Abstract
The widely used immunosuppressant cyclosporine A (CSA) blocks nuclear translocation of the transcription factor, NF-AT (nuclear factor of activated T cells), preventing its activity. mRNA for several NF-AT isoforms has been shown to exist in cells outside of the immune system, suggesting a possible mechanism for side effects associated with CSA treatment. In this study, we demonstrate that CSA inhibits biochemical and morphological differentiation of skeletal muscle cells while having a minimal effect on proliferation. Furthermore, in vivo treatment with CSA inhibits muscle regeneration after induced trauma in mice. These results suggest a role for NF-AT–mediated transcription outside of the immune system. In subsequent experiments, we examined the activation and cellular localization of NF-AT in skeletal muscle cells in vitro. Known pharmacological inducers of NF-AT in lymphoid cells also stimulate transcription from an NF-AT–responsive reporter gene in muscle cells. Three isoforms of NF-AT (NF-ATp, c, and 4/x/c3) are present in the cytoplasm of muscle cells at all stages of myogenesis tested. However, each isoform undergoes calcium-induced nuclear translocation from the cytoplasm at specific stages of muscle differentiation, suggesting specificity among NF-AT isoforms in gene regulation. Strikingly, one isoform (NF-ATc) can preferentially translocate to a subset of nuclei within a single multinucleated myotube. These results demonstrate that skeletal muscle cells express functionally active NF-AT proteins and that the nuclear translocation of individual NF-AT isoforms, which is essential for the ability to coordinate gene expression, is influenced markedly by the differentiation state of the muscle cell.
INTRODUCTION
Transcription factors play important roles in tissue development and maintenance by regulating the expression of genes required for cell function. The best studied transcription factors in skeletal muscle cells are the muscle regulatory factor (MRF)1 and myocyte enhancer factor 2 (MEF2) families implicated in establishing the myogenic lineage as well as controlling muscle differentiation. Far less is known about other transcription factors in skeletal muscle.
Nuclear factor of activated T cells (NF-AT) is a transcription factor discovered for its role in cytokine gene expression in lymphoid cells (for review see Rao et al., 1997). Four isoforms of NF-AT with a high degree of homology to each other are known: NF-ATc, NF-ATp, NF-AT4/x/c3, and NF-AT3 (Hoey et al., 1995). Under basal conditions, NF-AT exists as a phosphorylated form in the cytoplasm, but translocates to the nucleus upon dephosphorylation in response to increased intracellular calcium levels and activation of the phos- phatase, calcineurin. In the nucleus, NF-AT forms complexes with AP-1 family members on purine-rich enhancer elements of specific genes leading to transcriptional activation. The phosphatase activity of calcineurin is blocked by the immunosuppressive drugs, cyclosporine A (CSA) and FK506, thereby preventing the nuclear translocation of NF-AT.
Lymphoid cells are not the only cells that express NF-AT. Skeletal muscle tissue also expresses mRNA for NF-ATc, NF-ATp, and NF-AT4/x/c3 (Hoey et al., 1995). CSA, a potent immunosuppressive agent used to reduce organ transplant rejection and to manage autoimmune disease, can adversely affect skeletal muscle (Hardiman et al., 1993; Hokanson et al., 1995; Mercier et al., 1995). Paradoxically, CSA has been reported to have an ameliorative effect on the muscle weakness of Duchenne muscular dystrophy patients (Sharma et al., 1993). Together, these studies suggest that NF-AT proteins may play functional roles in skeletal muscle development or function.
In this report, we demonstrate that CSA has direct effects on both biochemical and morphological differentiation of skeletal muscle while having a minimal effect on cell proliferation. Skeletal muscle cells are shown to express functionally active NF-AT proteins that are activated by known pharmacological inducers of NF-AT in immune cells. Three isoforms of NF-AT are expressed by muscle cells, but each isoform undergoes calcium-induced nuclear translocation from the cytoplasm at only specific stages of myogenesis, suggesting specificity among NF-AT isoforms in gene regulation.
MATERIALS AND METHODS
Antisera and Reagents
A mouse monoclonal antibody against NF-ATc (clone 7A6, [Timmerman et al., 1997]) was purchased from Affinity Bioreagents, (Golden, CO). A mouse monoclonal antibody against NF-ATp (clone G1-D10, [Timmerman et al., 1997]) and a rabbit polyclonal antibody against NF-AT4/c3/x (Ho et al., 1995) were obtained from Dr. Gerald Crabtree. For immunoblots, a rabbit polyclonal antibody against NF-ATp was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse ascites and normal rabbit serum were purchased from Sigma Biosciences (St. Louis, MO). Secondary antibodies were obtained from Jackson Immunoresearch Laboratories, (West Grove, PA). Tyramide-green was purchased from NEN Dupont (Boston, MA). SJL mice were purchased from The Jackson Laboratory (Bar Harbor, ME), whereas C3H/HEN mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). NF-ATp null mice (Hodge et al., 1996) were a gift of Dr. Laurie Glimcher. Expression plasmids for NF-ATp (pSH210) (Ho et al., 1995), NF-ATc (pSH107c) (Northrop et al., 1994), and NF-AT 4/x/c3 (pSH250A) (Ho et al., 1995) were obtained from Dr. Gerald Crabtree. Except where noted, all cell culture supplies were purchased from Life Technologies (Grand Island, NY). FBS was obtained from Atlanta Biologicals (Norcross, GA). Basic fibroblast growth factor (bFGF) was purchased from Promega (Madison, WI). Calf skin collagen was purchased from Sigma. [methyl-3H]Thymidine was purchased from Amersham (Arlington Heights, IL). Amphotropic (ATCC CRL-11554) retroviral producer cells were obtained from the American Type and Culture Collection (Rockville, MD). d-Luciferin was purchased from Molecular Probes (Eugene, OR). Thapsigargin was purchased from LC Chemicals (San Diego, CA). Phorbol myristate acetate and ionomycin were purchased from Sigma. CSA was a gift of Sandoz (Basel, Switzerland).
Cell Culture
Primary cultures were derived from the tibialis anterior of adult SJL/J or NF-ATp null mice 2 d after induced muscle damage, and myoblasts were purified to >99% (Rando and Blau, 1994). Mouse myoblasts were grown in growth media (GM: Ham’s F10, 20% FBS, 5 ng/ml bFGF) on collagen-coated dishes in a humidified 5% CO2 atmosphere at 37°C. Dishes were coated with a solution of 0.01% calf skin collagen in 0.1 N acetic acid, allowed to stand overnight, and rinsed with sterile PBS. Differentiation was induced by switching confluent myoblast cultures to fusion medium (FM: DMEM, 2 or 15% horse serum) for 44–48 h. Primary human myoblasts were derived from a muscle biopsy taken from a 2-y-old donor and were purified to >99% using flow cytometry (Webster et al., 1988). Human myoblasts were grown in Ham’s F10, 15% FBS, 5 ng/ml bFGF on collagen-coated dishes in a humidified 5% CO2 atmosphere at 37°C. Differentiation of human myoblasts was induced by switching confluent myoblasts cultures to FM containing either 2 or 5% horse serum for 48–80 h.
Cell Proliferation Assays
Human myoblasts (2 × 104) were plated in 24-well collagen-coated tissue culture plates in muscle growth medium. At 24–72 h after plating, the cells were pulsed with 1 μCi/ml [methyl-3H]thymidine (specific activity, 25 Ci/mmol) for 2 h at 37°C. DNA synthesis was assayed by measuring the amount of radioactivity incorporated into trichloroacetic acid-insoluble material. The cells were washed twice with cold PBS, treated with cold 20% trichloroacetic acid for 30 min at 37°C, and lysed for 10 min with 0.1 N NaOH and 0.1% SDS. The lysates were mixed with Scintisafe Econo1 scintillation fluid (Fisher, Pittsburgh, PA) and counted in a Beckmann-LS6000IC liquid scintillation counter. Triplicate wells were used to analyze each experimental condition.
Embryonic Myosin Heavy Expression
Human myoblasts were plated in six-well collagen-coated culture plates in muscle growth medium and grown to high density. High density myoblasts were preincubated in either vehicle or CSA for 24 h before switching to FM, and drug treatment was continued for another 48–80 h. Treatment time was determined by the length of time necessary to obtain extensive myotube formation in the vehicle-treated samples. This time varied depending on the lot number and percentage of serum. Duplicate wells for each condition were lysed in ice-cold RIPA-2 (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS containing protease inhibitors [90 μg/ml phenylmethylsulfonylfluoride, 0.1 mM leupeptin, 0.2 trypsin inhibitor unit/ml aprotinin, 5 μg/ml pepstatin A, 0.2 mM sodium orthovanadate, and 50 μg/ml soybean trypsin inhibitor]). Insoluble material was pelleted by centrifugation.
Equal amounts of protein (10 μg) (Bradford, 1976) were loaded onto SDS-polyacrylamide minigels consisting of a 7.5% separating gel with a 4% stacking gel (Laemmli, 1970) and electrophoresed at 150 V for 70 min. Proteins were transferred to an Immobilon membrane (Millipore, Bedford, MA) using a Transblot apparatus (Bio-Rad, Richmond,CA) at 250 mA for 1 h as per the manufacturer’s protocol. After nonspecific binding was blocked by incubation of the membrane with 5% nonfat dry milk in Tris-buffered saline (TBS), the membrane was incubated overnight at 4°C with a mouse monoclonal antibody against embryonic myosin heavy chain (EyMHC) (Cho et al., 1994). The EMyHC antibody (F.1652) was used as an undiluted hybridoma supernatant. The membranes were washed in TBS containing 0.1% Tween 20 and further incubated with a 1:10,000 dilution of a peroxidase-conjugated anti-mouse IgG in TBS containing 0.1% Tween 20 and 5% horse serum for 1 h at room temperature. After further washing, the membrane was incubated with an ECL Western blotting detection kit (Amersham) as per the manufacturer’s directions, exposed to Hyperfilm-ECL x-ray film (Amersham), and developed using an automatic film processor. Densitometry of the films was performed using an optical scanner and NIH Image software.
Creatine Kinase Assays
SJL mouse myoblasts were plated at 1.5 × 105 cells per well in 12-well plates in GM. After 4 h, vehicle or CSA was added, and 12 h later, the GM was replaced with Insulin-Transferrin-Selenium FM (DMEM, 1:100 dilution of Insulin-Transferrin-Selenium Supplement [GIBCO BRL], 0.1% BSA, 200 U/ml penicillin G, 200 μg/ml streptomycin). Cells were lysed and sonicated 24 h later in 0.05 M glycylglycine (pH 7.5), 1% NP-40, 0.1% β-mercaptoethanol. Creatine kinase (CK) activity was determined using Sigma Diagnostics procedure number DG147-UV and standardized to protein levels (Bradford, 1976). Background, defined as nondifferentiation-specific CK activity in myoblasts maintained in GM, was subtracted from values determined in drug-treated samples (mean = 14.5% of vehicle CK activity). Values are reported as percent of vehicle CK activity (mU/mg protein). Triplicate samples were analyzed for each concentration of CSA in three independent experiments.
Induced Regeneration of Skeletal Muscle and Histological Analyses
C3H/HEN mice were anesthetized with an i.p. injection of a cocktail of 35 mg/kg ketamine and 5 mg/kg xylazine, and an incision of ∼3 mm was made overlying the tibialis anterior muscles. Muscle damage was induced by direct application of a small piece of dry ice to the surface of the exposed muscle for 5 s. Groups of two to three animals received either PBS or 45 mg/kg CSA i.p daily and were killed 10 d after damage. The muscles were removed, embedded in OCT mounting medium, and frozen in isopentane cooled in liquid nitrogen. Serial cross-sections were collected onto gelatin-coated slides at 400- to 500-μm intervals along the entire length of the muscle and analyzed histologically by hematoxylin and eosin staining.
Retroviral Reporter Plasmids
The retroviral NF-AT–responsive plasmid (pKA7) contains a luciferase coding sequence under the control of a minimal human IL-2 promoter with an upstream triplex of the distal IL-2 gene NF-AT response element (Northrop et al., 1993). The control plasmid (pKA9) is missing the NF-AT response elements, but contains the minimal IL-2 promoter. Both plasmids confer neomycin resistance and were created by modifications (Boss et al., 1998) to the retroviral plasmid pLNCX (Miller et al., 1993).
Retroviral Production and Infection
Retroviruses were prepared by transient transfection of helper-virus free amphotropic producer cells (Pear et al., 1993) with the plasmids pKA7 or pKA9. The Bing-CAK8 producer cells were grown in producer growth media (PGM) consisting of DMEM, 4.5 μg/ml glucose, 10% FBS in a humidified 5% CO2 atmosphere at 37°C. To produce infectious retroviral supernatants, cultured cells at 50–80% confluence in 100-mm diameter dishes were transfected with the retroviral plasmids using CaPO4 and 25 μM chloroquine for 6–12 h before refeeding with 20 ml PGM. Twenty hours after the start of the transfections, the PGM was aspirated and replaced with 9 ml fresh PGM before the dishes were placed in a humidified 5% CO2 atmosphere at 32°C to enhance the retroviral titer (Kotani et al., 1994). Supernatants containing infectious retroviruses were collected at 48, 60, and 72 h after transfection refeeding with 9 ml of PGM each time, filtered through a 0.45-μm syringe tip cellulose acetate filter, aliquoted, snap frozen in liquid nitrogen, and stored at −80°C.
SJL myoblasts in multiwell plates were infected by adding retroviral supernatant supplemented with 10% FBS, 5 ng/ml bFGF, and 4 μg/ml polybrene and spinning at 2500 rpm for 30 min at 32°C in a Beckman model GS-6R centrifuge in a swinging bucket rotor (Springer and Blau, 1997). The retroviral supernatant was aspirated, and the cells were refed with GM and returned to the incubator. The infection protocol was repeated once more 8–12 h later. Forty-eight hours after the last infection, the cells were fed with GM containing 50 μg/ml G418. After 3–5 d of selection, the surviving cells were expanded and used for experiments through approximately 10 more passages. The efficiency of the two rounds of retroviral infections was estimated at >90% as little cell death occurred during drug selection.
Drug Treatments and Luciferase Assays
Retrovirally infected SJL myoblasts were plated at 4–7 × 104 cells per well of 24-well collagen-coated plates and used in assays as indicated. Drugs were added to muscle cultures and incubated for 6–7 h at 37°C. The cells were washed with PBS, and 75 μl of lysis buffer (1% Triton X-100, 4 mM EGTA, 25 mM Tris/phosphate, 10% glycerol, 2 mM dithiothreitol) were added to each well and incubated for 10 min at room temperature. The cell lysates were centrifuged at 13,100 × g for 5 min at room temperature, and the supernatants were removed. Luciferase assays were performed by combining 50 μl of supernatant, 350 μl of assay buffer (25 mM Tris/phosphate, 40 mM MgSO4, 4 mM EGTA, 2 mM ATP, 1 mM dithiothreitol), and 100 μl of 0.75 mM d-luciferin. Light output was measured after a 5-s delay over a 10-s window using a Turner TD-20e luminometer (Turner Designs, Sunnyvale, CA).
Immunoblotting of NF-AT Proteins
SJL mouse muscle cells were lysed in RIPA-2 containing protease inhibitors (Complete Mini, Boehringer-Mannheim, Indianapolis, IN). Cellular proteins at 100 μg/lane for NF-ATc and NF-AT4/x/c3 analyses and 50 μg/lane for NF-ATp analysis were separated using SDS-PAGE. Immunoblots were processed as described for F.1652 detection except a peroxidase-conjugated anti-rabbit IgG was used for detection of NF-ATp and NF-AT4/x/c3. Specificity of the antibodies for NF-AT isoforms in immunoblots of muscle proteins was determined by preabsorbing the individual antibodies with extracts from HEK cells transfected with expression plasmids for NF-ATc(pSH107c), NF-AT4/x/c3 (pSH250A), or NF-ATp (pSH210) or control HEK extracts.
Immunohistochemistry
Human myoblasts were plated at approximately 30% confluency in GM. Myoblasts were assayed at high density (80–90% confluency). To induce the formation of multinucleated myotubes, myoblasts were grown to near confluence and switched to FM. Myotubes were assayed at two stages: nascent (24 h in FM) and mature (75–80 h in FM). Cultures were treated with vehicle (0.01% DMSO) or thapsigargin (10 nM) for 10 min at 37°C. In some experiments, CSA (1 μM) was added 10 min before the addition of thapsigargin.
Immediately after the drug treatments, the cells were fixed in −20°C methanol for 5 min. To block nonspecific protein binding, the cells were first incubated in blocking buffer (PBS, 2% horse serum, 0.5% Triton X-100) for 30 min. All antibodies were diluted in this blocking solution. The cells were further incubated with either a 1:1000 dilution of the anti-NF-ATc monoclonal antibody, a 1:500 dilution of the anti-NF-ATp monoclonal antibody, or a 1:1500 dilution of the anti-NF-AT4/x/c3 antibody for 1 h. After three washes, the cells were incubated with a 1:1000 dilution of either biotinylated anti-mouse IgG (for NF-ATc and NF-ATp antibodies) or biotinylated anti-rabbit IgG (for NF-AT4/x/c3) for 30 min. After washing, antibody binding was detected using TSA-Green according to the manufacturer’s directions, but with a 1:200 dilution of the streptavidin-conjugated horseradish peroxidase. Specific staining was tested by replacing the primary monoclonal antibodies with normal ascites or with normal rabbit serum in the case of the polyclonal NF-AT4/x/c3 antibody or by omitting the primary antibody. Further specificity was demonstrated by preabsorbing the NF-ATc and NF-AT4/x/c3 antibodies with extracts from HEK cells transfected with expression plasmids for these isoforms or control HEK extracts. Specificity of the NF-ATp antibody was shown by staining of muscle cultures derived from NF-ATp null mice. All analyses and photography were performed on a Axiovert microscope (Carl Zeiss, Thornwood, NY) equipped with a video camera (Optronics Engineering, Goleta, CA) and Scion Image software (Scion, Frederick, MD).
RESULTS
CSA Inhibits Differentiation of Skeletal Muscle
Myogenesis requires growth and differentiation of myoblasts. Myoblasts are proliferating mononucleated muscle precursor cells that can be induced to withdraw from the cell cycle and fuse with one another to form myotubes upon attaining high density in vitro and by a decrease in growth factors. Myotubes are multinucleated, postmitotic cells that express proteins characteristic of differentiated myofibers in vivo. To determine the consequences of CSA on skeletal muscle myogenesis, both growth and differentiation assays were performed. First, the effect of CSA on myoblast proliferation was measured. Myoblasts were treated with different doses of CSA for 24–72 h, and the cells were then pulse labeled with 3H-thymidine to quantitate DNA synthesis. As shown in Figure 1A, CSA minimally inhibits myoblast proliferation. We next determined the effect of CSA on the biochemical and histological differentiation of myoblasts into myotubes. Cultures of high-density myoblasts were treated with different doses of CSA in a low mitogen medium. Differentiation was assayed either by immunoblots using an antibody to embryonic myosin heavy chain (EMyHC) or enzymatic assays for CK activity. Both these assays reveal that biochemical differentiation of myoblasts is inhibited in a dose-dependent manner. Inhibition of differentiation is indicated by a decrease in EMyHC expression (Figure 1B) or CK activity (Figure 1C). Both these markers of differentiated myotubes are inhibited over a similar range of CSA doses. At the highest dose of CSA tested, biochemical differentiation is inhibited approximately 75–90% depending on the specific marker that was assayed. Histological analyses demonstrate that CSA also inhibits myoblast fusion as indicated by the few multinucleated differentiated myotubes in the drug-treated cultures (Figure 1D, panels A and B) in accordance with the studies by Hardiman et al. (1993).
Figure 1.
CSA inhibits myoblast differentiation with little effect on proliferation. (A) CSA minimally inhibits DNA synthesis in myoblasts. Human myoblasts were treated with different doses of CSA for 24–72 h. The cells were pulse labeled with 3H-thymidine for 2 h, and the number of TCA-precipitable counts per min was determined. The data are plotted as the amount of 3H-thymidine incorporation in the drug-treated cells as a percent of that in vehicle-treated cells. No difference was noted among the different treatment times, so the data for all timepoints were pooled. Each point represents the mean ± SD of four experiments each performed in triplicate. (B) CSA inhibits expression of embryonic myosin heavy chain, a marker of differentiated muscle cells, in a dose-dependent manner. Top panel, immunoblots of electrophorectically separated proteins of vehicle and CSA- treated human muscle cells for 48–80 h in fusion medium using an antibody to EMyHC are shown. Detection was with enhanced luminescence. Representative blot of three experiments is shown. Bottom panel, immunoblots were scanned and quantitated using NIH Image. Each point represents the mean ± SEM of EMyHC expression in CSA-treated samples relative to vehicle in three experiments, each performed in duplicate. (C) CSA inhibits CK enzyme activity in a dose-dependent manner. CK activity was determined in samples treated for 24 h in ITS FM. Each point represents the mean ± SEM of differentiation-specific CK activity in CSA-treated samples relative to vehicle in three experiments, each performed in triplicate. (D) CSA inhibits myoblast fusion both in vitro and in vivo. Top panels, high-density mouse myoblasts were treated either with vehicle (panel A) or 10−6 M CSA (panel B) for 24 h in ITS fusion medium. Few multinucleated myotubes are present in CSA-treated cultures. Bar, 20 μm. Bottom panels, CSA inhibits muscle regeneration in vivo. Localized damage was induced in the tibialis anterior muscles of mice. Groups of mice were either treated with vehicle (panel C) or 45 mg/kg CSA i.p. (panel D) daily for 10 d. Muscle from vehicle-treated animals is characterized by centrally nucleated muscle fibers of various sizes as expected at this timepoint. Centrally nucleated muscle fibers are a hallmark of muscle regeneration. Muscle from CSA-treated animals is greatly deficient in regenerated muscle fibers. Cryostat sections of muscle in cross-section stained with hematoxylin and eosin are shown.
To study the effect of CSA in skeletal muscle in vivo, we produced a localized damage within a limb muscle and then treated groups of animals with either vehicle or 45 mg/kg CSA i.p. on a daily basis for 10 d. The dose of CSA used in these experiments has previously been demonstrated to be immunosuppressive in C3H mice (Pavlath et al., 1994). Limb muscle completely regenerates in 12–14 d after local damage (Pavlath et al., 1998). As shown in Figure 1D, control muscles at day 10 (bottom panel, C) are characterized by centrally nucleated regenerating muscle fibers of various sizes. Centrally nucleated muscle fibers are a hallmark of muscle regeneration (Karpati et al., 1988). In contrast, at the same time point, the muscles of CSA-treated animals (Figure 1D, bottom panel, D) are greatly deficient in regenerated muscle fibers. Taken together, these results demonstrate that CSA has profound effects on myogenesis in skeletal muscle cells.
Functionally Active NF-AT Proteins Exist in Multinucleated Skeletal Muscle Cells
The principal effect of CSA in lymphocytes is to inhibit NF-AT–mediated transcription. To determine whether skeletal muscle cells express functionally active NF-AT proteins, an NF-AT–responsive luciferase reporter in a retroviral vector (Boss et al., 1998) was introduced into primary mouse myoblasts in vitro. A retrovirus-based system was necessary for these studies because primary myoblasts typically transfect with very low efficiency. However, with the retroviral method of gene transfer, nearly 99% of primary myoblasts can be stably transduced with the gene of interest (Springer and Blau, 1997). This NF-AT responsive reporter contains a very well characterized mimimal promoter and NF-AT enhancer (Northrop et al., 1993) and works with the same fidelity as has been observed in plasmids (Boss et al., 1996) (Boss et al., 1998).
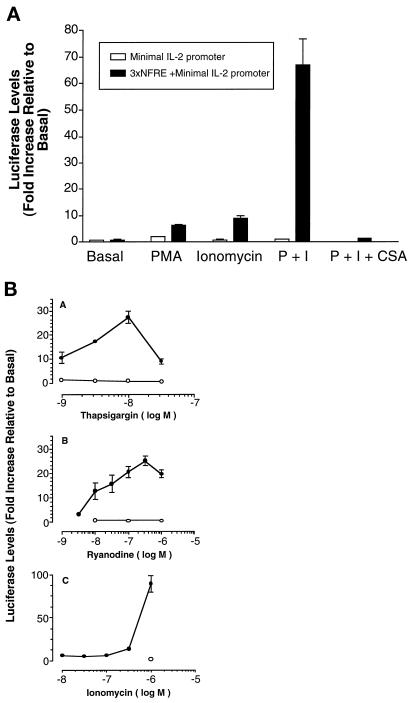
NF-AT–mediated transcription in lymphocytes is dependent on simultaneous activation of calcineurin and MAP kinase signaling. Under basal conditions, NF-AT exists as a phosphorylated form in the cytoplasm, but translocates to the nucleus upon dephosphorylation in response to increased intracellular calcium levels and activation of the phosphatase, calcineurin. CSA blocks the action of calcineurin. The MAP kinase pathway is dependent on activation of protein kinase c, which leads to an induction of AP-1 family members. In the nucleus, NF-AT forms complexes with AP-1 family members on enhancer elements of specific genes leading to transcriptional activation. To determine whether differentiated skeletal muscle cells contain functionally active NF-AT proteins, high-density cultures of myoblasts containing the NF-AT responsive reporter were allowed to form multinucleated myotubes. Myotubes were treated with ionomycin, a calcium ionophore, to induce nuclear translocation of NF-AT and phorbol myristate acetate (PMA), a protein kinase c activator, to induce AP-1 family members. Myotubes were treated for 6 h with an optimal dose of PMA or ionomycin (I) alone, or together in the absence or presence of CSA. NF-AT– mediated transcription is observed in myotubes containing the NF-AT responsive luciferase construct (Figure 2A, ▪). Both PMA or ionomycin alone induce luciferase expression, yet a marked synergistic effect is obtained in the presence of both drugs as expected for an NF-AT–mediated transcriptional response. This transcriptional response is blocked by CSA, further indicating that it is an NF-AT–mediated response. No luciferase activity is present in myotubes containing a control vector lacking the NF-AT response element (Figure 2A, □).
Figure 2.
Induction of NF-AT–mediated transcription in skeletal muscle cells. (A) Myotubes contain NF-AT proteins that are functionally active in response to known pharmacological inducers of NF-AT–mediated transcription in T cells. Primary mouse myotubes containing either the control (minimal IL-2 promoter only, □) or the NF-AT-responsive (triplex of the distal IL-2 gene NF-AT response element [NFRE] upstream of the minimal IL-2 promoter,▪) retroviral reporter plasmids were treated for 6 h with vehicle alone (basal), PMA (10 nM), or ionomycin (1 μM) alone or the two together (P+I). Some cultures were pretreated with cyclosporine A (CSA; 1 μM) for 1 h before addition of PMA and ionomycin to the medium. Luciferase values were subsequently measured. Luciferase activity is induced only in the cells containing the NF-AT response element upstream of the minimal IL-2 promoter. Both PMA and ionomycin stimulate NF-AT–mediated transcription in myotubes, but the two drugs together markedly syngerize. The P+I response is blocked in the presence of CSA. Each bar represents the mean ± SEM of two experiments, each performed in triplicate. (B) Drugs with different mechanisms of increasing intracellular calcium induce NF-AT– mediated transcription in myotubes. Primary mouse myotubes containing the NF-AT responsive reporter plasmid were treated with vehicle alone or different doses of thapsigargin (panel A), ryanodine (panel B), or ionomycin (panel C) in the presence of 10 nM PMA for 6 h before the measurement of luciferase activity. Cultures were either treated with these drugs alone (•) or together with 1 μM CSA (○). All three agents induce NF-AT–mediated transcription, but the maximum levels of induction differ, with ionomycin giving the greatest induction. Each point represents the mean ± SEM of three independent experiments, each performed in triplicate.
Two other drugs that increase intracellular calcium levels, but differ in their mechanism of action from ionomycin, were also tested to determine whether the induction of NF-AT–mediated transcription in myotubes is specific for ionomycin. Thapsigargin increases calcium levels by specifically inhibiting the sarcoplasmic reticulum Ca2+-ATPase (Thastrup et al., 1990), whereas ryanodine, in the micromolar range, stimulates ryanodine receptors involved in calcium release from the sarcoplasmic reticulum (Dulhunty et al., 1996). In the presence of PMA, both thapsigargin (Figure 2B, panel A) and ryanodine (Figure 2B, panel B) induce NF-AT–mediated transcription in a dose- dependent manner in myotubes as measured by the NF-AT responsive reporter plasmid (Figure 2B, •). The dose-response curve for ionomycin in the presence of PMA is shown for comparison in Figure 2B, panel C. CSA blocks the transcriptional responses obtained with all three drugs (Figure 2B, ○). Together these results indicate that multinucleated myotubes contain NF-AT proteins that are functionally active in response to known pharmacologic inducers of NF-AT–mediated transcription in T cells.
Induction of NF-AT–mediated Transcription Varies with the Stage of Myogenesis
To determine whether the induction of NF-AT–mediated transcription is restricted to a particular stage of myogenesis, primary mouse muscle cells were assayed either as myoblasts or myotubes. To discriminate more finely among muscle cells during specific stages of myogenesis, these experiments were performed on cells at three stages: myoblasts (80–90% confluent), nascent myotubes (24 h in fusion medium), and mature myotubes (48 h in fusion medium). Mature myotubes are distinguished from nascent myotubes by increased cell size and nuclear number. Muscle cells with the NF-AT responsive reporter plasmid at these three stages of myogenesis were treated for 6 h with an optimal dose of PMA and ionomycin and assayed for luciferase (Figure 3A). NF-AT–mediated transcription is detected at low levels in myoblasts, and these levels progressively increase as myotubes form. The highest level of induction is observed in mature myotubes. Similar differences between human myoblasts and myotubes were obtained.
Figure 3.
Induction of NF-AT–mediated transcription in muscle cells is developmentally regulated. (A) Primary mouse muscle cells were treated for 6 h with either vehicle alone or PMA (10 nM) and ionomycin (1 μM) together before the measurement of luciferase activity at the following stages of muscle development: myoblasts (Mb), nascent myotubes (N Mt), mature myotubes (M Mt). A twofold induction is observed in myoblasts, with the values progressively increasing to 10-fold and 19-fold in nascent and mature myotubes, respectively, as differentiation increases. Note that the observed level of induction in mature myotubes with PMA and ionomycin treatment varies among batches of retrovirally infected muscle cells (compare with 70-fold induction in Figure 2). Each bar represents the mean ± SEM of three independent experiments each performed in triplicate. (B) Muscle differentiation is not associated with an increase in the level of NF-AT proteins. Immunoblots were performed on electrophoretically separated proteins from myoblasts (Mb), nascent myotubes (NMt), and mature myotubes (MMt) using isoform-specific NF-AT antibodies to show that the increased levels of induction obtained with muscle differentiation are due to developmental differences and not simply protein levels.
To rule out that the increase in NF-AT–mediated transcription observed as muscle cells become more differentiated is due solely to an increase in the amount of NF-AT proteins, immunoblots were performed. Since three isoforms of NF-AT are expressed in skeletal muscle tissue as assessed from mRNA studies (Hoey et al., 1995), isoform-specific antibodies to NF-ATc, NF-ATp, and NF-AT4/x/c3 were used. The sizes of the NF-AT proteins in skeletal muscle cells (Figure 3B) are similar to those obtained in lymphoid cells (Timmerman et al., 1997). Furthermore, muscle differentiation is not accompanied by an increase in the level of any of the three NF-AT isoforms, indicating that protein content alone cannot account for the increase in NF-AT–mediated transcription with differentiation. Rather, NF-AT–mediated transcription is influenced by the developmental state of the muscle cells. It is most prominently associated with myotubes, but can occur in myoblasts at ∼10-fold lower level.
Nuclear Translocation of each NF-AT Isoform Occurs Only at Specific Stages of Myogenesis
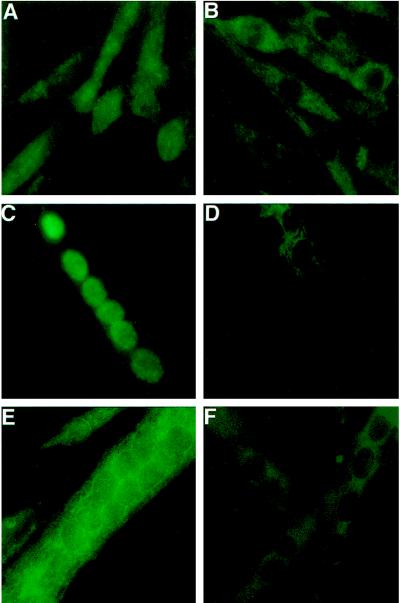
To determine whether muscle cells selectively activate individual NF-AT isoforms at specific stages of myogenesis, we next performed immunohistochemical analyses of cultured human muscle cells using isoform-specific antibodies to NF-ATc, NF-ATp, and NF-AT4/x/c3. Human muscle cells were used in these experiments rather than mouse cells because human myotubes are larger and flatter, facilitating their use in subsequent immunohistochemical analyses. The reported specificity of these antibodies (Timmerman et al., 1997) was verified using our immunohistochemical protocols by immunostaining of HEK293 cells transfected with expression plasmids for each of the individual isoforms (our unpublished results). In addition, we show that these antibodies are specific for NF-AT isoforms expressed in muscle cells (Figure 4). The nuclear staining obtained with the NF-AT4/x/c3 (Figure 4, A and B) and NF-ATc (Figure 4, C and D) antibodies in thapsigargin-treated skeletal muscle cells is eliminated by preabsorbing the antibodies with extracts prepared by transfection of HEK cells with the appropriate NF-AT expression plasmid (Figure 4, B and D). The specificity of the NF-ATp antibody in muscle cells is shown by intense cytoplasmic staining of wild-type (Figure 4E) but not NF-ATp null muscle cells (Figure 4F).
Figure 4.
Specificity of antibodies in immunohistochemistry of skeletal muscle cells. Human myoblasts (A and B) or myotubes (C and D) were treated with 10 nM thapsigargin for 1 h to concentrate the NF-AT proteins in the nucleus. Cells were fixed and stained with antibodies for NF-AT4/x/c3 (A and B) or NF-ATc (C and D) that had been preabsorbed with control extracts (A and C) or extracts from HEK cells transfected with specific NF-AT expression plasmids (B and D). For each antibody, the nuclear staining is eliminated by the extracts containing the specific NF-AT protein (B and D). The specificity of the NF-ATp antibody is shown by intense cytoplasmic staining of wild-type mouse muscle cells (E) but not of muscle cells from NF-ATp null mice (F).
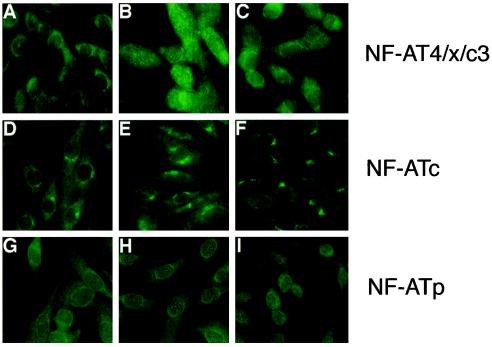
Immunohistochemical experiments were performed on muscle cells at three stages: myoblasts (80–90% confluent), nascent myotubes (24 h in fusion medium), and mature myotubes (75–80 h in fusion medium) in order again to finely discriminate among different stages of myogenesis. All three NF-AT isoforms are present in both myoblasts and myotubes in agreement with the immunoblot results (Figure 3B). Under basal culture conditions, the three NF-AT isoforms exist in the cytoplasm. Low amounts of the NF-AT isoforms may exist also in the nucleus but are undetectable by the immunochemical methods employed. Striking differences were observed among muscle cells in the calcium-induced nuclear translocation of NF-AT isoforms at all stages of myogenesis tested. Each isoform of NF-AT undergoes calcium-induced nuclear translocation only at specific stages of myogenesis. In myoblasts, calcium-induced, CSA-sensitive nuclear translocation of NF-AT 4/x/c3 is observed (Figure 5, A–C), but not of NF-ATc (Figure 5, D–F) or NF-ATp (Figure 5, G–I). Further selectivity among NF-AT isoforms in calcium-induced nuclear translocation was observed in multinucleated myotubes. Unlike myoblasts, nuclear translocation of NF-AT4/x/c3 is not observed in either nascent or mature myotube cultures (data not shown). Rather, NF-ATp and NF-ATc undergo nuclear translocation in multinucleated myotubes, but with clear differences. Only in 50–60% of nascent myotubes does NF-ATp undergo calcium-induced nuclear translocation (Figure 6B), which occurs to all nuclei within a multinucleated cell and is inhibited by CSA (Figure 6C). In contrast, NF-ATc undergoes calcium-induced nuclear translocation in 90–95% of both nascent and mature myotubes (Figure 7, B-D) that is CSA-sensitive (Figure 7E). Furthermore, in a population of myotubes, thapsigargin induces translocation of NF-ATc to all nuclei within the multinucleated cell (Figure 7B), but in another smaller population of myotubes in the same cultures (10–20%), NF-ATc translocates preferentially to some nuclei within the same cell and not to others (Figure 7, C and D). A nonrandom pattern in nuclear translocation is always observed: positive nuclei are clustered together in a region either at the end of a chain of nuclei (Figure 7C) or within an larger grouping of nuclei (Figure 7D). The frequency of myotubes with such nuclear selectivity is not influenced by the length of thapsigargin treatment. In addition, the number of nuclei contained within a myotube does not appear to influence whether such nuclear selectivity is present. The calcium-induced nuclear translocation of NF-AT isoforms at different stages of muscle development is summarized in Table 1. Together, these results indicate that nuclear translocation of individual NF-AT isoforms in response to calcium varies greatly and is influenced by the differentiation state of muscle.
Figure 5.
Only one NF-AT isoform undergoes calcium-dependent nuclear translocation in mononucleated myoblasts. Human myoblasts were analyzed at near confluence for the expression of NF-AT4/x/c3, NF-ATc, or NF-ATp using isoform-specific antibodies and immunohistochemistry. Cells are shown with vehicle (A, D, and G), 10 nM thapsigargin for 1 h (B, E, and H), or 1 μM CSA pretreatment before thapsigargin treatment (C, F, and I). The nuclear translocation of NF-AT4/x/c3 (top panels) observed with thapsigargin treatment (B) is blocked in the presence of CSA (C). Nuclear translocation of NF-AT 4/x/c3 is detected in 90–95% of the myoblasts. Under similar conditions, NF-ATc (middle panels) and NF-ATp (bottom panels) do not undergo nuclear translocation. The data shown are representative of three experiments.
Figure 6.
Calcium-dependent nuclear translocation of NF-ATp occurs only in nascent multinucleated myotubes. Human myotubes formed after 24 h in FM were treated with vehicle (panels A and A′), 10 nM thapsigargin (panels B and B′), or 10 nM thapsigargin and 1 μM CSA (panels C and C′) for 1 h before fixation and reaction with an antibody against NF-ATp. Top panels, immunofluorescent detection of NF-ATp in multinucleated myotubes; bottom panels, location of the individual nuclei in the same cell using the fluorescent DNA due, H33258. In nascent myotubes treated with vehicle (A), NF-ATp is cytoplasmic, but undergoes calcium-dependent nuclear translocation in 50–60% of myotubes (B), which is blocked by CSA (C). No nuclear selectivity is observed among the nuclei present within individual myotubes for NF-ATp. In mature myotubes (75–80 h in FM), NF-ATp does not undergo nuclear translocation in response to thapsigargin. The data shown are representative of two experiments.
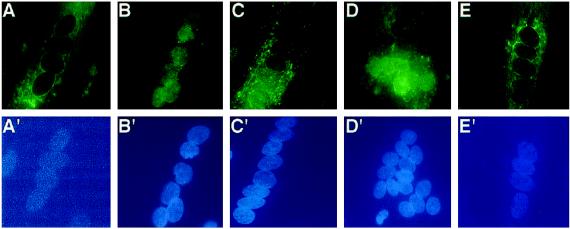
Figure 7.
NF-ATc can preferentially translocate to a subset of nuclei in multinucleated myotubes in response to calcium. NF-ATc undergoes calcium-dependent nuclear translocation in 90–95% of both nascent (24 h in FM) and mature (75–80 h in FM) human myotubes. Top panels, immunofluorescent detection of NF-ATc in multinucleated myotubes; bottom panels, location of the individual nuclei in the same cell using the fluorescent DNA due, H33258. In myotubes at both stages, NF-ATc is cytoplasmic (A). Thapsigargin treatment results in two types of nuclear translocation within the same population of cells. In some cells, NF-ATc translocates to all the nuclei within a single multinucleated myotube (B). In other cells, nuclear translocation is specific for a subset of nuclei within a single multinucleated myotube (C and D). CSA treatment blocks the nuclear translocation of NF-ATc in all myotubes (E). The data shown are representative of three experiments.
Table 1.
Each NF-AT isoform undergoes nuclear translocation at specific stages of muscle differentiation
| NF-AT isoform | Stage of myogenesis
|
||
|---|---|---|---|
| Myoblastsa | Myotubes (nascent)b | Myotubes (mature)c | |
| c | (−)d | (+)e | (+) |
| Nuclear selectivity | Nuclear selectivity | ||
| p | (−) | (+) | (−) |
| All nuclei | |||
| 4/x/c3 | (+) | (−) | (−) |
80–90% confluent.
24 h in FM.
75–80 h in FM.
(−) indicates lack of calcium-dependent nuclear translocation.
(+) indicates occurrence of calcium-dependent nuclear translocation.
DISCUSSION
In lymphocytes, the general role of NF-AT proteins in the induction of early gene expression in response to antigen receptor engagement is well defined (for review, see Rao et al., 1997). These early response genes include those coding for cytokines and cell surface receptors. Northern analyses suggest that mRNAs for various NF-AT isoforms exist also in nonlymphoid tissues (Hoey et al., 1995). NF-AT proteins are present in such diverse cell types as PC12 (Boss et al., 1996), smooth muscle (Boss et al., 1998), and endothelium (Cockerill et al., 1995). In these nonlymphoid cells, little is known about the role of NF-AT proteins in general or of individual isoforms.
In this study, we demonstrate that CSA, a widely used immunosuppressant and a well characterized inhibitor of NF-AT–mediated transcription, blocks the differentiation of skeletal muscle cells, suggesting a functional role for NF-AT proteins in skeletal muscle physiology. We hypothesize that the action of CSA is due to inhibition of a critical NF-AT activation event at some specific stage of differentiation. This work confirms and extends the in vitro findings of Hardiman (Hardiman et al., 1993) on CSA effects on morphological differentiation by demonstrating that muscle regeneration after induced trauma is inhibited at immunosuppressive doses of CSA. In addition, we used a combination of functional and immunohistochemical assays to demonstrate the activation and cellular localization of native NF-AT proteins in skeletal muscle cells in response to known pharmacological inducers in lymphoid cells. Differences were observed among developmental stages of muscle, among NF-AT isoforms and even among nuclei in multinucleated muscle cells. These results indicate complexity in the regulation of NF-AT in skeletal muscle cells compared with lymphoid cells.
Developmental Specificity
NF-AT proteins are expressed by skeletal muscle cells at all three stages of myogenesis tested: myoblasts and nascent and mature myotubes. The highest levels of NF-AT–mediated transcription in response to pharmacological stimulators of intracellular calcium and AP-1 are associated with mature myotubes. Several mechanisms acting alone or in combination could account for the differences between myoblasts and differentiated myotubes in the level of NF-AT–mediated transcription achieved with these drugs. First, different developmental stages of muscle may differ to the degree to which either intracellular calcium or AP-1 can be elevated. Second, we have shown that NF-AT 4/x/c3 is the only isoform in high-density myoblasts that undergoes nuclear translocation in response to a stimulator of intracellular calcium. The low levels of NF-AT–mediated transcription in myoblasts, therefore, may reflect the lower affinity of the NF-AT 4/x/c3 isoform for the NF-AT response element that was derived from the IL-2 promoter (Ho et al., 1995; Timmerman et al., 1997) and used in the reporter construct. Third, in myoblasts, NF-AT4/x/c3 may form transcriptional complexes with nuclear proteins other than AP-1. Finally, activation of JNK, a member of the MAP kinase family, has been recently shown to block nuclear accumulation of recombinant NFAT4/x/c3 in BHK cells (Chow et al., 1997). Drugs, such as PMA, that induce the expression of AP-1 serve to activate the MAP kinase pathway in cells.
Isoform Specificity
Three isoforms of NF-AT (p, c, and 4/c3/x) are expressed by muscle cells at all stages of myogenesis studied. Since all the cells in the population express each isoform, this implies that each individual cell must coexpress all three isoforms, as do lymphocytes (Timmerman et al., 1997). However, in lymphocytes each isoform undergoes nuclear translocation with the same kinetics in response to a given stimulus. In contrast, in skeletal muscle cells each isoform undergoes nuclear translocation only at specific stages of myogenesis. The ability of individual NF-AT isoforms to undergo calcium-induced nuclear translocation at only specific stages of myogenesis suggests that individual NF-AT isoforms may regulate distinct subsets of genes necessary for muscle cell physiology. The genes that are regulated by NF-AT in skeletal muscle cells are unknown but currently under investigation.
The role of individual isoforms of NF-AT in regulating expression of specific genes in lymphoid cells is unclear. Two lines of evidence suggest that each NF-AT family member may regulate distinct subsets of genes. First, in NF-ATp null mice, the expression of some cytokines is greatly affected, whereas the expression of others is minimally or not at all affected (Hodge et al., 1996). Second, differences exist among isoforms in the ability to form transcriptional complexes on the NF-AT–binding sites in the enhancers of several early response genes (Timmerman et al., 1997).
The differential ability of natively expressed NF-AT isoforms to undergo nuclear translocation during muscle development suggests specificity in the activation of NF-AT beyond simply that of the amplitude and duration of changes in intracellular calcium (Dolmetsch et al., 1997). Such specificity may occur at the level of dephosphorylation of NF-AT isoforms by calcineurin or in the subsequent nuclear import or export of NF-AT isoforms (Chow et al., 1997). Possibly, individual isoforms of NF-AT exist in different subcellular compartments that differ in accessibility to calcium (Thomas et al., 1996) or downstream effectors.
Nuclear Specificity
Differences were noted among the ability of NF-ATp and NF-ATc to translocate to individual nuclei within a multinucleated myotube. In all cases, NF-ATp underwent translocation to all nuclei within a single multinucleated myotube. In contrast, NF-ATc could preferentially translocate to only a subset of nuclei within a single multinucleated myotube in some cases. Calcium-induced nuclear translocation of a transcription factor to specific nuclei of a multinucleated muscle cell may be a mechanism for localization of gene products to nuclear domains or specialized regions of muscle cells (Hall and Ralston, 1989; Pavlath et al., 1989). Nuclei in the synaptic region of adult muscle fibers are transcriptionally distinct from other myofiber nuclei (Changeux, 1991; Moscoso et al., 1995). The nuclei of myotubes in culture are also not transcriptionally equivalent; some genes are expressed by a subset of nuclei, whereas other genes are transcribed by all nuclei equivalently under both aneural (Harris et al., 1989; Berman et al., 1990; Tsim et al., 1992; Dutton et al., 1993; Su et al., 1995) or neural-like (Chu et al., 1995; Grubic et al., 1995) conditions. How transcriptional differences between nuclei in myotubes are established is unknown, but appears to be controlled by a program already active before myoblast fusion (Su et al., 1995). Thus, gene transcription in multinucleated cells is regulated not only by classic cis and trans-acting elements, and chromatin structure, but also, as suggested in this study, by nuclear selectivity in the translocation of transcription factors.
In summary, studies of native NF-AT isoforms in skeletal muscle reveal similiar pharmacological properties to those found in lymphocytes but identify additional complexities in the regulation of individual isoforms. The study of muscle cells isolated from mice genetically deficient in individual NF-AT isoforms will help define the role of specific isoforms in myogenesis. Future studies will delineate the genes that are regulated by NF-AT in skeletal muscle cells and how signal transduction via such NF-AT–regulated pathways contributes to muscle development and function.
ACKNOWLEDGMENTS
We thank Dr. Gerald Crabtree for the antibodies against NF-ATp and NF-AT 4/x/c3, as well as the expression plasmids for each NF-AT isoform, and Dr. Laurie Glimcher for the NF-ATp null mice. T.J.M. is an Established Investigator of the American Heart Association. Supported by grants from the NIH (T.J.M.) and NIH grant AR-43410, Muscular Dystrophy Association, and Emory University Research Council (G.K.P.).
Abbreviations used:
- bFGF
basic fibroblast growth factor
- CK
creatine kinase
- CSA
cyclosporine A
- EMyHC
embryonic myosin heavy chain
- FBS
fetal bovine serum
- FM
fusion medium
- GM
growth medium
- MEF2
myocyte enhancer factor 2
- MRF
myogenic regulatory factor
- NF-AT
nuclear factor of activated T Cells
- PGM
producer growth medium
- PMA
phorbol myristate acetate
- TBS
Tris-buffered saline
REFERENCES
- Berman SA, Bursztajn S, Bowen B, Gilbert W. Localization of an acetylcholine receptor intron to the nuclear membrane. Science. 1990;247:212–214. doi: 10.1126/science.1688472. [DOI] [PubMed] [Google Scholar]
- Boss V, Abbott KA, Wang X-F, Pavlath GK, Murphy TJ. Expression of the cyclosporin A-sensitive factor NFAT in cultured vascular smooth muscle cells: differential induction of NFAT-mediated transcription by phospholipase C-coupled cell surface receptors. J Biol Chem. 1998;273:19664–19671. doi: 10.1074/jbc.273.31.19664. [DOI] [PubMed] [Google Scholar]
- Boss V, Talpade DJ, Murphy TJ. Induction of NFAT-mediated transcription by Gq-coupled receptors in lymphoid and non-lymphoid cells. J Biol Chem. 1996;271:10429–10432. doi: 10.1074/jbc.271.18.10429. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991;3:413–429. [PubMed] [Google Scholar]
- Cho M, Hughes SM, Karsch-Mizrachi I, Travis M, Leinwand LA, Blau HM. Fast myosin heavy chains expressed in secondary mammalian muscle fibers at the time of their inception. J Cell Sci. 1994;107:2361–2371. doi: 10.1242/jcs.107.9.2361. [DOI] [PubMed] [Google Scholar]
- Chow C-W, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Chu GC, Moscoso LM, Sliwkowski MX, Merlie JP. Regulation of the acetylcholine receptor epsilon subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Cockerill GW, Bert AG, Ryan GR, Gamble JR, Vadas MA, Cockerill PN. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood. 1995;86:2689–2698. [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration [see comments] Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Junankar PR, Eager KR, Ahern GP, Laver DR. Ion channels in the sarcoplasmic reticulum of striated muscle. Acta Physiol Scand. 1996;156:375–385. doi: 10.1046/j.1365-201X.1996.193000.x. [DOI] [PubMed] [Google Scholar]
- Dutton EK, Simon AM, Burden SJ. Electrical activity-dependent regulation of the acetylcholine receptor delta-subunit gene, MyoD, and myogenin in primary myotubes. Proc Natl Acad Sci USA. 1993;90:2040–2044. doi: 10.1073/pnas.90.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubic Z, Komel R, Walker WF, Miranda AF. Myoblast fusion and innervation with rat motor nerve alter distribution of acetylcholinesterase and its mRNA in cultures of human muscle. Neuron. 1995;14:317–327. doi: 10.1016/0896-6273(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell. 1989;59:771–772. doi: 10.1016/0092-8674(89)90597-7. [DOI] [PubMed] [Google Scholar]
- Hardiman O, Sklar RM, Brown RH., Jr Direct effects of cyclosporin A and cyclophosphamide on differentiation of normal human myoblasts in culture. Neurology. 1993;43:1432–1434. doi: 10.1212/wnl.43.7.1432. [DOI] [PubMed] [Google Scholar]
- Harris DA, Falls DL, Fischbach GD. Differential activation of myotube nuclei following exposure to an acetylcholine receptor-inducing factor. Nature. 1989;337:173–176. doi: 10.1038/337173a0. [DOI] [PubMed] [Google Scholar]
- Ho SN, Thomas DJ, Timmerman LA, Li X, Francke U, Crabtree GR. NFATc3, a lymphoid-specific NFATc family member that is calcium-regulated and exhibits distinct DNA binding specificity. J Biol Chem. 1995;270:19898–19907. doi: 10.1074/jbc.270.34.19898. [DOI] [PubMed] [Google Scholar]
- Hodge MR, Ranger AM, de la Brousse CF, Hoey T, Grusby MJ, Glimcher LH. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Hokanson JF, Mercier JG, Brooks GA. Cyclosporine A decreases rat skeletal muscle mitochondrial respiration in vitro. Am J Respir Crit Care Med. 1995;151:1848–1851. doi: 10.1164/ajrccm.151.6.7767529. [DOI] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Prescott S. Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle & Nerve. 1988;11:795–803. doi: 10.1002/mus.880110802. [DOI] [PubMed] [Google Scholar]
- Kotani H, Newton PB, III, Zhang S, Chiang YL, Otto E, Weaver L, Blaese RM, Anderson WF, McGarrity GJ. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mercier JG, Hokanson JF, Brooks GA. Effects of cyclosporine A on skeletal muscle mitochondrial respiration and endurance time in rats. Am J Respir Crit Care Med. 1995;151:1532–1536. doi: 10.1164/ajrccm.151.5.7735611. [DOI] [PubMed] [Google Scholar]
- Miller AD, Miller DG, Garcia JV, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- Moscoso LM, Merlie JP, Sanes JR. N-CAM, 43K-rapsyn, and S-laminin mRNAs are concentrated at synaptic sites in muscle fibers. Mol Cell Neurosci. 1995;6:80–89. doi: 10.1006/mcne.1995.1008. [DOI] [PubMed] [Google Scholar]
- Northrop JP, Ho SN, Chen L, Thomas DJ, Timmerman LA, Nolan GP, Admon A, Crabtree GR. NF-AT components define a family of transcription factors targeted in T-cell activation [see comments] Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Northrop JP, Ullman KS, Crabtree GR. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- Pavlath GK, Rando TA, Blau HM. Transient immunosuppressive treatment leads to long-term retention of allogeneic myoblasts in hybrid myofibers. J Cell Biol. 1994;127:1923–1932. doi: 10.1083/jcb.127.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Thaloor D, Rando TA, Cheong M, English AW, Zheng B. Heterogeneity among muscle precursor cells in adult skeletal muscles with differing regenerative capacities. Dev Dynam. 1998;212:495–508. doi: 10.1002/(SICI)1097-0177(199808)212:4<495::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Sharma KR, Mynhier MA, Miller RG. Cyclosporine increases muscular force generation in Duchenne muscular dystrophy. Neurology. 1993;43:527–532. doi: 10.1212/wnl.43.3_part_1.527. [DOI] [PubMed] [Google Scholar]
- Springer ML, Blau HM. High-efficiency retroviral infection of primary myoblasts. Somatic Cell Mol Genet. 1997;23:203–209. doi: 10.1007/BF02721371. [DOI] [PubMed] [Google Scholar]
- Su X, Berman SA, Sullivan T, Bursztajn S. Myoblast and myotube nuclei display similar patterns of heterogeneous acetylcholine receptor subunit mRNA expression. J Cell Biochem. 1995;58:22–38. doi: 10.1002/jcb.240580105. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AP, Bird GS, Hajnoczky G, Robb-Gaspers LD, Putney JW., Jr Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Timmerman LA, Healy JI, Ho SN, Chen L, Goodnow CC, Crabtree GR. Redundant expression but selective utilization of nuclear factor of activated T cell family members. J Immunol. 1997;159:2735–2740. [PubMed] [Google Scholar]
- Tsim KW, Greenberg I, Rimer M, Randall WR, Salpeter MM. Transcripts for the acetylcholine receptor and acetylcholine esterase show distribution differences in cultured chick muscle cells. J Cell Biol. 1992;118:1201–1212. doi: 10.1083/jcb.118.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Pavlath GK, Parks DR, Walsh FS, Blau HM. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res. 1988;174:252–265. doi: 10.1016/0014-4827(88)90159-0. [DOI] [PubMed] [Google Scholar]