Abstract
The aim of this study was to analyze the scope of pre-surgical high resolution ultrasound in basal cell carcinoma (BCC). BCC is the most common human cancer. According to recent large prospective studies incomplete excision of BCC is higher than expected. Pre-operative imaging may aid surgical planning by identifying the extent and location of a neoplasm, which can be interesting at zones with higher risk of recurrences such as the face. These are slow growing tumors but there are some aggressive types described that can involve deeper structures. Twenty-five patients were studied (10 F/15 M, 48–91 years old; mean age 69.5 ± 11.5 years) with suspicious facial lesions ≤1.5 cm. Pre-surgical ultrasound with compact linear 15–7 MHz probe was performed. Ultrasound reported the morphology and thickness of the tumors. The results were correlated with the histology. Ultrasound identified 29 suspicious facial lesions that were removed with tumor-free borders at the first surgery and confirmed by histology. The main location was the nose. Two subclinical satellite lesions at the nasal zone were detected under ultrasound which led to a change in the surgery plan. The intraclass correlation coefficient (ICC) value was used to compare tumor thickness measurements between ultrasound and histology. ICC was considered as very good (0.9). Therefore, ultrasound can be useful to plan BCC surgery, it can recognize lesions, layers of involvement and vascularity patterns in a non-invasive way. It can show subclinical satellite lesions, even though the number of subclinical cases is small and require further investigations. It has a good thickness correlation with histology and may be used as a technique to monitor disease changes following non-invasive medical treatments in the future.
Keywords: Basal cell carcinoma ultrasound; BCC ultrasound; skin cancer imaging; basocellular carcinoma imaging; dermatology ultrasound; skin ultrasound, non-invasive imaging skin
Introduction
Basocellular carcinoma (BCC) is the most common cancer overall in humans and constitutes 75–90% of all skin cancers. The tumor's malignant characteristics depend on the destructive extension of the generally slow growing primary tumor, rather than on metastasis[1–3], even though there are aggressive forms of BCC associated with extensive dermal invasion and destruction of collagen, which in certain cases can grow into deep tissues and even metastasize, and identification of tumor borders can be a challenge[4–8].
Although rarely a life-threatening disease, this predominantly facial tumor may cause considerable morbidity related to functional and aesthetic problems if untreated or recurring. High risk areas for recurrence are for, e.g. the skin of the eyes, nose and ears. Prevention of a recurrence, particularly within the facial area, is therefore an important goal in the treatment of BCC, because it may have a considerable impact on a patient's quality of life[9].
As a measure of the standard of surgical care, incomplete excision of skin malignancy is an important clinical indicator. Reported rates of incomplete excision of BCC vary widely (5–25%) among centers worldwide. Risk factors for incomplete excision are: location on the head; morpheiform, superficial, and infiltrative subtypes; lesions larger than 20 mm in diameter; the presence of multiple lesions; repair by skin graft; and recurrent and previously incompletely excised BCCs[10–16].
It may be speculated that these risk factors can be better controlled for by a more accurate pre-operative assessment of the tumor size, depth and relation to surrounding structures. As in other tissues, surgical planning with previous knowledge of the tumor margins is often the key to avoiding incomplete excision and post-surgical re-intervention or functional and aesthetical defects in the treatment of skin tumors.
Several therapeutic modalities have been used to treat BCC with different cosmetic results, complications and recurrences. The treatment modalities most frequently used are surgical excision (SE), cryosurgery, curettage and electrodesiccation, radiotherapy, Mohs micrographic surgery (MMS) and photodynamic therapy[17]. SE constitutes the accepted standard[10,11]. In recent years non-surgical therapies such as photodynamic therapy and immunomodulation with imiquimod have also become available. Because of their essentially non-scarring outcome these treatments are gaining increased acceptance by dermatologists worldwide.
A broad variety of diagnostic technologies are becoming available for non-invasive diagnosis of non-melanoma skin cancer (NMSC)[18]. Penetration, resolution, adaptation to the different convexities and concavities of the body surface, that are relevant on the face and worldwide availability are among the challenges for the different imaging techniques.
With the recent developments within high-resolution ultrasound equipment, which allow visualization of skin layers, it may be possible to recognize skin tumors and in addition to describe their morphological and topographical characteristics as well as their size more accurately, thereby helping the surgeon to plan the surgery[18–30].
Lateral and longitudinal extension of a skin tumor could be defined by clinical observation within a safety margin, but depth and the possibility of involvement of deeper structures may remain unknown.
High frequency ultrasound has been used in dermatology with frequencies between 20 and 100 MHz, but when the frequency is increased, axial resolution improves with a loss of depth of the image, which can be a problem for detecting tumor thickness and involvement of deep structures. Penetration at a frequency of 20 MHz is no more than 6–7 mm.
The high resolution ultrasound in general use in radiology or diagnostic imaging departments, uses variable frequency transducers which can adapt to the depth of different tissue layers by modifying the applied frequency and focus without losing resolution. New probes with variable frequencies from 17 to 7 MHz or from 15 to 7 MHz can visualize the skin layers in real time[19] (Fig. 1) as well as musculo-tendinous, cartilaginous and bony structures at the same resolution, with quality images that are more understandable and clearer from the anatomical point of view. The attached color Doppler function further permits observation of vascular morphology and perfusion in real time.
Figure 1.
Compact linear probe, 15–7 MHz (variable frequency) used for the study.
There are few reports in the medical literature about diagnostic imaging techniques used for pre-surgical visualization of skin tumors[18,22,31]. Ultrasound is not meant to replace histologic evaluation of BCCs, but may be a useful adjunct in surgical planning. The objective of this study was therefore to analyze the potential of pre-surgical high-resolution ultrasound technology in BCC.
Materials and methods
We recruited 25 patients, 10 females and 15 males, ranging in age from 48 to 91 years (mean ± SD 69.5 ± 11.5 years) from the Dermatology Department of our institutions, with one or more lesions that conformed with the following inclusion criteria: (1) lesion clinically apparent BCC; (2) lesion located on the face; (3) lesion diameter ≤1.5 cm; (4) lesions without any previous treatment or biopsy. The study was approved by the ethics committees of the institutions, and informed consent was obtained from all patients.
A color Doppler ultrasound study of the lesions was performed using a Philips HDI 5000 (Bothel, WA), with a 15–7 MHz compact linear probe and copious amounts of gel over the surface of the lesions, on the same day as the surgery. The ultrasound report included: shape, composition, described as solid or cystic, according to overall echogenicity (hypoechoic for solid and anechoic for cystic), homogeneity (homogeneous or heterogeneous), size, level of invasion, presence, type and location of vascularity. Thickness was measured from the epidermal surface to the deepest infiltration point of the tumor on two axis (one longitudinal view and one transverse view). The same value was used as the annotation value for both axes. In the case of nasal lesions, any presence or absence of nasal cartilage involvement was described.
The same radiologist (XW) with 15 years of experience in ultrasound performed all the sonographic studies. The same dermatologist (FB) performed all the surgeries and removed the lesions after having seen the ultrasound report, with conventional narrow safety borders (5 mm) and included the data in the pre-operative planning. The decisions to remove or not the adjacent nasal cartilage in nasal lesions and the depth of the incision during the surgery were based on the ultrasound report.
Tissue samples were sent to the histology laboratory fixed in 10% buffered formalin. The samples were stained with hematoxylin/eosin and examined with a microscope (Olympus BX 40, Olympus Optical, Tokyo, Japan).
After surgery, the same dermatopathologist (LS) looked at all the tissue samples throughout the study. The histology report included: cancer subtype, size, level of invasion and thickness, the presence of inflammation, blood vessels, sebaceous glands and collagen. In the case of nasal lesions, the presence or absence of nasal cartilage involvement was also reported. Tumor thickness measurements were made from the epidermal granulous layer to the deepest point in the slide with major tumoral infiltration. One axis depth annotation value was obtained given the nature of the procedure which involves observation on a slide under a microscope.
Measurements in ultrasound and histology were done at the deepest thickness infiltration point of the tumors to achieve the closest possible similar measurements for both methods.
There was one operator for the ultrasound, one surgeon and one pathologist, throughout the study. The results were blinded between the radiologist who made the ultrasound examinations and the dermatopathologist, but the radiologist had access to the histological reports after they were released.
The intraclass correlation coefficient (ICC) was calculated to compare thickness measurements for the same tumors for the two diagnostic methods: ultrasound and histology. The formula used was:
Where k is the number of observations for each patient (depth annotation values), SC is the sum of squares between cases and SS is the sum of total squares. ICC values ≥ 0.9 are very good, 0.70–0.89 good, 0.50–0.69 moderate, 0.30–0.49 mediocre, ≤ 0.29 bad. The k value is the number of annotation values made for each subject. There were 2 depth annotation values for the same lesion (k = 2), one for the ultrasound depth and one for histology. Depth annotation values, not the number of measurements in the axial views, were taken for the intraclass coefficient calculation.
Coefficient analysis for the ultrasound operator was performed under the same analysis but divided in two stages during the time of the study. The first group contained patients who had ultrasound and surgery earlier during the study (n = 12) and then a second group who had ultrasound and surgery later (n = 13). Thus, the second group represents an analysis of the ultrasound operator's work after a period with close contact with BCC lesions.
The ICC was calculated using a one-way ANOVA table with the SPSS 11.0 software program and all the statistical analysis was performed by the same statistical analyst (ME).
Results
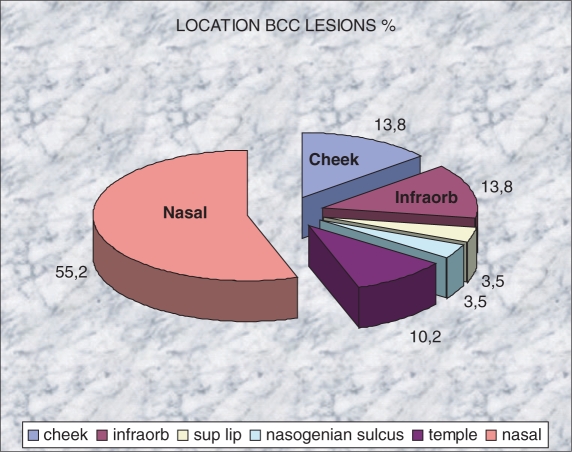
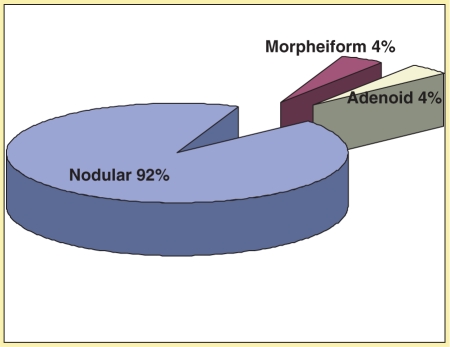
Of the 25 patients recruited (Fig. 2), 10 were female and 15 were male, with an age range from 48 to 91 years (mean 69.5 years; SD ±11.5).Clinically, 27 lesions suspicious of BCC were identified and ultrasound was able to recognize 29 lesions suspicious for BCC. The BCCs were located (Fig. 3) mainly on the nose (55%) followed by the infraorbitary zone (10%) and the cheek (10%). The histological subtypes (Fig. 4) were distributed as follows: 92% nodular (27 cases), 4% morpheiform (1 case), and 4% adenoid (1 case). No handling or fixation artifacts were seen on the tissue samples.
Figure 2.
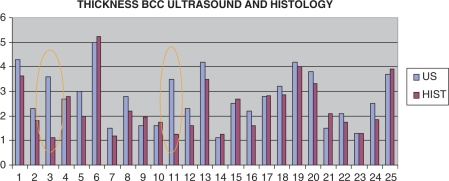
Thickness (mm) correlation distribution between ultrasound and histology. Inside the orange circles are the two pitfall cases of the study.
Figure 3.
Distribution of the location of the BCC lesions (%).
Figure 4.
Histological distribution of the BCC lesions.
Two of the patients had more than one lesion. One patient presented two clinically visible tumors. The other patient had three lesions, with only one clinically visible and two satellite neoplasms that were found only with ultrasound. In both patients the lesions were located on the nose. The 29 tumors were completed excised and the histology confirmed the diagnosis of BCC with tumor-free borders in 100% of the cases.
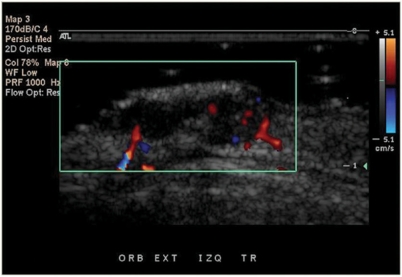
Sonography showed hypoechoic, heterogeneous, oval shaped, solid tumors with irregular borders in 100% of the cases. All the lesions presented arterial vessels inside and at the periphery of the lesion, mainly surrounding the deep area of the tumor. Since most of the cases were nodular type (n = 27), which is the most common subtype, and only 2 cases corresponded to different histological subtypes (1 morpheiform and 1 adenoid) there were no significant sonographic differences between the cancer subtypes. Nasal cartilage was sonographically visible in all cases and showed no signs of tumor involvement, which was confirmed by histology showing free margin reports (Fig. 5). Arterial flow peak systolic velocities and resistance indexes were measured in every patient. Mean peak systolic velocity for BCC patients was 9.1 ± 4.09 cm/s (range 4–22.2), and mean resistance index was 0.53 ± 0.1 (range 0.37–0.67) (Figs. 6 and 7).
Figure 5.
BCC tumor ultrasound shows an oval and hypoechoic lesion. Three thickness measurements (between calipers) demonstrate the irregularities at the deep borders that generate different distances. For the purpose of this study the largest distance was considered.
Figure 6.
Color Doppler ultrasound shows the distribution of vessels (in red and blue) inside and at the deep portion of the tumor.
Figure 7.
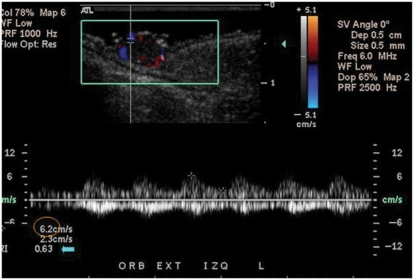
Spectral curve analysis color Doppler ultrasound of the arterial flow inside the tumor demonstrates the peak systolic velocity (circle) and the resistance index (arrow).
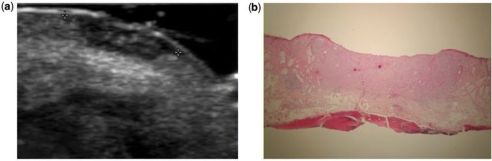
Thickness (depth) was measured by histology and ultrasound. Mean ultrasound thickness was 3.7 ± 1.1 mm (range 1.1–5.0) and 3.9 ± 1.0 mm (range 1.1–5.2) from histology (Fig. 8). All except two cases had a very good correlation (ICC) between ultrasound and histology. In one case histology showed sebaceous gland hypertrophy around the tumor, which produced fuzzy sonographic tumoral borders. After seeing the histology, the radiologist was able to identify the ultrasound morphology pattern most likely associated with the sebaceous gland hypertrophy, which was a coarse hypoechoic tissue surrounding the tumor (Figs. 9 and 10). In the second case the histology presented an inflammatory giant cell reaction surrounding the deep portion of the tumor with prominent vessels. This area was taken at the ultrasound to be part of the whole tumor, but this type of surrounding inflammatory pattern with deep vessels that belongs to the inflammation process forms an angle with the tumor and it was subsequently possible to recognize it in the following ultrasound thickness measurement cases (Fig. 11a and b).
Figure 8.
Ultrasound (a) and histology (b) of the same case showing a thickness difference of less than 1 mm (0.7 mm) between the methods and corresponding to a patient with a tumor measuring 4.2 mm thick by ultrasound and 3.5 mm by histology. Calipers are marking the lateral extension of the lesion, not the depth. Note the similar shape of the tumor under ultrasound and under 2 × zoom hematoxylin/eosin stain in histology.
Figure 9.
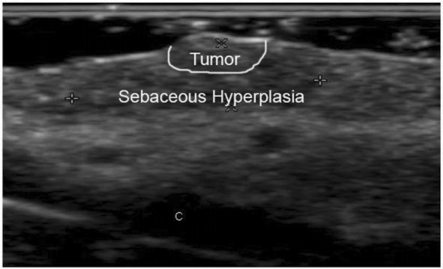
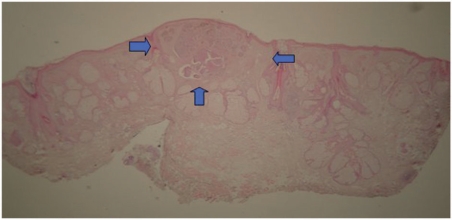
Ultrasound of a case later described by histology as surrounded with sebaceous hyperplasia that overestimated the size of the tumor. Calipers show the measurements that were included in the ultrasound report. The (C) mark corresponds to the cartilage at the nasal ala. Notice the coarse, fuzzy and heterogeneous echoic pattern of the tissue, which differs from the lesions previously seen. Tumor and sebaceous hyperplasia zones are marked.
Figure 10.
Histology of the same tumor as the patient in Fig. 9. The tumor is located between arrows and the rest was reported as sebaceous hyperplasia.
Figure 11.
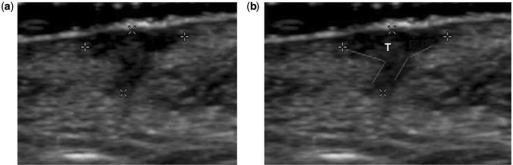
Ultrasound of a case described in histology as thickness overestimation because of the presence of a giant cell inflammatory reaction at the deepest portion. (a) Calipers show the actual measurements done by the ultrasound operator. In (b) the red lines show the angles formed by the inflammatory reaction at the profound part of the lesion. The tumor (T) was only located at the superficial area before the angles are formed.
Ultrasound depth measurements compared to histological depth measurements excluding the two pitfall cases had an excellent correlation (ICC 0.9); the correlation for all the cases including the two pitfalls was good (ICC 0.7). In addition, correlation of the ultrasound operator's measurements with histology gave an ICC of 0.7 (good) in the first 12 cases, which included the two pitfall cases, and 0.9 (very good) in the second 13 cases.
Surgical management was different in all the nasal cases (55%) because with the sonographic information on the absence of nasal cartilage infiltration the surgeon decided to not remove the cartilage adjacent to the lesion. The main surgical technique was completely changed in only one case; a cutaneous frontal flap to the nose was carried out to remove two subclinical lesions near to the main nasal tumor in one time surgery.
Discussion
All of the 29 BCC lesions that we studied had recognizable images on ultrasound, even though clinically they measured less than 15 mm. In all cases it was possible to visualize the morphology, exact localization and thickness in a reliable way before surgery with a good histological correlation. The hypoechoic and oval shaped sonographic appearance of the tumors was consistent with previous reports in the literature[20,32].
The relatively low arterial flow peak systolic velocities (mean 9.1 cm/s) and low resistance indexes (less than 0.7) for BCC cases could make them suitable for another aspect of non-invasive imaging, i.e. dynamic imaging. The morphology patterns of BCC vascularity in ultrasound have not been described previously. The predominant deep location of vessels can be compared in further investigations on other types of skin cancer in the future.
The big standard deviation observed in histology and ultrasound arises because we are comparing different sizes of lesions in different patients, therefore the lesions cannot be compared between each other. Therefore, the intraclass coefficient is used for a concordance analysis between the two modalities measuring the same tumor.
The purpose of the study was not to measure the diagnostic accuracy of ultrasound in the diagnosis of BCC; the aim was to analyze the scope of ultrasound in the definition of the sonographic morphology of BCC and to determine the possibility of measuring the unknown axis (depth) of the lesion. It was the surgeon's experience that pre-surgical color Doppler ultrasound was a useful and reliable technique to plan the surgery; it permitted the sonographer to depict the skin tumors in real time and in a non-invasive way, both visible and subclinical lesions, thereby optimizing treatment. Information for the surgical management in nasal cases (55%) concerning the removal of cartilage was useful and permitted conservative one time surgery in all cases. The surgical technique was altered in one case with subclinical lesions near the main tumor.
In other types of skin cancer, e.g. melanoma, tumor vascularity may be of strong prognostic significance and prominent perfusion has been described[33]. Further investigations and comparisons between NMSC and melanoma tumoral vessel morphology, velocity and distribution probably can improve our diagnostic accuracy and could also be a prognostic factor in NMSC.
Solitary and multiple satellite subclinical BCC lesions in the same patient were detected in this study, which may be advantageous with regard to achieving adequate surgical planning and optimizing the results, similar to e.g. hidradenitis[34]. In one case, this observation allowed the surgeon to change the technique and to extract three skin cancers in one session. However, the number of subclinical lesions were low in this study and this observation therefore requires further investigations (Figs. 12 and 13). The detection of subclinical cases of BCC lesions using ultrasound has not been previously described in the literature, but identification of subclinical lesions has been described using ultrasound for studying melanoma[18,35,36].
Figure 12.
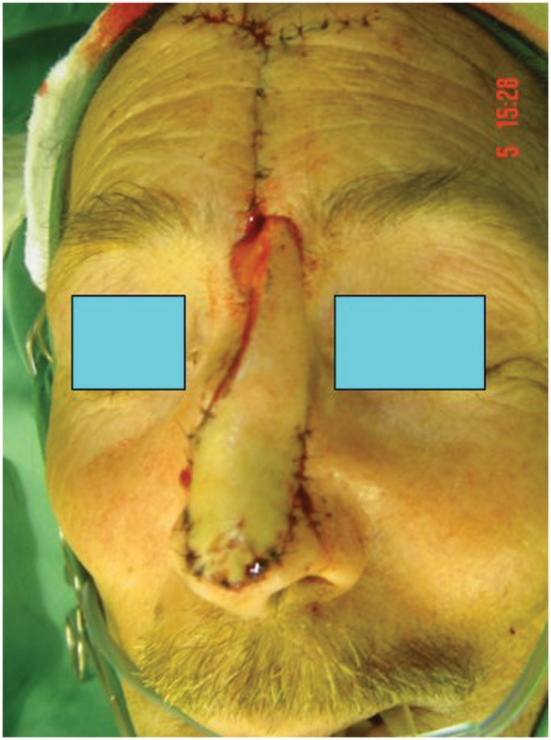
Cutaneous frontal outflap surgery in a case with three BCC focuses on the nose. Two of the three focuses were subclinical and detected only at the ultrasound examination.
Figure 13.
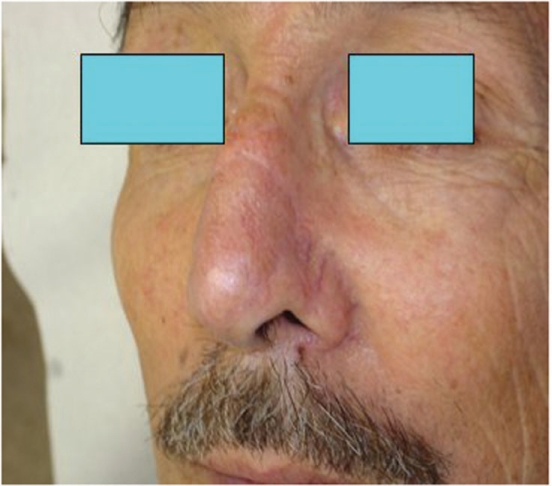
Post op result of the same patient as Fig. 9 after removal of all three BCC focuses in one time surgery.
The tumors reviewed were all <15 mm, suggesting that even small tumors can be correctly identified. The fact that it was possible to study small tumors suggests that this procedure would also be valid for larger tumors with deeper type of involvement and where the changes may be expected to be more pronounced; these observations suggest that ultrasound, at the current stage of its development, may be useful in the pre-operative assessment of BCC in high-risk areas.
One of the aspects of physical examination, which is offered by ultrasound imaging over clinical examination alone, is that of quantification. We measured the size of the tumors, and found that the intraclass coefficient values were good even though the measurements were comparing real time live tissues by ultrasound with dehydrated tissues in histology. A strong correlation between histology and ultrasound thickness has already been reported for melanoma[37,38].
However, in previous studies some overestimation of tumor thickness appears when ultrasound measurements are compared directly with the histology measurements. This may represent tissue shrinkage occurring during preparation of the histological slides, due to the inclusion of echoic paratumoral inflammatory infiltrates, or because tissue measurements were not made at the exact same point of the usually irregular borders in neoplastic lesions. Comparing ultrasound and histology, the results are good and the differences between the methods are small and less than the mean safety security margins.
All these mechanisms may play a role, but as in other imaging methods, the results are to some degree operator dependent. The ultrasound operator analysis in this study suggests a learning curve for the discrimination of potential measurement pitfalls, i.e. once the pitfalls are recognized, it is possible to avoid them and improve accuracy for the next cases. The detection of potential pitfalls for the ultrasound operator can be a useful topic for further investigations. Our experience suggests that echoic inflammatory paratumoral infiltrates can be recognized and distinguished from actual tumor tissue by a trained sonographer based on the ultrasound morphology.
Multiple hypersonographic spots have been proposed recently as a characteristic to differentiate between NMSC and melanoma[39].These spots were observed but not quantified in our study, because it was not the purpose of our work. This fact could be a subject for further investigations to analyze the histological causes and the amount of hyperechoic spots that can make the difference in the diagnosis.
Tumor location is one of the factors associated with incomplete excision of BCC mainly on the nose (particularly the nasal ala) and the eyelids (particularly the inner canthus)[9,40]. In our study all the anatomical sites, including the nose and eyelids, were easily reached with the compact linear probe shape and a copious amount of gel, which is important in order to have easy access to the most common locations for BCC.
Conclusion
Presurgical high resolution color Doppler ultrasound was a useful and reliable technique to plan surgery; it permitted real time non-invasive detection of all visible lesions and also some subclinical lesions, making it possible to optimize treatment. Previous knowledge of the margins and shape, including thickness and distribution of vascularity, made it feasible to excise the tumors in a single session with tumor-free borders. Access to determine the surface skin lesion morphology was easy and the involvement of deeper tissues could be ruled out. There are some sonographic pitfalls that can be recognized and avoided by the operator in order to achieve more accurate measurements. Although the number of cases in this study is small, these results are promising in terms of non-invasive, earlier detection of subclinical lesions and a better description of clinical lesions in the most common type of human cancer. Ultrasound is not meant to replace histologic evaluation, but it is can be used as another diagnostic and support tool, as well as treatment planner and for stratifying high risk patients. It permits good visualization of the nasal cartilage that could avoid incomplete excisions in this location. It is also an imaging method that could be used for all the new medical non-invasive treatments in skin cancer that will require of non-invasive techniques to monitor the evolution of the disease.
References
- 1.Miller SJ. Biology of basal cell carcinoma (Part I) J Am Acad Dermatol. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- 2.Chuang TY, Popescu A, Su WP, Chute CG. Basal cell carcinoma: a population-based incidence study in Rochester, Minnesota. J Am Acad Dermatol. 1990;22:413–17. doi: 10.1016/0190-9622(90)70056-n. [DOI] [PubMed] [Google Scholar]
- 3.Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794–8. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasswimmer J, Pierce MC, Park BH, Neel V, de Boer JF. Polarization-sensitive optical coherence tomography of invasive basal cell carcinoma. J Biomed Opt. 2004;9:292–8. doi: 10.1117/1.1644118. [DOI] [PubMed] [Google Scholar]
- 5.Preminger BA, Talmor M, Specht MC, Suzman M, Hoffman LA. The legacy of Icarus in the 21st century: report of a case of aggressive submental basal cell carcinoma resulting from frequent use of a metallic ultraviolet reflector. Ann Plast Surg. 2001;46:192–3. doi: 10.1097/00000637-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Cernea CR, Ferraz AR, de Castro IV, et al. p53 and skin carcinomas with skull base invasion: a case-control study. Otolaryngol Head Neck Surg. 2006;134:471–5. doi: 10.1016/j.otohns.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Villalon-Lopez JS, Valle-Mejia CA, Patino-Lara A, Moreno-Perez BA, Munoz-Lopez JA, Alcantar-Andrade A. Supraestructure maxillectomy and orbital exenteration for treatment of basal cell carcinoma of inferior eyelid: case report and review. J Cancer Res Ther. 2006;2:140–3. [PubMed] [Google Scholar]
- 8.Ansarin H, Daliri M, Soltani-Arabshahi R. Expression of p53 in aggressive and non-aggressive histologic variants of basal cell carcinoma. Eur J Dermatol. 2006;16:543–7. [PubMed] [Google Scholar]
- 9.Essers BA, Dirksen CD, Nieman FH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142:187–94. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- 10.Su SY, Giorlando F, Ek EW, Dieu T. Incomplete excision of basal cell carcinoma: a prospective trial. Plast Reconstr Surg. 2007;120:1240–8. doi: 10.1097/01.prs.0000279148.67766.e1. [DOI] [PubMed] [Google Scholar]
- 11.Bogelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Dermatol Venereol. 2007;87:330–4. doi: 10.2340/00015555-0236. [DOI] [PubMed] [Google Scholar]
- 12.Farhi D, Dupin N, Palangie A, Carlotti A, Avril MF. Incomplete excision of basal cell carcinoma: rate and associated factors among 362 consecutive cases. Dermatol Surg. 2007;33:1207–14. doi: 10.1111/j.1524-4725.2007.33255.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Orton CI, McWilliam LJ, Watson S. Incidence of incomplete excision in surgically treated basal cell carcinoma: a retrospective clinical audit. Br J Plast Surg. 2000;53:563–6. doi: 10.1054/bjps.2000.3394. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanov-Berezovsky A, Cohen AD, Glesinger R, Cagnano E, Krieger Y, Rosenberg L. Risk factors for incomplete excision of basal carcinomas. Acta Derm Venereol. 2004;84:44–7. doi: 10.1080/00015550310020585. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths RW. Audit of histologically incompletely excised basal cell carcinomas: recommendations for management by re-excision. Br J Plast Surg. 1999;52:24–8. doi: 10.1054/bjps.1998.3018. [DOI] [PubMed] [Google Scholar]
- 16.Nagore E, Grau C, Molinero J, Fortea JM. Positive margins in basal cell carcinoma: relationship to clinical features and recurrence risk. A retrospective study of 248 patients. J Eur Acad Dermatol Venereol. 2003;17:167–70. doi: 10.1046/j.1468-3083.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 17.Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177–83. doi: 10.1001/archderm.135.10.1177. [DOI] [PubMed] [Google Scholar]
- 18.Mogensen M, Jemec GB. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: a review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol Surg. 2007;33:1158–74. doi: 10.1111/j.1524-4725.2007.33251.x. [DOI] [PubMed] [Google Scholar]
- 19.Wortsman XC, Holm EA, Wulf HC, Jemec GB. Real-time spatial compound ultrasound imaging of skin. Skin Res Technol. 2004;10:23–31. doi: 10.1111/j.1600-0846.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 20.Wortsman X, Jemec GBE. [High resolution ultrasound applications in dermatology] Rev Chilena Dermatol. 2006;22:37–45 (in Spanish). [Google Scholar]
- 21.Schmid-Wendtner MH, Burgdorf W. Ultrasound scanning in dermatology. Arch Dermatol. 2005;141:217–24. doi: 10.1001/archderm.141.2.217. [DOI] [PubMed] [Google Scholar]
- 22.Lassau N, Spatz A, Avril MF, et al. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics. 1997;17:1559–65. doi: 10.1148/radiographics.17.6.9397463. [DOI] [PubMed] [Google Scholar]
- 23.Ruocco E, Argenziano G, Pellacani G, Seidenari S. Noninvasive imaging of skin tumors. Dermatol Surg. 2004;30((2 Pt 2):):301–10. doi: 10.1111/j.1524-4725.2004.30092.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Turnbull DH, Foster FS, et al. High frequency 40-MHz ultrasound. A possible noninvasive method for the assessment of the boundary of basal cell carcinomas. Dermatol Surg. 1996;22:131–6. doi: 10.1111/j.1524-4725.1996.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 25.Cammarota T, Pinto F, Magliaro A, Sarno A. Current uses of diagnostic high-frequency US in dermatology. Eur J Radiol. 1998;27(suppl 2):S215–23. doi: 10.1016/s0720-048x(98)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Rallan D, Harland CC. Ultrasound in dermatology—basic principles and applications. Clin Exp Dermatol. 2003;28:632–8. doi: 10.1046/j.1365-2230.2003.01405.x. [DOI] [PubMed] [Google Scholar]
- 27.Stiller MJ, Gropper CA, Shupack JL, Lizzi F, Driller J, Rorke M. Diagnostic ultrasound in dermatology: current uses and future potential. Cutis. 1994;53:44–8. [PubMed] [Google Scholar]
- 28.Fornage BD, McGavran MH, Duvic M, Waldron CA. Imaging of the skin with 20 MHz US. Radiology. 1993;189:69–76. doi: 10.1148/radiology.189.1.8372222. [DOI] [PubMed] [Google Scholar]
- 29.Clement A, Hoeffel C, Fayet P, et al. Value of high frequency (20 mHz) and Doppler ultrasound in the diagnosis of pigmented cutaneous tumors. J Radiol. 2001;82:563–71. [PubMed] [Google Scholar]
- 30.Jemec GB, Gniadecka M, Ulrich J. Ultrasound in dermatology. Part I. High frequency ultrasound. Eur J Dermatol. 2000;10:492–7. [PubMed] [Google Scholar]
- 31.Desai T, Desai A, Horowitz D, Kartono F, Wahl T. The use of high frequency ultrasound in the evaluation of superficial and nodular basal cell carcinomas. Dermatol Surg. 2007;33:1220–7. doi: 10.1111/j.1524-4725.2007.33257.x. [DOI] [PubMed] [Google Scholar]
- 32.Vaillant L, Grognard C, Machet L, et al. [High resolution ultrasound imaging: value in treatment of basocellular carcinoma by cryosurgery] Ann Dermatol Venereol. 1998;125:500–4. in French. [PubMed] [Google Scholar]
- 33.Humphrey S, Walsh NM, Delaney L, Propperova I, Langley RG. Prognostic significance of vascularity in cutaneous melanoma: pilot study using in vivo confocal scanning laser microscopy. J Cutan Med Surg. 2006;10:122–7. doi: 10.2310/7750.2006.00031. [DOI] [PubMed] [Google Scholar]
- 34.Wortsman X, Jemec GB. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340–2. doi: 10.1111/j.1524-4725.2007.33286.x. [DOI] [PubMed] [Google Scholar]
- 35.Machet L, Ossant F, Bleuzen A, Grégoire JM, Machet MC, Vaillant L. [High-resolution ultrasonography: utility in diagnosis, treatment, and monitoring dermatologic diseases] J Radiol. 2006;87((12 Pt 2):):1946–61. doi: 10.1016/s0221-0363(06)74180-4. in French. [DOI] [PubMed] [Google Scholar]
- 36.Solivetti FM, Di Luca Sidozzi A, Pirozzi G, Coscarella G, Brigida R, Eibenshutz L. Sonographic evaluation of clinically occult in-transit and satellite metastases from cutaneous malignant melanoma. Radiol Med (Torino) 2006;111:702–8. doi: 10.1007/s11547-006-0067-7. in English, Italian. [DOI] [PubMed] [Google Scholar]
- 37.Lassau N, Chami L, Peronneau P. [Imaging of melanoma: accuracy of ultrasonography before and after contrast injection for diagnostic and early evaluation of treatments] Bull Cancer. 2007;94:93–8. in French. [PubMed] [Google Scholar]
- 38.Essers BA, Dirksen CD, Nieman FH, et al. Cost-effectiveness of Mohs micrographic surgery vs surgical excision for basal cell carcinoma of the face. Arch Dermatol. 2006;142:187–94. doi: 10.1001/archderm.142.2.187. [DOI] [PubMed] [Google Scholar]
- 39.Uhara H, Hayashi K, Koga H, Saida T. Multiple hypersonographic spots in basal cell carcinoma. Dermatol Surg. 2007;30:1215–19. doi: 10.1111/j.1524-4725.2007.33256.x. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani E, Doro D, Milizia E, Steindler P. Recurrent eyelid basal cell carcinoma with sclerochoroidal infiltration: echographic findings. Ophthalmologica. 1998;212(Suppl 1):40–1. doi: 10.1159/000055421. [DOI] [PubMed] [Google Scholar]