments. Spine. 1998;23(4):403–411. doi: 10.1097/00007632-199802150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto K, Matsuda H. Nerve growth factor and wound healing. Prog Brain Res. 2004;146:369–384. doi: 10.1016/S0079-6123(03)46023-8. [DOI] [PubMed] [Google Scholar]
- 32.Keller JT, Marfurt CF. Peptidergic and serotoninergic innervations of the rat dura mater. J Comp Neurol. 1991;309:515–534. doi: 10.1002/cne.903090408. [DOI] [PubMed] [Google Scholar]
- 33.Kinkelin I, Mötzing S, Koltzenburg M, Bröcker E-B. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–37. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- 34.Kiviluoto O. Use of free fat transplants to prevent epidural scar formation: an experimentsl study. Acta Orthop Scand Suppl. 1976;164:73–75. doi: 10.3109/ort.1976.47.suppl-164.01. [DOI] [PubMed] [Google Scholar]
- 35.Konnai Y. Nerve innervation of peptidergic fibers in the spinal dura mater of rats. Acta Anat Nipponica. 1994;69:89. [Google Scholar]
- 36.Konnai Y, Honda T, Sekiguchi Y, Kikuchi S, Sugiura Y. Sensory innervation of the lumbar dura mater passing through the sympathetic trunk in rats. Spine. 2000;25(7):776–782. doi: 10.1097/00007632-200004010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27(1–2):35–69. doi: 10.1016/S0306-4530(01)00035-X. [DOI] [PubMed] [Google Scholar]
- 38.Kuivila TE, Berry JL, Bell GR, Steffee AD. Heparinized materials for control of the formation of the laminectomy membrane in experimental laminectomies in dogs. Clin Orthop Relat Res. 1988;236:166–173. [PubMed] [Google Scholar]
- 39.Kumar R, Berger R, Dunsker S, Keller J. Innervation of the spinal dura: myth or reality? Spine. 1996;21(1):18–25. doi: 10.1097/00007632-199601010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Langenskiöld A, Kiviluoto O. Prevention of epidural scar formation after operations on the lumbar spine by means of free fat transplants. A preliminary report. Clin Orthop Relat Res. 1976;115:92–95. [PubMed] [Google Scholar]
- 41.Leslie TA, Emson PC, Dowd PM, Woolf CJ. Nerve growth factor contributes to the up-regulation of growth-associated protein 43 and preprotachykinin. A messenger RNAs in primary sensory neurons following peripheral inflammation. Neuroscience. 1995;67:753–761. doi: 10.1016/0306-4522(95)00101-N. [DOI] [PubMed] [Google Scholar]
- 42.Lindholm D, Hengerer B, Heumann R, Carroll P, Thoenen H. Glucocorticoid hormones negatively regulate nerve growth factor expression in vivo and in cultured rat fibroblasts. Eur J Neurosci. 1990;2:795–801. doi: 10.1111/j.1460-9568.1990.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 43.Loeser J. Pain due to nerve injury. Spine. 1985;10:232–235. doi: 10.1097/00007632-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, et al. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187(3):297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarron RF, Wimpee MW, Hudkins PG, Laros GS. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine. 1987;12(8):760–764. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Mearow KM, Kril Y. Anti-NGF treatment blocks the upregulation of NGF receptor mRNA expression associated with collateral sprouting of rat dorsal root ganglion neurons. Neurosci Lett. 1995;184:55–58. doi: 10.1016/0304-3940(94)11167-H. [DOI] [PubMed] [Google Scholar]
- 47.Meßlinger K, Hanesch U, Baumgärtel M, Trost B, Schmidt RF. Innervation of the dura mater encephali of cat and rat: ultrastructure and CGRP/SP-like immunoreactivity. Anat Embryol (Berl) 1993;188:219–237. doi: 10.1007/BF00188214. [DOI] [PubMed] [Google Scholar]
- 48.Mosconi T, Krüger L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain. 1996;64(1):37–57. doi: 10.1016/0304-3959(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 49.Oaklander AL, North RB. Failed back surgery syndrome. In: Loeser JD, editor. Bonica`s management of pain. Philadelphia: Williams & Wilkins; 2001. pp. 1540–1549. [Google Scholar]
- 50.O’Connor TP, Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci. 1988;8(7):2468–2476. doi: 10.1523/JNEUROSCI.08-07-02468.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parke WW, Watanabe R. Adhesions of the ventral lumbar dura. An adjunct source of discogenic pain? Spine. 1990;15(4):300–303. doi: 10.1097/00007632-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen HE, Blunck CF, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerve (sinu-vertebral nerves); with an experimental study of their functions. J Bone Joint Surg Am. 1956;38:377–391. [PubMed] [Google Scholar]
- 53.Petrie JL, Ross JS. Use of ADCON-L to inhibit postoperative fibrosis and related symptoms following lumbar disc surgery: a preliminary report. Eur Spine J. 1996;5(Suppl1):10–17. doi: 10.1007/BF00298567. [DOI] [PubMed] [Google Scholar]
- 54.Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur J Neurosci. 1999;11(3):837–846. doi: 10.1046/j.1460-9568.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 55.Reinert A, Kaske A, Mense S. Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Exp Brain Res. 1998;121:174–180. doi: 10.1007/s002210050449. [DOI] [PubMed] [Google Scholar]
- 56.Rossi F, Want JJ, Wiklund L, Strata P. Reinnervation of cerebellar Purkinje cells by climbing fibres surviving a subtotal lesion of the inferior olive in the adult rat. II. Synaptic organization on reinnervated Purkinje cells. J Comp Neurol. 1991;308(4):536–554. doi: 10.1002/cne.903080404. [DOI] [PubMed] [Google Scholar]
- 57.Saxler G, Krämer J, Barden B, Kurt A, Pförtner J, Bernsmann K. The long-term clinical sequelae of incidental durotomy in lumbar disc surgery. Spine. 2005;30(20):2298–2302. doi: 10.1097/01.brs.0000182131.44670.f7. [DOI] [PubMed] [Google Scholar]
- 58.Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O`Neill C. Failed back surgery: etiology and diagnostic evaluation. Spine J. 2003;3(5):400–403. doi: 10.1016/S1529-9430(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 59.Sekiguchi Y, Kinnai Y, Kikuchi S, Sugiura Y. An anatomic study of neuropeptide immunoreactivities in the lumbar dura mater after lumbar sympathectomy. Spine. 1996;21(8):925–930. doi: 10.1097/00007632-199604150-00004. [DOI] [PubMed] [Google Scholar]
- 60.Songer MN, Rauschnig W, Carson EW, Pandit SM. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in dogs. Spine. 1995;20(5):571–580. doi: 10.1097/00007632-199503010-00012. [DOI] [PubMed] [Google Scholar]
- 61.Stelzner DJ, Baisden RH, Goodman DC. The ventral lateral geniculate nucleus, pars lateralis of the rat. Synaptic organization and conditions for axonal sprouting. Cell Tissue Res. 1976;170(4):435–454. doi: 10.1007/BF00361703. [DOI] [PubMed] [Google Scholar]
- 62.Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, et al. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stilwell DL. The nerve supply of the vertebral column and its associated structures in the monkey. Anat Rec. 1956;125(2):139–169. doi: 10.1002/ar.1091250202. [DOI] [PubMed] [Google Scholar]
- 64.Vaquero J, Arias A, Oy S, Martinez R, Zurita M. Effect of fibrin glue on postlaminectomy scar formation. Acta Neurochir (Wien) 1993;120(3–4):159–163. doi: 10.1007/BF02112036. [DOI] [PubMed] [Google Scholar]
- 65.Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1949;19:211–221. doi: 10.3109/17453674908991094. [DOI] [PubMed] [Google Scholar]
- 66.Wiklund L, Rossi F, Strata P, Want JJ. The rat olivocerebellar system visualized in detail with anterograde PHA-L tracing technique, and sprouting of climbing fibers demonstrated after subtotal olivary lesions. Eur J Morphol. 1990;28(2–4):256–267. [PubMed] [Google Scholar]
- 67.Wilkinson HA. The Failed back surgery syndrome. New York: Springer; 1992. pp. 1–2. [Google Scholar]
- 68.Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495(6):679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]