Abstract
A specific marker for early prostate cancer would fill an important void. In initial evaluations of the prostate cancer antigen 3 (PCA3) gene vis-à-vis serum prostate-specific antigen (PSA) levels, the gene offers great promise. At the cellular level, PCA3 specificity for cancer is nearly perfect because of the gross overexpression of the gene by cancer cells. As a clinical test for early prostate cancer, heightened specificity is also seen in urine containing prostate cells from men with the disease. PCA3 gene testing holds valuable potential in PSA quandary situations: (1) men with elevated PSA levels but no cancer on initial biopsy; (2) men found to have cancer despite normal levels of PSA; (3) men with PSA elevations associated with varying degrees of prostatitis; and (4) men undergoing active surveillance for presumed microfocal disease.
Key words: Prostate cancer, Prostate cancer antigen 3 gene, Prostate-specific antigen, Benign prostatic hyperplasia, Prostatitis, Microfocal disease, Watchful waiting
Prostate cancer detection has increased over the past 2 decades through 1 million biopsies per year, mostly prostate-specific antigen (PSA) driven; tens of thousands of men with early disease have been treated with curative intent; and mortality from the disease has declined. Nevertheless, some 28,000 men died from prostate cancer during 2007.1 A specific marker of early disease is not yet available, and such a test would be highly desirable. One candidate to become that marker is the prostate cancer antigen 3 (PCA3) gene, a segment of noncoding messenger ribonucleic acid (mRNA) from chromosome 9q21–22 that is overexpressed by more than 95% of all prostate cancers tested.2
PCA3 expression, which is 60- to 100-fold greater in cancerous than in benign prostate tissues,3 gives the gene a cancer specificity lacking with PSA. At the cellular level, PCA3 determination can separate benign from malignant prostate cells with an accuracy approaching 100%.4 Moreover, no PCA3 transcripts have been detected in a wide range of human extraprostatic benign and cancerous tissues, indicating that PCA3 is the most specific prostate cancer gene identified to date. Overexpression of PCA3 by cancer cells has allowed diagnostic use of gene levels in tissues or fluids containing prostate cellular material.3,5 In clinical studies to date, urinary PCA3 scores (PCA3-mRNA/PSA-mRNA) are consistently superior to serum PSA levels in diagnosis of prostate cancer.5–8 These studies have been reviewed recently in this journal.9
The PCA3 test requires collection of the first 20 to 30 mL of voided urine after a digital rectal examination (DRE) (Figure 1); without DRE the test provides valid results in only approximately 80% of cases, but DRE increases this yield to more than 98% of cases. Approximately 2 mL of the urine sample is then placed into a transport tube with lysis buffer that contains ribonuclease inhibitors. This is the most critical step; the assay evaluates mRNA, a very labile sample that is destroyed in approximately 20 minutes in the absence of inhibiting agents. Then the specimen is transported overnight to the testing center in frozen gel packs. A sufficient sample was defined as a PSA mRNA copy number of 7500/mL or greater. Unlike the first-generation test based on the PCA3 gene (uPM3™, DiagnoCure, Quebec, Canada), which was qualitative (results were either positive or negative), the current second-generation product, now referred to as PCA3 (Gen-Probe, San Diego, CA), provides a quantitative result from the ratio of mRNA transcripts of PCA3 to PSA, thereby controlling for the number of prostatic epithelial cells in the urine (only these cells contain PSA mRNA transcripts).
Figure 1.
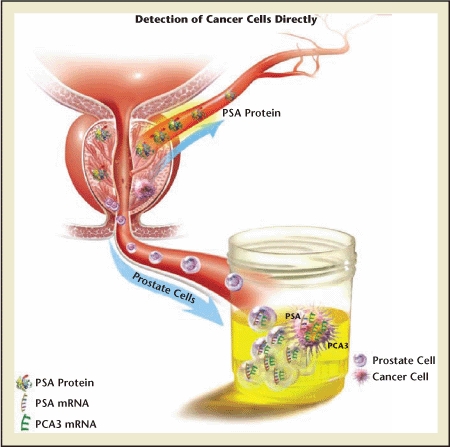
Diagram showing urinary prostate cancer antigen 3 (PCA3) (lower arrow) versus serum prostate-specific antigen (PSA) (upper arrow). Whereas PSA is a glycoprotein that may enter the bloodstream, PCA3 is a gene that exists in the nuclear material of prostate epithelial cells and that may be shed into the urine. Those cells, if cancerous, overexpress the gene. That overexpression, which may be many times that found in benign prostate cells, is detected by the assay. Importantly, PCA3 expression is normalized against a background of prostate-specific nuclear material (PSA messenger ribonucleic acid [mRNA]), yielding a PCA3 score. The PCA3 score is much more cancer-specific than serum PSA levels, which are confounded by factors such as prostate volume, age, trauma, and certain drugs.
A collaboration between the Urological Sciences Research Foundation and the manufacturer, Gen-Probe, was begun in 2004 with the goal of evaluating the PCA3 gene as a specific marker for early prostate cancer. The first public presentation of the Gen-Probe research test was at a Gordon Research Conference the next year; test methodology was published by Groskopf and associates in 2006.10 Soon after the methodology was published, several commercial laboratories in the United States began offering a laboratory-developed PCA3 test using class I analyte-specific reagents, which although not formally approved by the US Food and Drug Administration, is covered by Medicare and insurance plans. At that point, we began testing patient specimens outside of research protocols to assist clinical decision making. Approximately 200 PCA3 tests were performed by Bostwick Laboratories (Richmond, VA) on patients from southern California in 2006 and 2007. DRE was performed before each urine collection, as described elsewhere.7
In the following case reports, drawn from that early clinical experience, the potential value of PCA3 gene testing in management of individual patient quandaries is demonstrated. A cutoff score of 35 was adopted, because among 570 men undergoing prostate biopsy, this score combined the greatest cancer sensitivity and specificity6 (Table 1).
Table 1.
PCA3 Assay Sensitivity and Specificity at Various Cutoffs
| PCA3 Cutoff | Sensitivity (%) | Specificity (%) |
| 5 | 96 | 14 |
| 20 | 71 | 56 |
| 35 | 54 | 74 |
| 50 | 40 | 83 |
| 65 | 32 | 91 |
| 90 | 20 | 95 |
N = 570 men undergoing prostate biopsy; 36% biopsy positive.
PCA3, prostate cancer antigen 3.
Modified from Deras IL et al.6
Development of the PCA3 gene test is described online at the Urological Sciences Research Foundation Web site at http://www.usrf.org/news/PCA3/PCA3.html.
Case 1: Elevated PSA Level, Biopsy Demonstrates Benign Prostatic Hyperplasia
An estimated 1 million prostate biopsies are now performed annually in the United States (though no registry exists). The great majority of these biopsies are stimulated by increased serum PSA levels. A total of 186,000 new cases of prostate cancer were reported in 2007.1 Thus, for every 5 prostate biopsies, at least 4 will show no cancer. However, some of the men with negative biopsy results, perhaps 25%, will be found to harbor cancer upon subsequent biopsy.11
Figure 2 shows the PSA and biopsy events of a man with a paternal history of prostate cancer who at the age of 53 years was found to have a serum PSA level of 4.5 ng/mL. Over this period, PSA increased from 4.5 ng/mL to 12.1 ng/mL, while prostate volume increased from 43 cm3 to 58 cm3. During this period, abnormalities were noted in age-adjusted PSA, PSA density, and PSA velocity. Percentage free PSA remained in a mid-range throughout. The patient underwent 6 biopsies over 12 years because of PSA abnormalities. Each biopsy demonstrated benign prostatic hyperplasia (BPH), with no evidence of cancer.
Figure 2.
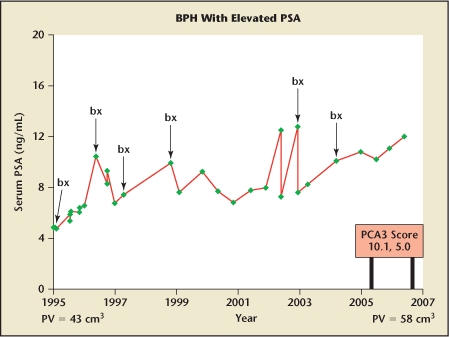
Serum PSA levels over a 12-year interval in a white man whose prostate grew from 43 cm3 at age 53 years (1995) to 58 cm3 at age 65 years (2007). PSA levels ranged from 4.5 ng/mL at age 53 years to 12.1 ng/mL at age 65 years. Over this period 6 sets of biopsies (bx) were performed, but no cancer was ever found. Toward the end of this story, the PCA3 gene test became available. On 2 separate occasions the PCA3 score was low (10.1 and 5.0). Had the test been available early on, repeated biopsies might have been avoided and a degree of reassurance provided to the patient. BPH, benign prostatic hyperplasia; PCA3, prostate cancer antigen 3; PSA, prostate-specific antigen; PV, prostate volume.
Late in this man’s story, the PCA3 gene test became available. The score was low (10.1 and 5.0) on 2 separate occasions. Had the test been available early on, the biopsies might have been avoided and the patient given a degree of reassurance. The value of the PCA3 test in men with elevated PSA levels and prior negative biopsies has been detailed previously.7
Case 2: Normal PSA Level, Biopsy Reveals Cancer
In 15% of men ultimately found to have prostate cancer, followed with serial PSA determinations for as long as 7 years in a large clinical trial, PSA concentration greater than 4.0 ng/mL was never seen.12 Serum PSA levels are not perfect screening tests for cancer, exhibiting false-negative and false-positive values. PSA refinements, such as density and velocity measures and percentage free PSA determinations, may help in some cases, but misleading test results are commonplace with any PSA-based testing.
Figure 3A shows PSA levels in a man who was found to have prostate cancer when his PSA level of 2 ng/mL increased to 3 ng/mL. Abnormal PSA velocity stimulated the concern, and the patient was unwilling to wait for a confirmatory PSA determination at a later date. The PCA3 score was 71.7. A biopsy demonstrated adenocarcinoma with a Gleason score of 3 + 4 = 7. Radical prostatectomy was performed; the specimen was found to contain extensive carcinoma with a Gleason score of 7 in both lobes; margins and lymph nodes were clear.
Figure 3.
(A) Serum prostate-specific antigen (PSA) levels in a man whose level was 1.2 ng/mL when first seen at age 61 years (1998), rising gradually to approximately 2.0 ng/mL over the next several years, with an additional increase to approximately 3.0 ng/mL at age 69 years (2006).
Urinary PCA3 score was 71.7 ng/mL, which confirmed suspicion of increased PSA velocity, leading to discovery of adenocarcinoma with a Gleason score of 7 on biopsy. Radical prostatectomy was performed; cancer was extensive within the prostate but was organ confined. Relatively low levels of PSA are found in some 15% of men with localized prostate cancer.12
(B) Serum PSA level was 1.0 ng/mL in this 63-year-old physician who was a member of a family with known mutation in the BRCA2 gene. Prostate examination was unrevealing. His prostate cancer antigen 3 (PCA3) score was 123. Biopsy (bx) revealed adenocarcinoma with a Gleason score of 7, and radical prostatectomy was performed (case courtesy of Jack Schalken, University of Nijmegen, The Netherlands). In neither of these patients did the PSA level exceed 4.0 ng/mL, but both had other indications of concern; PCA3 score was helpful in suggesting cancer and mandating biopsy.
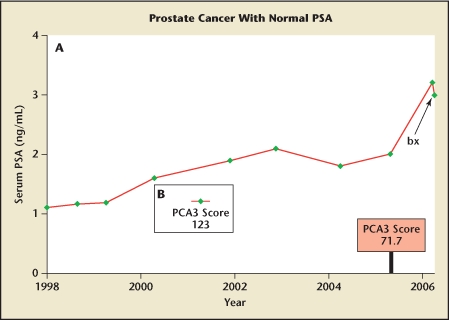
Figure 3B shows the PSA level in a 63-year-old physician who underwent prostate biopsy because he was a proband in a family with a known BRCA2 mutation, which increases both risk13 and virulence14 of prostate cancer. His biopsy was performed despite normal results on DRE and a PSA level of 1.0 ng/mL. The PCA3 score was 123. Biopsy revealed extensive adenocarcinoma with a Gleason score of 7, and radical prostatectomy was performed.
PCA3 scores, as opposed to serum PSA levels, do not increase with increasing prostate volume.6 Therefore, in any given individual, an increased PCA3 score cannot be attributed simply to prostate enlargement. In a recent multicenter study of 570 men who had PCA3 specimen collection just before undergoing ultrasound-guided prostate biopsy, no relationship was found between ultrasound-measured prostate volume and PCA3 score.6 Men with prostate volume greater than 50 cm3 were found to have essentially the same PCA3 score as men with prostate volume less than 30 cm3 (25 vs 23).
Thus, the confounding influence of an enlarging prostate on serum PSA levels does not occur with PCA3 scores. Serum PSA level is an excellent marker of prostate volume,15 but PCA3 score is not. The concentration of PSA in BPH cells is several times higher than in prostate cancer cells.16 With PCA3 the opposite is seen: prostate cancer cells express 60 to 100 times more PCA3 than benign prostate cells.3 The false-positive PSA level caused by an enlarged prostate is not seen with PCA3 scores.
Case 3: Elevated PSA Level, Irritative Voiding Symptoms (Prostatitis)
Acute prostatitis is known to cause marked elevation in serum PSA levels; less dramatic is the potential effect that inflammation found incidentally in prostate tissue specimens may exert on serum PSA levels.17 Between these extremes lies a wide spectrum of clinical presentations, ranging from minimal to severe, and open to subjective interpretation of symptoms by both patient and physician. Complicating this matter is the difficulty in establishing a specific diagnosis of subacute or chronic prostatitis.
Figure 4 shows the PSA levels in a 55-year-old man under observation over years with mild voiding symptoms (lower urinary tract symptoms) not requiring treatment. He then returned with a mild-to-moderate increase in irritative voiding symptoms over the previous few weeks. DRE revealed a slightly enlarged, smooth, symmetric, nontender prostate. Urinalysis was acellular, and results from culture were negative. His serum PSA level was increased to 9.1 ng/mL. The PCA3 score was 22.5, which is below the cutoff of 35 for optimal sensitivity and specificity.6 A presumptive diagnosis of prostatitis was made, and he was treated with 3 weeks of a quinolone antibiotic. Eventually his serum PSA levels returned to former levels. Biopsy was not performed.
Figure 4.
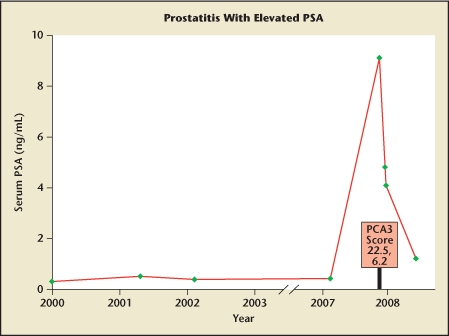
Prostate-specific antigen (PSA) levels in a 55-year-old man with stable, low values until he returned at age 62 years with a mild increase in voiding symptoms over the past several weeks. Results from digital rectal examination and urinalysis were normal. PSA evaluations showed a marked increase to 9.1 ng/mL from earlier levels. His prostate cancer antigen 3 (PCA3) score was 22.5 (and 6.2 on repeat test), levels suggesting absence of cancer. With antibiotic treatment, PSA declined, and biopsy was deferred. Acute or subacute prostatitis, with symptoms ranging from severe to mild, may elevate serum PSA levels for months, confounding the utility of serum PSA level as a cancer marker.
Along with direct trauma (eg, biopsy, cystoscopy), prostatitis is the most common cause of sudden, marked elevation in serum PSA levels. However, prostatitis is not always acute, and the symptoms are not always severe, and even mild or moderate inflammation may be sufficient to alter cellular integrity and cause leakage of PSA into serum. Though data are limited, urinary levels of the PCA3 gene seem unaffected by acute or subacute prostatitis, and in a recent publication, chronic inflammation found on prostate biopsy was also not associated with PCA3 level.6 Thus, at this early stage of clinical evaluation, cancer specificity of the PCA3 gene seems to be maintained in the face of the 2 major causes of noncancerous PSA elevations-BPH and prostatitis.
Case 4: Microfocal Disease, Active Surveillance
Many men with small foci of prostate cancer may not need immediate treatment and are candidates for active surveillance.18,19 Guidelines for selecting and following such patients have been established by Carter and colleagues,20 Kattan and colleagues,21 Klotz,22 and others. However, patient anxiety over cancer uncertainty is an overriding influence for many men undergoing active surveillance.23 A recent literature survey from the United Kingdom found that microfocal disease does not always equate with insignificant disease, with some patients demonstrating adverse outcomes despite low-volume lesions on initial biopsy.24 A specific cancer marker in these patients would be of immense value.
In Figure 5, the potential value of PCA3 in identifying insignificant disease is shown. Two patients with well-differentiated microfocal lesions help to exemplify the value of a low PCA3 score in confirming the presence of indolent disease. Patient 5A is a 31-year-old man who underwent 12-core biopsy because of a serum PSA level of 3.4 ng/mL. A single core contained 1 mm of adenocarcinoma with a Gleason score of 3 + 3 = 6. The initial PCA3 score was 6.6 (extremely low), and the patient has been followed with repeat biopsies annually for 3 years with no sign of disease progression. At recent follow-up, his PCA3 score was 2.5 and PSA level was 0.8 ng/mL.
Figure 5.
(A) A 34-year-old man presented at age 31 years with a serum prostate-specific antigen (PSA) level of 3.4 ng/mL, leading to biopsy showing 1 mm of adenocarcinoma with a Gleason score of 6. Prostate cancer antigen 3 (PCA3) scores were low (6.6 initially, 2.5 at 2-year follow-up). Serial biopsy showed no evidence of progression over the next 3 years. During that time, PSA values ranged from 3.4 ng/mL to 0.8 ng/mL (current level). Active surveillance continues.
(B) A 64-year-old man with a serum PSA level of 5.2 ng/mL. His PCA3 score was 27.2. Biopsy showed 2 mm of adenocarcinoma with a Gleason score of 6. Active surveillance was considered, but the patient elected radical prostatectomy; the 60-g prostate contained tiny foci (< 1% of volume) of adenocarcinoma with a Gleason score of 6, an unlikely threat. Thus, the low PCA3 score accurately predicted insignificant disease. The cancer specificity of PCA3 may allow gene scores to be used as a marker of disease severity and a guide for active surveillance.25,26
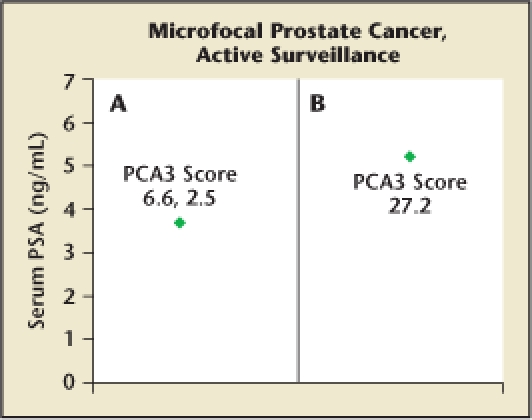
Patient 5B is a 64-year-old man with a microfocal lesion similar to that seen in patient 5A. His PSA level was 5.2 ng/mL and his PCA3 score 27.2 (cutoff = 35). The patient was advised that a program of active surveillance was a reasonable consideration, but cancer anxiety drove him to undergo radical prostatectomy. His 60-g prostate contained several tiny foci of adenocarcinoma with a Gleason score of 3 + 3 = 6 (< 1% of tissue), which would most likely not have become a serious threat during his projected lifetime.
The above cases lend confirmation to the recent work of Nakanishi and colleagues,25 who obtained blinded PCA3 scores in 96 men before radical prostatectomy. When preoperative PCA3 scores were greater than 25 (n = 66), 94% had significant cancers, defined as dominant tumor volume greater than 0.5 cm3 and/or final Gleason score of 7 or greater. Furthermore, PCA3 scores in men with insignificant cancers were not different from those in other men without cancer: both had scores lower than in men with significant lesions (P < .01), as described above. The investigators concluded that the PCA3 score is “potentially an effective tool for determining which men are candidates for active surveillance.”25
The above findings have been extended in a similar study of 72 men undergoing radical prostatectomy at Walter Reed Army Medical Center.26 In this study, PCA3 score was found to correlate with final tumor volume (r = .38, P < .01) and also was an independent predictor of extracapsular extension (ECE). Independently, a PCA3 score of 47 or higher predicted ECE with a specificity of 94% and a positive predictive value of 80%. Remarkably, when PCA3 score was combined with preoperative PSA level and biopsy Gleason score, the area under the receiver operating characteristic curve for prediction of ECE approximated 90%. Thus, PCA3 gene expression seems to function synergistically with other clinical information.
Summary and Conclusions
A marker specific for early prostate cancer would fill an important void. In initial evaluations of the PCA3 gene vis-à-vis serum PSA levels, the gene offers great promise. At the cellular level, PCA3 specificity for cancer is nearly perfect because of the gross overexpression of the gene by cancer cells. As a clinical test for early prostate cancer, heightened specificity is also seen in urine containing prostate cells from men with the disease. PCA3 gene testing holds valuable potential in PSA quandary situations: (1) men with elevated PSA levels but no cancer on initial biopsy; (2) men found to have cancer despite normal levels of PSA; (3) men with PSA elevations associated with varying degrees of prostatitis; and (4) men undergoing active surveillance for presumed microfocal disease. In the present article, examples from clinical practice show how urinary levels of the PCA3 gene can help establish a correct diagnosis in such situations. A much larger experience will be required to identify regulators of the PCA3 gene and to fully define sensitivity and specificity of PCA3 gene testing.
Main Points.
A specific marker of early prostate cancer is not yet available, and such a test would be highly desirable. One candidate to become that marker is the prostate cancer antigen 3 (PCA3) gene.
PCA3 expression, which is 60- to 100-fold greater in cancerous than in benign prostate tissues, gives the gene a cancer specificity lacking with prostate-specific antigen (PSA).
The PCA3 test requires collection of the first 20 to 30 mL of voided urine after a digital rectal examination (DRE); without DRE the test provides valid results in only approximately 80% of cases, but DRE increases this yield to more than 98%.
At this early stage of clinical evaluation, cancer specificity of the PCA3 gene seems to be maintained in the face of the 2 major causes of noncancerous PSA elevations-benign prostatic hyperplasia and prostatitis.
PCA3 gene testing holds valuable potential in PSA quandary situations: (1) men with elevated PSA levels but no cancer on initial biopsy; (2) men found to have cancer despite normal levels of PSA; (3) men with PSA elevations associated with varying degrees of prostatitis; and (4) men undergoing active surveillance for presumed microfocal disease.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–5979. [PubMed] [Google Scholar]
- 3.Hessels D, Klein Gunnewiek JM, van Oort I, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8–15. doi: 10.1016/s0302-2838(03)00201-x. discussion 15–16. [DOI] [PubMed] [Google Scholar]
- 4.Schalken JA, Hessels D, Verhaegh G. New targets for therapy in prostate cancer: differential display code 3 (DD3(PCA3)), a highly prostate cancer-specific gene. Urology. 2003;62:34–43. doi: 10.1016/s0090-4295(03)00759-3. [DOI] [PubMed] [Google Scholar]
- 5.Fradet Y, Saad F, Aprikian A, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology. 2004;64:311–315. doi: 10.1016/j.urology.2004.03.052. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 6.Deras IL, Aubin SM, Blase A, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587–1592. doi: 10.1016/j.juro.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532–535. doi: 10.1016/j.urology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine—a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182–186. doi: 10.1016/j.eururo.2004.06.004. discussion 187. [DOI] [PubMed] [Google Scholar]
- 9.Shappell SB. Clinical utility of prostate carcinoma molecular diagnostic tests. Rev Urol. 2008;10:44–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–1095. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- 11.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–2439. [PubMed] [Google Scholar]
- 12.Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 13.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 14.Tryggvadottir L, Vidarsdottir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 15.Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–589. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 16.Magklara A, Scorilas A, Stephan C, et al. Decreased concentrations of prostate-specific antigen and human glandular kallikrein 2 in malignant versus nonmalignant prostatic tissue. Urology. 2000;56:527–532. doi: 10.1016/s0090-4295(00)00621-x. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami J, Siemens DR, Nickel JC. Prostatitis and prostate cancer: implications for prostate cancer screening. Urology. 2004;64:1075–1080. doi: 10.1016/j.urology.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Villers A, McNeal JE, Freiha FS, Stamey TA. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992;70:2313–2318. doi: 10.1002/1097-0142(19921101)70:9<2313::aid-cncr2820700917>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–374. [PubMed] [Google Scholar]
- 20.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–2364. doi: 10.1016/j.juro.2007.08.039. discussion 2364–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kattan MW, Cuzick J, Fisher G, et al. Nomogram incorporating PSA level to predict cancer-specific survival for men with clinically localized prostate cancer managed without curative intent. Cancer. 2008;112:69–74. doi: 10.1002/cncr.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klotz L. Active surveillance with selective delayed intervention for favorable risk prostate cancer. Urol Oncol. 2006;24:46–50. doi: 10.1016/j.urolonc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178:826–831. doi: 10.1016/j.juro.2007.05.039. discussion 831–832. [DOI] [PubMed] [Google Scholar]
- 24.Harnden P, Naylor B, Shelley MD, et al. The clinical management of patients with a small volume of prostatic cancer on biopsy: what are the risks of progression? A systematic review and meta-analysis. Cancer. 2008;112:971–981. doi: 10.1002/cncr.23277. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–1809. doi: 10.1016/j.juro.2008.01.013. discussion 1809–1810. [DOI] [PubMed] [Google Scholar]
- 26.Whitman EJ, Groskopf J, Ali A, et al. PCA3 scores in urine before radical prostatectomy predict extra-capsular extension and tumor volume. J Urol. doi: 10.1016/j.juro.2008.07.060. [DOI] [PubMed] [Google Scholar]


