Abstract
Current fusionless scoliosis surgical techniques span the intervertebral disc. This alters the spine stiffness, disc pressure equilibrium and possibly may lead to disc degeneration. A new fusionless physeal device was developed that locally modulates vertebral growth by compressing the physeal ring, while maintaining maximum segmental spinal mobility without spanning the intervertebral disc. This study’s objective was to test the feasibility of the device on a small animal model by inducing a scoliotic deformity (inverse approach) while analyzing the growth modifications. This study was conducted on caudal vertebrae of 21 rats (26-day-old) divided into 3 groups: (1) “experimental” (n = 11) with 4 instrumented vertebrae, (2) sham (n = 5) and (3) control (n = 5). Radiographs were taken at regular intervals during the 7-week experimental period. Tissues were embedded in methyl metacrylate (MMA), prepared by the cutting/grinding method, and then stained (Toluidine blue). The discs physiological alterations were qualitatively assessed and classified by inspection of the histological sections. A mean maximum Cobb angle of 30º (±6º) and a mean maximum vertebral wedge angle of 10º (±3º) were obtained between the 23rd and 35th day postoperative in the subgroup that underwent a long-term response from the device. The sham group underwent no growth alterations when compared to the control group. Descriptive histological analyses of the operated segments showed that 69% had no alterations to the intervertebral disc. This study presents experimental evidence that the device induces a significant and controlled wedging of the vertebrae while maintaining regular flexibility. In most discs, there were no visible morphological alterations induced. Further analysis of the discs and testing of this device on a larger animal is recommended with the long-term objective of developing an early treatment of progressive idiopathic scoliosis.
Keywords: Scoliosis, Fusionless correction, Minimally invasive, Growth modulation, Hemiepiphysiodesis
Introduction
In the past years there have been many attempts to develop and test fusionless devices for the correction of spinal deformities [1, 3–6, 18, 19]. These minimally invasive approaches maintain a certain spinal flexibility depending on the rigidity of the implant. Wall et al. [18] introduced a rigid 2-prong vertebral staple that fixes two adjacent vertebral bodies, while slowing down growth on one side of the spine. These were tested on a pig model and showed the ability to induce significant spinal curvature (reverse problem). Braun et al. [1, 5, 6] tested two different fusionless scoliotic treatments on previously induced spinal curvatures produced by an asymmetric tether in a goat model. These consisted of a flexible ligament tether attached to bone anchors and a rigid-shape memory alloy staple with two prongs. Both the systems allowed for successful correction of the deformity via growth modulation. The shape memory alloy stapling technique, with a 2- and 4-prong design, was also used by Betz et al. [3, 4] in a pilot study in humans for the treatment of AIS.
These new promising fusionless implants have shown their capacity to modulate growth and correct spinal deformities. However, they all span the intervertebral disc space, which may change the spine kinematics due to an applied compression of the discs. This in turn may alter end plate health and local blood vessel density, which can lead to disc degeneration [7, 8].
In order to address this important concern, we have developed a new minimally invasive and fusionless concept: a device that compresses only the physes without crossing the intervertebral disc. The objective of this study was to test this device’s feasibility in an animal model by inducing a spinal deformity (inverse scoliosis model approach) to analyze the growth modification.
Materials and methods
The chosen animal model was the rat tail because of the accessibility to the caudal vertebrae and the numerous growth modulation studies done on this model [14, 15]. Caudal rat vertebrae have a second ossification center and therefore the device was adapted to fit the physiology and size of this subject. The developed device is an L-shaped body with a large and thin prong designed to fit between the intervertebral disc and the bony endplate. It is made of titanium (grade 5, 6Al4 V) and has 2 small holes for manipulation with a holding tool during installation and a bigger hole for the insertion of a 1-mm diameter maxillofacial-type screw to fix the device against the vertebral body. This device spans only one growth plate (i.e. the prong is between the intervertebral disc and the bony endplate and the screw is in the vertebral body) and acts as a growth inhibitor on one side of the vertebra. This local growth modulation is intended to induce a local disc wedging via the Hueter–Volkmann principle [11, 14, 16].
All procedures were reviewed and approved by the institutional committee for care and use of animals. This study was performed on 21 immature rats (Male, Sprague–Dawley) divided into 3 groups: (1) the experimental group (n = 11) in which the physeal devices were installed on four adjacent caudal vertebrae Cd7–Cd10; (2) The sham group (n = 5) in which the rats had incisions at the sites where the device would have been implanted on Cd7–Cd 10 and (3) The control group (n = 5) in which the rats had no surgery at all.
The rats were operated at the age of 26 days and kept alive for 7 weeks. They were anesthetized with isoflurane (induction at 3–4%, maintained at 2–3% during the procedure) and the surgeries were performed under a binocular microscope. For the rats from group 1 (experimental), an incision of approximately 2 mm deep was made (11-blade) between the intervertebral disc and the bony endplate. The prong of the physeal device was then inserted and the device fixed to the vertebra with the screw (length of 3 or 4 mm depending on the size of the operated vertebra). The skin was then continuously sutured (vicryl 5–0). The rats from group 2 (sham) underwent the same intervention, but without implantation of the device or screw. In these two first groups, postoperative pain control was achieved with buprenorphine injections (sc, 0.1–0.5 mg/kg). One injection was made an hour before the surgery and another 5 hours after the surgery.
Operated rats were radiographed before and after the surgery as well as at 12-day intervals postoperative. Each time, the animals were in the same ventral decubitus position and a postero–anterior radiograph was taken. All radiographs were digitized and analyzed using dedicated image analysis software to perform local measurements (SliceOMatic, version 4.2; Tomovision, Montreal, QC, Canada). The global deformations were measured with the Cobb method on the Cd7–Cd10 segments (angle from the superior endplate of the proximal instrumented vertebra to the inferior endplate of the distal instrumented vertebra). Local measurement on Cd6–Cd11 was also recorded through vertebral wedging measurements by fitting a line on the proximal vertebral plate and one on the distal vertebral plate. The angle between theses lines provided the wedging angle. The vertebral heights were also measured in the middle of the vertebrae.
After killing (CO2), all rat tails were radiographed using a high-resolution radiographic system (Faxitron MX-20, Faxitron X-ray Corporation, Wheeling, IL, USA). CT scans were performed on three samples using a desktop micro-CT instrument (Model 1072, Skyscan, Aartselaar, Belgium). Samples were scanned at a magnification resulting in a pixel size of 15.95 μm. The cross-sections along the specimen axis were reconstructed using Nrecon Software (SkyScan), with a distance between each cross-section of 31.90 μm.
For histological analyses, the tissues were fixed in 5% paraformaldehyde solution and dehydrated in solutions of increasing alcohol concentrations. Tissues were then cleared in xylene and embedded in methyl methacrylate solutions (MMA) with increasing concentration of polymerization agents. Eight specimens showing at least three harvested vertebrae [6 specimens of the experimental group presenting significant deformation (resulting in 16 intervertebral discs), 1 of the sham group (with 3 intervertebral discs) and 1 of the control group (with 3 intervertebral discs)] were prepared for histological observation of the intervertebral discs. The cutting/grinding method was used to cut thick slices (30–40 μm) containing the implant using the Technovit 7200 (EXAKT Technologies, Inc. Oklahoma City, OK) and the slices were stained with Toluidine blue. Pictures of the intervertebral discs were taken using an optical microscope (Leika DMR) and classified into three categories: discs with no visible alteration, discs showing the presence of fibrous tissue and discs showing prominent alterations such as a hernia.
Results
Nine of the animals from the experimental group (9/11) and all of the others were followed during the entire 7-week-period. One preoperative death occurred because of anesthesia related issues. One of the instrumented rats had to be killed because of a severe postoperative infection. Five animals had minor postoperative infections, which were healed within 2 days by applying local antibiotics (Polysporin Ophthalmic Ointment).
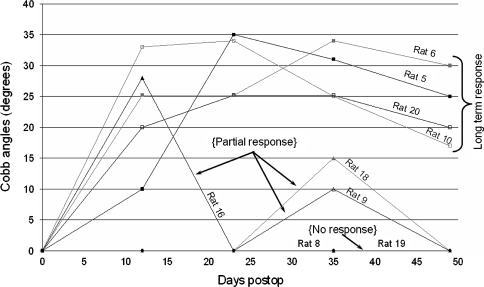
The 9 animals from the experimental group were divided into 3 groups depending on their growth modulation response (see Fig. 1): (1) the “long term response” group (n = 4), in which the device induced a growth modulation response from the first radiograph taken immediately after the surgery until sacrifice, (2) the “partial response” group (n = 3) in which the device induced a growth modulation response over a short period of time and that was observed in only one radiograph and (3) the “non response” group (n = 2) in which the device induced no growth modulation response.
Fig. 1.
Cobb angles versus time postoperative for all the rats of the experimental group
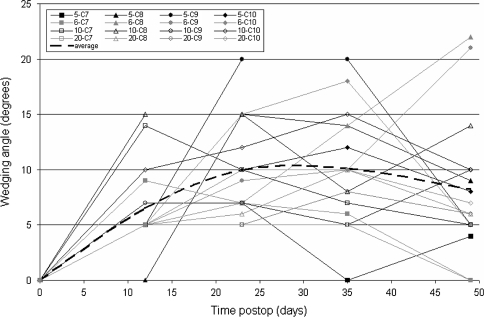
The long-term response group showed vertebral wedging angles in the instrumented vertebrae which reached a maximum of 22, 20, 15, and 10° between 12 and 35 days postoperative (Figs. 2, 3). These local growth modulations lead to global deformations (Fig. 4). A maximum Cobb angle of 35° was obtained 23 days postoperative in one of the four rats, the other had maximums of 34, 34 and 25° between 23 and 35 days postoperative. The spinal curve was slightly reduced to an average of 23° after 49 days postoperative.
Fig. 2.
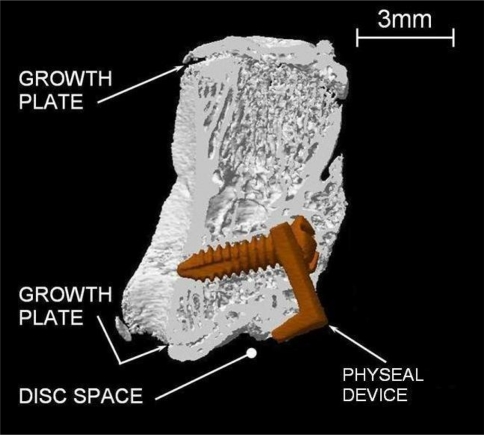
Effective growth modulation of the 7th caudal vertebra in Rat 18 (3D reconstruction of micro CT Scan images, after sacrifice)
Fig. 3.
Vertebral wedging of versus time. Seventh to tenth vertebrae are plotted for the four rats from long-term response subgroup
Fig. 4.
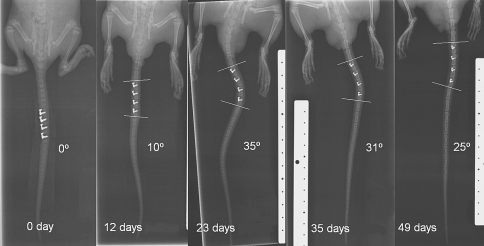
Global growth deformations in the tail of rat 5 presenting a long term growth modulation response. The fives pictures show the evolution of the curvature in time
In the partial response group, a spinal curvature was induced after the instrumentation: 28° (12 days postoperative), 10 and 15° (35 days postoperative) but disappeared subsequently (Fig. 1).
In the sham group, three had no deformity while two projected a small deformity (10 and 16°) 12 days postoperative. These angles were significant when compared to the absence of angle in the control group (P = 0.01) but they disappeared within the next 12 days.
Figure 2 shows the 3D reconstruction after microCT scan of an instrumented vertebra. This image is a good example of the physeal device in proper position and effectively invoking a local growth modulation. The vertebral wedging of this vertebra is 14°.
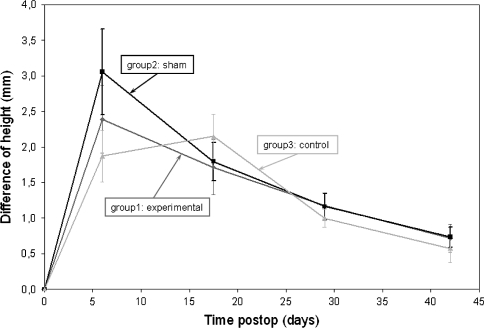
The sham and experimental groups experienced their growth peak at the same time, within the 12 first days postoperative, but the growth peak of the experimental group was significantly larger than the shams (3.1 ± 0.6 mm vs. 2.4 ± 0.5 mm). The control group underwent its growth peak later (12–23 days postoperative) with a smaller average of 2.2 ± 0.3 mm (Fig. 5).
Fig. 5.
Growth rate estimation versus days postoperative for the rats from all three experimental groups
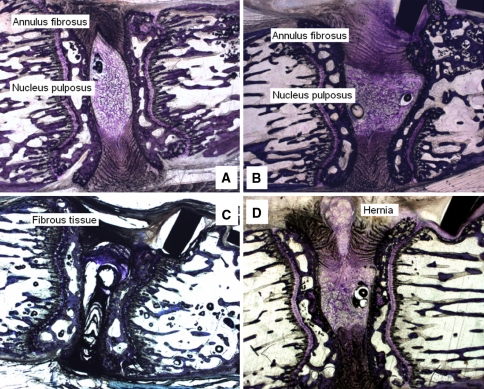
The discs that underwent histological analysis were classified as follows. Eleven of the 16 discs from the instrumented vertebrae showed no visible alteration (Fig. 7a), with 4 of them being deformed but not altered (Fig. 7b). As for the remaining five images from the instrumented group, three of them (from the same rat) showed fibrous tissues (Fig. 7c) and two showed a hernia (Fig. 7d). The three discs from the sham group and the three discs from the control groups showed no alterations at all.
Fig. 7.
a Intervertebral disc showing no visible alteration. b Deformed intervertebral disc showing no visible alteration. c Intervertebral disc with fibrous tissues. d Intervertebral disc with a hernia
Discussion
The present study demonstrated the feasibility of a fusionless growth modulation technique in a small animal model. Significant spinal deformities were induced to the caudal vertebrae by the physeal device. Global deformities reached a maximum of 35° and vertebral deformities a maximum of 22°. Decreasing deformities are observed at the end of the experiment. It is believed that the strength of the physeal plate during its growth pushed the device out of its position. The vertebrae overgrew the device and it became less efficient therefore the deformities decreased. Also, the observed displacement of the device was often angulated compared to its initial position. The device was a single rigid piece fixed to the vertebra by a rigid screw. The junction between these two sections was not completely fixed. As the vertebra grew, it pushed the device out but the screw was tightly fixed in the vertebral body (Fig. 6b). This evidence of growth beneath the implant suggests that the location of the incision does not bring to an end epiphyseal growth.
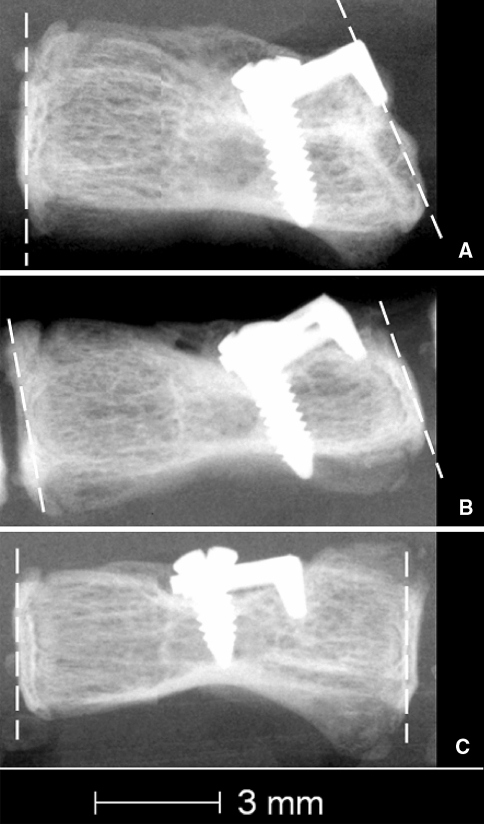
Fig. 6.
High resolution radiographs showing the position of 3 devices. a Efficient position. b Position of the device being pushed out by the vertebra. c Inefficient position
Three of the nine instrumented rats had a partial growth modulation response (Fig. 1) and two of the nine had no growth response at all. The high-resolution radiographs taken after killing showed that the devices implanted in these animals were not properly positioned. A proper position appears to be the key to an effective growth modulation. Figure 6, shows three typical device positions: (1) a proper and efficient position, (2) a device, which was overgrown by the vertebra and (3) a device in an inadequate and inefficient position. Improperly positioned devices did not provide a growth modulation. At the time of surgery the animals were very young (26 days), the caudal vertebrae very small (approximately 4 mm of length; 3 mm of diameter) and the physis (approx. 0.1 mm) was difficult to differentiate from the rest of the cartilage (height of the intervertebral disc: approx. 0.5 mm). The very small and cartilaginous vertebrae made it difficult to position the device properly even under a microscope. This brings a limit of surgical precision for this animal model.
Animals from the sham group showed no significant long-term growth modulation. A local disturbance, with no device implanted to compress the growth plate, did not induce a significant long-term growth modulation. However, this disturbance may have encouraged blood flow and cellular activity around the wound, which in turn could have lead to the highest growth peak of all the three tested groups. A similar cellular activity probably occurred in the experimental group but the physeal device interrupted any growth acceleration. The growth peak of the control group appeared later around the age of 40 days as reported by others [2, 9].
Two intervertebral discs showed a hernia. It is believed that these alterations are probably due to the surgical procedure. The incision that should have helped the insertion of the device might have been done in the intervertebral disc, which led to a clear hernia. One of the herniated discs came from an animal from the long-term response subgroup regarding growth modulation (Rat 20). Although the global deformity of its tail is important (25°), the local wedging of the herniated vertebra reached a maximum of 5° and ended with no wedging. The other herniated disc came from an animal in the partial response subgroup (Rat 16). The three discs that showed fibrous tissues all came from Rat 5 which is one of the animals that had a postoperative infection. The presence of the fibrous tissue could be due to healing of this infection. All the other discs showed no alteration; their annulus fibrosus and nucleus pulposus seemed intact and functional. However, stab and rim lesions have previously been shown to induce intervetebral disc degeneration in various animal models [10, 13]. Further, in a study performed on a same rat model, evidence of the disc remodeling occurred as early as 5 weeks [17]. Therefore, a longer postoperative follow-up may have revealed a more convincing insight into the discs condition. Different methods of analysis were performed in this study, thus, further investigation of the intervertebral discs such as immunohistochemistry to label type X collagen could be done in a future complementary phase to more accurately evaluate the health of the intervertebral discs. Also, histomorphometric analysis could be performed in future work to better analyze the effects of the device on the vertebra biology.
Although the animals were always positioned in a similar way during the X-rays (ventral decubitus), the position of their tails may have varied slightly from one radiograph to the other. The tail was in an optimal position on the X-ray if the view was normal to the device. Two X-rays of a rat showing no deformation at all were taken in a way that could not permit any angle measurement. For the other animal the measurement errors were estimated to be less than 5°. This Cobb angle acquisition error defined experimental results with a power of 0.9 and a significance level of 0.1. Even if small, the experimental group, consisting of 11 subjects, allowed demonstrating the feasibility of the device to provide local growth modulation. It should also be noted that results from this study are reported in a 1D observation plane whereas scoliosis consists of a 3D deformation. However, the number of devices, their geometry and location of insertion may be modified to address control of other anatomical planes if desired.
The results of this study are promising for early treatment of progressive idiopathic scoliosis in human. The use of a physeal device could correct spinal deformations that are detected before the adolescent growth peak to avoid a later surgery that involves the fusion of multiple spinal segments. It is difficult for the clinician to predict which patients will progress rapidly when they are seen in an orthopedic clinic at the age of 11–13 years. Serous tests are being developed by Moreau et al. [12] (personal communication) with the objective of diagnosing which patient’s scoliosis is likely to progress rapidly and which is not. In the future, the clinical use of this physeal device could be combined with these serous tests to choose which patients need a minimally invasive surgery that could avoid a rapid progression of their scoliosis. A device with a limited time effect could also be a new research way. This intelligent device could bring an anatomical and time targeted correction.
Conclusion
This study presents experimental evidence that this new vertebral physeal device, when properly positioned, induced a significant controlled wedging to vertebrae, while maintaining segmental flexibility. The intervertebral discs were not altered, except one animal having grown lots of fibrous tissue and two discs presenting hernia which are probably due to the surgical procedure. Deeper analysis of the intervertebral discs needs to be undertaken. This new surgical concept and device could then be tested on larger animal models before possibly transferred and adapted to humans with the clinical strategic goal of early treatment of scoliosis by controlled modulation of vertebral growth.
Acknowledgments
Funded by the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chair Program, and by an educational/research grant from Medtronic Sofamor Danek. Special thanks to Archana Sangole Ph.D. and Mark Driscoll for the editorial revision of the document.
References
- 1.Akyuz E, Braun JT, Brown NAT, et al. Static versus dynamic loading in the mechanical modulation of vertebral growth. Spine. 2006;31(25):E952–E958. doi: 10.1097/01.brs.0000248810.77151.22. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez J, Balbin M, Santos F, et al. Different bone growth rates are associated with changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J Bone Miner Res. 2000;15(1):82–94. doi: 10.1359/jbmr.2000.15.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Betz RR, D’Andrea LP, Mulcahey MJ, et al. Vertebral body stapling procedure for the treatment of scoliosis in the growing child. Clin Orthop Relat Res. 2005;434:55–60. doi: 10.1097/01.blo.0000163472.46511.a8. [DOI] [PubMed] [Google Scholar]
- 4.Betz RR, Kim J, D’Andrea LP, et al. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety and utility study. Spine. 2003;28:S255–S265. doi: 10.1097/01.BRS.0000092484.31316.32. [DOI] [PubMed] [Google Scholar]
- 5.Braun JT, Akyuz E, Udall H, et al. Three-dimensional analysis of two fusionless scoliosis treatments: a flexible ligament tether versus a rigid-shape memory alloy staple. Spine. 2006;31(3):262–268. doi: 10.1097/01.brs.0000197569.13266.fe. [DOI] [PubMed] [Google Scholar]
- 6.Braun JT, Ogilvie JW, Akyuz E, et al. Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine. 2006;31(13):1410–1414. doi: 10.1097/01.brs.0000219869.01599.6b. [DOI] [PubMed] [Google Scholar]
- 7.Grunhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88:30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 8.Hunt K, Braun J, Christensen B (2007) The effect of two clinically relevant fusionless scoliosis implant strategies on the health of the intervertebral disc. In: SRS 42nd Annual Meeting. Edinburg, Scotland [DOI] [PubMed]
- 9.Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocye regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaapa E, et al. Collagens in the injured porcine intervertebral disc. J Othop Res. 1994;12:93–102. doi: 10.1002/jor.1100120112. [DOI] [PubMed] [Google Scholar]
- 11.Mente PL, Stokes IAF, Spence H, et al. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine. 1997;22:1292–1296. doi: 10.1097/00007632-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Moreau A, Wang DS, Forget S, et al. Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine. 2004;29(16):1772–1781. doi: 10.1097/01.BRS.0000134567.52303.1A. [DOI] [PubMed] [Google Scholar]
- 13.Osti OL, Vernon-Roberts B, Fraser RD. Annulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15(8):762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Sarwark J, Aubin CE. Growth considerations of the immature spine. J Bone Joint Surg. 2007;89:8–13. doi: 10.2106/JBJS.F.00314. [DOI] [PubMed] [Google Scholar]
- 15.Stokes IAF, Gwadera J, Dimock A, et al. Modulation of vertebral and tibial growth by compression loading: diurnal versus full-time loading. J Orthopaed Res. 2005;23:188–195. doi: 10.1016/j.orthres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Stokes IAF, Spence H, Aronsson DD, et al. Mechnical modulation of vertebral body growth: implications for scoliosis progression. Spine. 1996;21:1161–1167. doi: 10.1097/00007632-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Stokes IA, et al. Intervertebral disc adaptation to wedging deformation. Stud Health Technol Inform. 2006;123:182–187. [PubMed] [Google Scholar]
- 18.Wall EJ, Bylski-Austrow DI, Kolata RJ et al (2005) Endoscopic mechanical spinal hemiepiphysiodesis modifies spine growth. Spine 30(10):1148–1153 [DOI] [PubMed]