Abstract
Subchondral signal abnormalities have been suggested to play an important role in chronic low back pain (LBP) syndromes. Their natural course is not well known. In this study the morphology and natural course of isolated subchondral signal abnormalities in the lumbosacral spine were analyzed with MRI. Twenty-four chronic LBP patients with a subchondral hypointensity on T1-weighted image (hyperintense on T2), indicating edema, were selected from a base population of 1,015 consecutive LBP patients to a follow-up MRI study within 18–72 months. Exclusion criteria were age >60 years, nerve root compression, a more specific back disease or a recent or major spine operation. The size and location of each subchondral signal abnormality and endplate lesion and the degree of degenerative disc changes were evaluated and compared between the baseline and follow-up studies. Most subchondral hypointensities were found at the L4/L5 or L5/S1 disc space, anteriorly and in both adjacent endplates. Almost all (53/54) hypointensities were associated with an endplate lesion. Twelve of the 54 subchondral hypointensities enlarged, six remained constant and 36 decreased or disappeared while five new ones appeared. Twenty-two (41%) hypointensities changed totally to hyperintensities or to mixed lesions. If the hypointensity increased, decreased or changed into hyperintensity, a change tended to develop in the adjacent endplate. If the hypointensity was absent or unchanged, endplate lesions did not tend to progress. In the absence of disc herniation or other specific spinal disease, subchondral hypointensities indicating edema are uncommon. They seem to have a highly variable course. There appears to be a link between endplate lesions and subchondral signal abnormalities. Further study is needed to explain the contribution of these findings to low back symptoms.
Keywords: Low back pain, Lumbar spine, Intervertebral disk, Follow-up studies, Magnetic resonance imaging, Abnormalities
Introduction
Lumbar disc degeneration plays a major role in low back pain (LBP) syndromes [24], but the magnetic resonance imaging (MRI) findings of intervertebral discs have not been found to explain well the symptoms among such patients. Subchondral signal abnormalities have been found to be associated with LBP [2, 3, 5, 11, 26], particularly with hypointensities [2, 13, 23]. Subchondral edema-like hypointensity on T1 weighted image (T1WI), being hyperintense on T2 weighted image (T2WI), has been found in association with vascularized fibrous tissue and with fissuring or disruption in the adjacent end plates [17]. Hyperintensity both on T1WI and T2WI has been found in association with fatty replacement of the subchondral marrow [17] and hypointensity both on T1WI and T2WI with sclerotic subchondral lesions in advanced degeneration.
Subchondral hypointensities have been mainly found to convert to hyperintensities on T1WI [16, 17], but some of them in contrast appear to enlarge [2, 16]. Most follow-up studies on the natural course of subchondral signal abnormalities include patients with disc herniations [2, 12, 16, 17, 25].
Subchondral bone marrow edema found in many specific diseases like spondylodiscitis or ankylosing spondylitis may be difficult to differentiate radiologically from the degenerative subchondral signal abnormalities [9, 17]. The course of such signal abnormalities in a specific back disease, even in disc herniation, may differ from that in degenerative disc disease in general. It is important for the physicians treating LBP patients to be familiar with the appearance and the natural course of the subchondral signal abnormalities in degenerative disc disease. Attempts to subclassify patients with LBP should have high priority [4, 7, 15], since it may result in a better targeting of treatment [4]. Therefore, we chose to study the morphology and the natural course of degenerative subchondral signal abnormalities, indicating edema, during a long-term follow-up in a specifically selected group of patients suffering from chronic non-specific LBP.
Materials and methods
The subjects were selected from 1,015 consecutive LBP patients referred for standard lumbar spine MRI at a university hospital, to study the association between subchondral signal abnormalities and LBP, which will be accomplished later. The inclusion criteria were chronic non-specific LBP of at least 3 months’ duration and a clearly detectable subchondral hypointensity on T1WI, hyperintense on T2WI, covering at least 5% of the area of the vertebra in a sagittal MR image. Lumbar T1 and T2 weighted MR images were retrospectively evaluated by an experienced musculoskeletal radiologist (LK), according to the criteria by Modic et al. [17], for the presence of subchondral signal abnormalities and other findings possibly explaining the LBP. The exclusion criteria were as follows: age ≥60 years, a specific back disease like fracture, neoplasia, a known or detected infectious or rheumatic spine disease, spondylolisthesis (5 mm or more), spinal stenosis, disc extrusion, any other finding with neural compression, a recent spine operation (less than 6 months ago) and a major spine operation like spondylodesis or disc prosthesis at any time. Small marginal signal changes beneath osteophytes were excluded. The mean age of the selected patients (16 females, 8 males) was 43 (28–60) years. Three had had an earlier operation 3–7 years before the MRI. All patients included gave a written informed consent to use their clinical data for the study purposes. The study protocol was approved at the local ethical committee.
The baseline MR images were obtained with high field MR units by using T1- and T2-weighted TSE and FSE sequences. Two Gyroscan units (1.0 T), one Sonata (1.5 T) and one Signa (1.5 T) unit were used. The follow-up (sagittal) images were obtained with the Gyroscan 1.0 T unit and TSE-sequences with an average interval of 42 (18–74) months.
An independent and blind reading of sagittal T1- and T2-weighted images (hard copies) was performed by an experienced radiologist (KL). She read 20 images twice and so did another radiologist (LK) once to estimate the intra- and interobserver agreements. The location and maximal area (as a percentage of the corresponding area of the vertebral body) of any subchondral signal abnormality was visually estimated and classified as M1 (hyperintense on T2WI, hypointense on T1WI), M2 (hyperintense on T1WI and T2WI) and M3 (hypointense on T1WI and T2WI). Lesions in each endplate (n = 240) were assessed and classified as irregularity (uneven border between the intervertebral disc and vertebral body) or focal defect (focal subchondral lesion or defect with appearance of Schmorl lesion or erosion) or both. The location of each endplate lesion in relation to M1 was assessed by comparing whether it was located in the same or adjacent sagittal slice as the largest M1 (in contact) or more distant.
Signal intensity of the nucleus pulposus was estimated by comparing it with the adjacent cerebrospinal fluid (CSF) and cortical bone as normal, slightly decreased, decreased, or strongly decreased. Disc height (anterior, posterior and middle) was visually estimated as normal (higher than, or as high as the upper not degenerated disc space), slightly decreased (≤33% lower than the upper disc space), clearly decreased (34–66% lower), or strongly decreased (>66% lower). Anterior and posterior bulges or protrusions reaching clearly beyond the interspace were assessed. Examples of degenerated discs are shown in Figs. 1, 2.
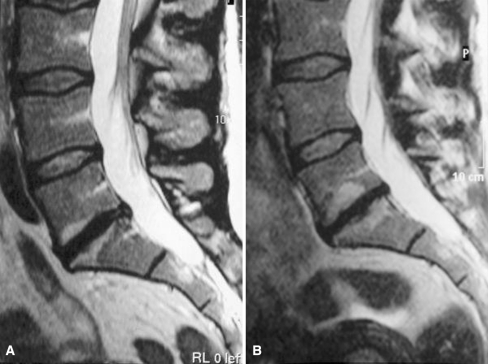
Fig. 1.
a On T2-weighted image (T2WI) at baseline, a subchondral hyperintensity (M1) adjacent to both endplates of L5/S1 disc space and a focal hypointense lesion subchondrally adjacent to the lower endplate. Disc signal intensity slightly decreased at L4/5, decreased at L3/4 and strongly decreased at L5/S1. Disc height decreased at L5/S1. b After follow-up the focal hypointense lesion and the surrounding subchondral hyperintensity adjacent to the lower endplate have enlarged as well as the hyperintensity adjacent to the upper endplate and a new focal lesion has appeared subchondrally in its center. Disc height at L5/S1 is strongly decreased (further)
Fig. 2.
a On T2WI at baseline the small focal subchondral hypointensity surrounded by a hyperintensity (MI) on the upper and lower endplate of L3/L4 disc space slightly enlarges after follow-up (b) and a new MI surrounding a focal hypointensity appears on the lower endplate. Disc signal intensity is decreased at L3/L4 and L4/L5 and slightly decreased at L5/S1, and disc height slightly decreased at L3/L4 and normal at L4/L5 and L5/S1 at baseline (a) and decreased (further) at L3/L4 after follow-up (b)
Then a consensus reading was performed by two experienced radiologists (KL, TV) to evaluate the development of M1, endplate lesions and degenerative disc changes in each disc space (n = 120), by comparing the baseline and follow-up images.
The presence and change of the size of M1 was classified as follows: constantly no M1, M1 remaining constant, M1 disappeared or decreased, M1 enlarged, or a (new) M1 appeared. M2 and M3 were evaluated accordingly. Endplate lesion was evaluated as follows: constantly no lesion, lesion persisted, focal defect disappeared and endplate lesion appeared. Anterior and posterior bulges were evaluated accordingly. The constancy in signal intensity of the nucleus pulposus was classified as follows: constantly normal, constantly decreased but unchanged, intensity (further) decreased and intensity locally increased. The constancy in disc height was classified as follows: constantly normal, constantly decreased but unchanged, height (further) decreased. A visually estimated height decrease of at least 20% in any region was considered a true change.
Statistical analysis
The intraobserver and interobserver agreements of assessing the degenerative changes at each disc level on MR images were estimated by calculating kappa or intraclass correlation coefficients (ICC). The size of each M1 adjacent to the upper endplate was correlated with that adjacent to the corresponding lower endplate by Spearman’s correlation. The size of each M2 was correlated accordingly. Statistically significant difference was set at P < 0.05.
The independent effect of the presence or change of the endplate lesion (No lesion/lesion persisted/focal defect disappeared/lesion appeared) on the dichotomized change in the M1 lesion (No M1 or M1 remained constant/M1 decreased, disappeared, enlarged or appeared) during follow-up was studied with logistic regression taking into account other possible predictors such as disc changes (anterior/posterior bulge, height and signal intensity of the disc) and endplate location (upper/lower). Patient age, gender and imaging interval were included in the original analysis but then eliminated due to their non-significant effect. A single endplate was regarded as an observational unit (n = 240). Endplates within patients are likely to be more similar than those between patients due to genetic and environmental factors. Also endplates located at the same disc level (e.g., all presacral endplates) are likely to be affected by more similar physical loading factors than those at different levels (e.g., L1/2 and L4/L5) due to biomechanical factors. The above facts violate the basic assumption of independency of observational units within a group, which is required in most statistical analyses. Logistic regression analyses were, therefore, conducted by defining patients as clusters. The disc level (n = 5) was also set as a cluster. SPSS 14.0.1. (SPSS Inc., Chicago, Illinois, USA) with its complex samples module was used. P < 0.005 were considered significant.
Results
Baseline
The 24 patients had 54 M1s, 19 of them at a single disc space and five at two disc spaces. M1 was adjacent to a single endplate in four cases and to both endplates in 25 cases of those 29 affected disc spaces. Location of M1 was disc space L5/S1 in thirteen cases, L4/L5 in eleven, L3/L4 in three, L2/L3 in one and L1/L2 in one case. Twenty-eight of the 54 M1s were uniformly hypointense on T1WI and 26 of mixed hypo- and hyperintense (M1/M2) type.
Nineteen patients had 41 M2s, 15 of which uniformly hyperintense on T1WI, 12 at a single disc level, four at two levels and three at three levels. At 14 (48%) of the 29 affected disc spaces M2 was located adjacent to the upper endplate only and at three (10%) to the lower one only while at 12 (41%) to both. Seven patients had M3s, all associated with M1.
The presence and size of subchondral signal abnormalities adjacent to all the 240 endplates is detailed in Table 1. Forty-four percent of the M1s covered at least 20% of the sagittal area of the vertebral body. M2s and M3s were smaller. The size of the M1 adjacent to the upper endplate correlated with that adjacent to the lower one: r = 0.925, P = 0.01 at L5/S1 level and r = 0.818, P = 0.01 at L4/L5 level. The correlation with regard to the M2 lesions was somewhat weaker, but still significant: r = 0.450, P = 0.05 at L5/S1 level and r = 0.640, P = 0.01 at L4/L5 level. M1 lesions were slightly more common in the anterior half than the posterior half or central part of the endplate (Table 2). At disc space L5/S1, the largest four M1 lesions were located adjacent to the upper endplates.
Table 1.
Presence and size of the subchondral Modic type signal intensity changes (M1, M2 and M3) adjacent to 240 lumbar end plates of 24 chronic LBP patients at baseline
| Size (% of area) | M1 | M2 | M3 | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 5–19 | 30 | 56 | 38 | 93 | 14 | 100 |
| 20–39 | 17 | 31 | 2 | 5 | 0 | 0 |
| 40–60 | 7 | 13 | 1 | 2 | 0 | 0 |
| All | 54 | 100 | 41 | 100 | 14 | 100 |
Size estimated as the maximal percentual area of the signal change in the sagittal plane in relation to that of the vertebral body
Table 2.
Location of the Modic type 1 signal changes (M1) in the subchondral marrow adjacent to the upper and lower endplates of the 120 disc spaces L1/L2–L5/S1 at baseline and after follow-up
| Location of M1 | Endplate with M1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||||
| Upper n |
Lower n |
Both n |
Both % | Upper n |
Lower n |
Both n |
Both % | |
| Anterior | 1 | 1 | 2 | 4 | 3 | 4 | 7 | 15 |
| Antero-central | 15 | 9 | 24 | 44 | 10 | 8 | 18 | 39 |
| Central | 2 | 4 | 6 | 11 | 6 | 4 | 10 | 22 |
| Postero-central | 1 | 3 | 4 | 7 | 1 | 1 | 2 | 4 |
| Posterior | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 2 |
| Whole end plate | 8 | 9 | 17 | 32 | 3 | 5 | 8 | 18 |
| All | 28 | 26 | 54 | 100 | 23 | 23 | 46 | 100 |
There was an endplate lesion in 41/120 upper endplates and in 42/120 lower ones. Forty-seven of lesions were irregularities, 19 focal lesions and 17 irregularities with focal lesions. Endplate lesion was in contact with 53/54 (98%) M1s (Figs. 1, 2, 3, 4). Some M1s had a semilunar shape surrounding the centrally located endplate lesion (Figs. 2, 3). Some of the focal lesions had an appearance of an erosion or Schmorl lesion (Fig. 4), some a focal small hypointensity (Figs. 1, 2, 3).
Fig. 3.
a On T2WI at baseline a hyperintensity (M1) with a semilunar shape surrounds the small hypointense end plate lesion on the upper end plate of the L4/L5 disc space. b After follow-up the hypointense lesion on the upper endplate enlarges along with the surrounding hyperintensity. A new small hypointense lesion and a surrounding hyperintensity (M1) appear adjacent to the lower end plate. c On T1WI after follow-up, a focal defect has appeared in the center of the M1 type subchondral hypointensity adjacent to the upper end plate. A new hypointensity (M1) has appeared adjacent to the lower one
Fig. 4.
At baseline a subchondral hyperintensity (M1) on T2WI (a), hypointense on T1WI (b) is surrounding a Schmorl-type defect adjacent to the lower end plate of the L4/L5 disc space and a small irregularity adjacent to the upper end plate. After follow-up the subchondral signal changes have enlarged anteriorly on T2WI (c) and partly changed from M1 to M2 type, hyperintense on T1WI (d). The small irregularity adjacent to the upper end plate has clearly enlarged along with the surrounding M1, resembling now an erosion or Schmorl lesion. Posteriorly, the irregularity adjacent to the lower endplate is decreased and the Schmorl type defect become more clearly demarcated while the subchondral hypointensity (M1) has totally changed to a hyperintensity (M2)
Signal intensity was normal in 32 (44%), slightly decreased in 25 (21%), decreased in 28 (23%) and strongly decreased in 15 (12%) of the 120 lumbar discs. Disc height was normal in 58 (65%), slightly decreased in 18 (15%), clearly decreased in 13 (11%) and strongly decreased in 11 (9%) discs. Thirty discs (25%) had a posterior bulge, 29 (24%) an anterior bulge.
The inter- and intraobserver agreements were 0.72 and 0.76 (ICC) concerning disc signal intensity, 0.91 and 0.91 (ICC) concerning disc height, 0.80 and 0.89 (kappa) concerning no endplate lesion/endplate lesion, 0.82 and 0.88 (ICC) concerning the size of M1 on T1WI, 0.80 and 0.89 (ICC) concerning the size of M2 on T1WI, 0.89 and 0.95 (ICC) concerning hyperintense subchondral change on T2WI and 0.40 and 0.51 concerning disc bulges, respectively.
Follow-up
Most (36/54, 67%) M1s disappeared or decreased during the follow-up (Fig. 4d), but 12 of them (22%) enlarged (Figs. 1b, 3b) and five new ones appeared (Figs. 2b, 3b, Table 3). The longer the interval, the more likely M1 tended to decrease or disappear (Table 3). 22/23 new M2s developed from M1s (Fig. 4d) and the only new one not detectable at baseline, was of mixed type (Table 4). 18/41 (43%) M2s remained unchanged while 17/41 (41%) enlarged. In the three cases in which M2 disappeared or decreased, an adjacent M1 enlarged. Most M3s remained unchanged or increased. Six developed from M1. The changes of M1 varied with time and between regions of the vertebral body (Table 2).
Table 3.
The development of the Modic type 1 subchondral signal intensity lesions (M1) of 24 LBP patients: percentages of lesions with different types of development and the according mean interval (in months) between baseline and follow-up MRI studies
| Development of M1 | M1 | Interval in months | |
|---|---|---|---|
| n | % | Mean (range) | |
| Same size persists | 6 | 10 | 34.8 (24–61) |
| Size increased | 14a | 24 | 37.4 (24–45) |
| New change appeared | 3 | 5 | 42.7 (29–62) |
| Disappeared | 13 | 22 | 44.1 (29–61) |
| Size decreased | 23 | 39 | 45.4 (18–74) |
| All lesions at follow-up (at baseline) | 59 (54) | 100 | 41.8 (18–74) |
aIncludes two Modic one lesions that appeared adjacent to Modic 2 lesions during follow-up
Table 4.
The development of the Modic type 2 subchondral signal intensity lesions (M2) of 24 LBP patients: percentages of lesions with different types of development and the according mean interval (in months) between baseline and follow-up MRI studies
| Development of M2 | M2 | Interval in months | |
|---|---|---|---|
| n | % | Mean (range) | |
| Size decreased | 2 | 3 | 31.5 (24–39) |
| New change appeared | 1 | 2 | 39.0 |
| Same size persists | 18 | 28 | 40.3 (18–74) |
| Size increased | 39a | 61 | 44.7 (25–74) |
| Disappeared | 4 | 6 | 55.6 (37–74) |
| All M2 lesions at follow-up (at baseline) | 64 (41) | 100 | 41.8 (18–74) |
aIncludes 22 M2 lesions that were developed from type 1 to type 2 during follow-up
Endplate lesion increased during follow-up in 19/53 (36%) endplates in which it was in contact with M1 (Figs. 3c, 4c, d) and in 35/240 (15%) of all the endplates. The signal intensity decreased in 18 (15%) and the height in 17 (14%) of the total 120 discs. A posterior bulge appeared in seven discs and disappeared in ten, an anterior bulge in six and seven, respectively. There was a statistically significant positive association between the presence or change in the endplate lesion (when compared to endplates without lesion) and any change in the adjacent M1 lesion as studied with logistic regression (P = 0.032). Thus, if the size of M1 increased or decreased, or its signal intensity changed, an adjacent endplate lesion often was present (OR = 17.1, 95% CI 2.7–109.6), increased or a new one appeared (OR = 10.1, 95% CI 1.5–68.7), or the lesion changed in appearance (OR 12.9, 95% CI 1.5–114.5).
Discussion
This is a detailed mid- to long-term prospective MRI-study on subchondral signal abnormalities in the lumbar spine of LBP patients without disc herniation or other specific spine disorders. Studying subgroups of the usually very heterogeneous LBP patients has been given a high priority in LBP research [4, 7, 15].
Only patients with a relatively large, conclusive M1 were included, since such more definite hypointensities (on T1WI) have been found to correlate with LBP [5, 13]. Elderly people (over 60 years old) were excluded because of the common, age-dependent, degenerative changes in intervertebral discs and bone marrow, which may hamper the assessment of the subchondral abnormalities. The number of patients in this study was limited because M1 as a single finding is not common among patients with chronic LBP even though it is in association with disc herniation [2, 22]. In our study, the carefully selected group of LBP patients with M1 consisted of 24 (2.4%) patients of the 1,015 base population. In previous studies, the reported prevalence of subchondral signal abnormalities varies, that of hypointensities between 4% and 16% [2, 6, 11, 13, 17, 22]. This is obviously due to varying criteria for abnormalities and selection of subjects.
In the present study the radiological analysis was more detailed and the follow-up longer than in most previous studies although it varied somewhat between patients. There was a high intra- and interobserver agreement with respect to subchondral signal abnormalities. The agreement on detecting bulges was fair. Although the initial MRI equipments and imaging protocol were not identical in the participating clinics, all baseline images were obtained with a high-field unit and all follow-up MRI images with the same 1.0 T equipment and imaging protocol. We believe that the equipment dependent differences did not essentially affect the results of the follow-up because our classification was chosen to detect only large M1s and unquestionable signal changes.
In our study, M1 had a more dynamic course than other signal abnormalities or degenerative disc changes. The majority decreased or disappeared and 41% of those changed into hyperintensities (M2). Hypointensities (M1) did neither change simultaneously in all regions nor after same time interval (Table 4). The finding of three new hypointensities appearing within previous hyperintensities may be explained by new subchondral edema in a region with earlier fatty degeneration. Reverse transformation of M2 to M1 has been reported earlier [12, 14]. Thirty-four percent of M1s persisted or enlarged, suggesting that the phase of subchondral edema may last even for years.
In one study, during a follow-up of 14 months subchondral hypointensities increased from 9 to 29% [2] but in another study, during a longer follow-up of 72 months most subchondral hypointensities converted to hyperintensities [16]. We found a similar trend: most (67%) hypointensities seemed to turn into hyperintensities or disappear, while most (65%) hyperintensities persisted or increased. It has been suggested that they may represent different consecutive phases of the degenerative process in the subchondral marrow [3, 17].
The importance of the endplate [21, 22] and subchondral bone [8] for the integrity of the intervertebral disc has been pointed out. An injury to the vertebral body endplate has been found to provide a potent mechanical stimulus for disc degeneration [20] and tissue trauma following fracture has been suggested to cause accelerated intervertebral disc degeneration [10]. Schmorl lesions have been found to be associated with disc degeneration [10, 19].
Modic et al. [17], found fissures in the subchondral marrow adjacent to the endplate in patients with subchondral edema on MRI. A microtrauma to the endplate has been suggested to be the initiating event for a progressive deteriorating process with inflammatory or autoimmune effects, by allowing a contact between the noxious substances in the degenerative disc and cells in the bone matrix via the injured endplate [1, 20]. An endplate defect may also develop as a non-specific reaction to an insufficient healing process, beginning with bone marrow edema and necrosis [8]. An inflammatory process in endplates with M1 subchondral signal changes has been suggested to be induced by tumor necrosis factor (TNF) [18]. In a 3-year follow-up of sciatica patients [12], new subchondral signal abnormalities were seldom found to be associated with endplate lesions, in contrast to our study, but the follow-up was shorter and the prevalence of subchondral hypointensities was lower and none was uniformly hypointense at baseline.
Conclusions
Subchondral signal abnormalities have a highly variable course during a mid- to long-term follow-up. Endplate lesions appear to change in parallel with them, suggesting that endplate lesions may be an essential feature of the degenerative process manifesting as subchondral signal abnormalities.
Acknowledgments
This study was financially supported by Finska Läkaresällskapet.
Contributor Information
Katariina Luoma, Phone: +358-9-4747423, FAX: +358-9-4747-423, Email: katariina.luoma@hus.fi.
Tapio Vehmas, Email: tapio.vehmas@ttl.fi.
References
- 1.Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16(7):977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braithwaite I, White J, Saifuddin A, Renton P, Taylor BA. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363–368. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan GP, Fritz J, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain results of a randomized clinical trial. Spine. 2006;31(6):623–631. doi: 10.1097/01.brs.0000202807.72292.a8. [DOI] [PubMed] [Google Scholar]
- 5.Carragee E, Alamin T, Miller J, Carragee JM. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine. 2005;5(1):24–35. doi: 10.1016/j.spinee.2004.05.250. [DOI] [PubMed] [Google Scholar]
- 6.Chung C, Berg B, Tavernier T, Cotten A, Laredo J, Vallee C, Malghem J. End plate marrow changes in the asymptomatic lumbosacral spine: frequency, distribution and correlation with age and degenerative changes. Skeletal Radiol. 2004;33(7):399–404. doi: 10.1007/s00256-004-0780-z. [DOI] [PubMed] [Google Scholar]
- 7.Dankaerts W, O’Sullivan P, Burnett A, Straker L. Differences in sitting postures are associated with nonspecific chronic low back pain when patients are subclassified. Spine. 2006;31:698–704. doi: 10.1097/01.brs.0000202532.76925.d2. [DOI] [PubMed] [Google Scholar]
- 8.Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581–588. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jevtic V, Kos-Golja B, Rozman B. Marginal erosive discovertebral “Romanus” lesions in ankylosing spondylitis demonstrated by contrast enhanced Gd-DTPA magnetic resonance imaging. Skeletal Radiol. 2000;29:27–33. doi: 10.1007/s002560050005. [DOI] [PubMed] [Google Scholar]
- 10.Kerttula LI, Serlo WS, Tervonen OA, Paakko EL, Vanharanta HV. Post-traumatic findings of the spine after earlier vertebral fracture in young patients: clinical and MRI study. Spine. 2000;25(9):1104–1108. doi: 10.1097/00007632-200005010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen J, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year old men and women. Spine. 2005;30(10):1173–1180. doi: 10.1097/01.brs.0000162396.97739.76. [DOI] [PubMed] [Google Scholar]
- 12.Kuisma M, Karppinen J, Niinimäki J, Kurunlahti M, Haapea M, Vanharanta H, Tervonen O. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine. 2006;31(15):1714–1718. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 13.Kuisma M, Karppinen J, Niinimäki J, Ojala R, Haapea M, Heliövaara M, Korpelainen R, Taimela S, Natri A, Tervonen O. Modic changes in endplates of lumbar vertebral bodies. Prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32(10):1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 14.Marshman LA, Trewhella M, Friesem T, Bhatia CK, Krishna M. Reverse transformation of Modic type 2 changes to Modic type 1 changes during sustained chronic low-back pain severity. Report of two cases and review of the litterature. J Neurosurg Spine. 2007;6(2):152–155. doi: 10.3171/spi.2007.6.2.152. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy CJ, Rushton A, Billis V, Arnall F, Oldham JA. Development of a clinical examination in non-specific low back pain: a Delphi technique. J Rehabil Med. 2006;38:263–267. doi: 10.1080/16501970600632768. [DOI] [PubMed] [Google Scholar]
- 16.Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004;14:1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 17.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 18.Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic type 1 or type 2 changes on MRI. Spine. 2006;31(9):1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. [DOI] [PubMed] [Google Scholar]
- 19.Paajanen H, Alanen A, Erkintalo M, Salminen J, Katevuo K. Disc degeneration in Scheuermann disease. Skeletal Radiol. 1989;18:523–526. doi: 10.1007/BF00351753. [DOI] [PubMed] [Google Scholar]
- 20.Przybala A, Pollintine P, Bedzinski R, Adams MA. Outer annulus tears have less effect than endplate fracture on stress distribution inside intervertebral discs: relevance to disc degeneration. Clin Biomech. 2006;21:1013–1019. doi: 10.1016/j.clinbiomech.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the end plate on diffusion in normal and degenerate discs. Spine. 2004;29(23):2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 22.Schmid G, Witteler A, Willburger R, Kuhnen C, Jergas M, Koester O. Lumbar disk herniation: correlation of histologic findings with marrow signal intensity changes in vertebral endplates at MR imaging. Radiology. 2004;231:352–358. doi: 10.1148/radiol.2312021708. [DOI] [PubMed] [Google Scholar]
- 23.Toyone T, Takahashi K, Kithara H, Yamagata M, Murakami M. Vertebral bone-marrow changes in degenerative lumbar disc disease: an MRI study of 74 patients with low back pain. J Bone Joint Surg (Br) 1994;76:757–764. [PubMed] [Google Scholar]
- 24.Vanharanta H, Guyer R, Ohnmeiss D, Stith J, Sachs B, Aprill C, Spivey M, Rashbaum R, Hochschuler S, Videman T, Selby D, Terry A, Mooney V. Disc deteriorating in low-back syndromes. A prospective, multi-center CT/discography study. Spine. 1988;13(12):1349–1351. doi: 10.1097/00007632-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Vital J, Gille O, Pointillart V, Pedram M, Bacon P, Razanabola F, Schaelderle C, Azzouz S. Course of Modic 1 six months after lumbar posterior osteosynthesis. Spine. 2003;28:715–721. doi: 10.1097/00007632-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 26.Weishaupt D, Zanetti M, Hodler J, Min K, Fuchs B, Pfirrman CW, Boos N. Painful lumbar disk derangement: relevance of endplate abnormalities at MR imaging. Radiology. 2001;218:420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]