Abstract
The qualitative ECG strain pattern of ST depression (STD) and T-wave inversion is strongly associated with coronary heart disease and left ventricular (LV) hypertrophy and is an independent predictor of new-onset heart failure in hypertensive patients. However, whether quantitative measures of STD in the lateral precordial predict new heart failure is unclear. Digital ECGs were examined in 2,059 American Indian participants in the second Strong Heart Study examination with no history of heart failure. The absolute magnitude of ST segment deviation was measured by computer to the nearest 5 μV in leads V5 and V6. During 5.7±1.4 years follow-up, heart failure developed in 77 participants (3.7%). Participants who developed heart failure had greater STD in leads V5 or V6 (−11±35 vs 12±27 μV, p<0.001) than those who did not. In univariate Cox analyses, STD was a significant predictor of new heart failure, with each 10 μV greater STD associated with a 31% greater risk of heart failure (hazard ratio [HR] 1.31, 95% CI 1.24-1.39). Increasing STD grouped according to quartiles was strongly associated with the development of heart failure, with step-wise increasing risk of heart failure compared with the lowest quartile of STD for the second (HR 2.39, 95% CI 0.77-7.40), third (HR 3.01, 95% CI 1.00-9.08) and fourth quartile of STD (HR 9.06, 95% CI 3.26-25.16). In Cox multivariate analyses controlling for age, gender, diabetes, coronary heart disease, albuminuria and for other baseline risk factors, STD remained a significant predictor of incident heart failure (HR 1.22, 95% CI 1.13-1.32, per 10 μV increment in STD, p<0.001). In conclusion, increasing STD in the lateral precordial leads is strongly associated with an increased risk of developing heart failure, independent of other risk factors for new heart failure.
Keywords: electrocardiogram, heart failure, hypertrophy
Computerized measurement of the degree of ECG ST depression (STD) in the lateral precordial leads has demonstrated that increasing magnitude of STD in leads V5 and/or V6 is associated with higher left ventricular (LV) mass and greater prevalence of anatomic LV hypertrophy (1). Although the magnitude of STD has been demonstrated to predict all-cause and cardiovascular mortality independent of the predictive value of ECG and echocardiographic LV hypertrophy (2), whether greater STD in the lateral precordial leads on the standard rest ECG is associated with increased risk of new-onset heart failure has not been examined. Therefore, the present study examined the relation of the magnitude of STD in the lateral precordial leads to the development of heart failure, adjusting for the severity of ECG LV hypertrophy and other potential risk factors for heart failure.
The Strong Heart Study is a population-based study of cardiovascular disease and its risk factors in American Indians from 13 communities in Arizona, Oklahoma, and North and South Dakota. Detailed information about the population, methods, and enrollment procedures for the study have been previously reported in detail (1-3). The current study examined 2059 participants in the second Strong Heart Study examination (64% women, mean age 59±8 years) with digital ECG records in sinus rhythm with no bundle branch block and no history of heart failure.
Digital 12-lead ECGs were performed as previously described (1,2). Absolute ST segment deviation was measured by computer at the midpoint of the ST segment on median complexes in leads V5 and V6 and participants divided into quartiles based on the maximal magnitude of ST deviation in these leads (1). ECG LV hypertrophy was determined using Cornell voltage duration product ([RaVL + SV3]*QRS duration) (4) and Sokolow-Lyon voltage criteria (SV1 + RV5/6) (5).
Development of heart failure was a prespecified endpoint in the Strong Heart Study (3), with the diagnosis of heart failure made using clinical and diagnostic findings based on Framingham criteria (6). Each case was reviewed and verified by investigators who were blinded to ECG STD results when classifying possible morbid events (3).
Data management and analysis were performed with SPSS version 12.0. Data are presented as mean±SD for continuous variables and proportions for categorical variables. Prevalences were compared using χ2 analyses and mean values of continuous variables were compared using two-sample t-tests. Heart failure rates were calculated and plotted for ST deviation quartiles according to the Kaplan-Meier product limit method and compared with the log-rank test. The relation of ST deviation to the risk of developing heart failure was assessed using Cox proportional hazards models. Hazard ratios for heart failure incidence were computed as the antilog of the estimated coefficient for ST deviation quartiles and per 10 μV of STD as the antilog of the product of this and the estimated coefficient. The 95% CI of each hazard ratio, Wald χ2 statistics and p-values were calculated. To test the independence of ST deviation as a predictor of new-onset heart failure, either STD as a continuous variable or quartiles of ST deviation were entered into multivariable Cox models that included as covariates significant univariate Cox predictors of new heart failure. A stepwise selection procedure was used to identify significant covariates.
Analyses were repeated stratifying the population by sex, age, diabetes, obesity, albuminuria and by the presence or absence of LV hypertrophy by Cornell product on the ECG. Interaction between the magnitude of ST deviation as a continuous variable and these variables was formally tested by adding cross-product terms of ST depression and these variables into the models in the total population. For all tests, a two-tailed p-value < 0.05 was required for statistical significance.
Results
During 5.7±1.4 years follow-up, heart failure developed in 77 participants (3.7%). Clinical and demographic characteristics of participants in relation to development of heart failure are shown in Table 1. Participants who developed heart failure were older, more likely to be female and diabetic, had greater baseline severity of ECG LV hypertrophy, higher fasting glucose, hemoglobin A1C, fibrinogen and triglyceride levels, greater albuminuria, higher systolic pressures and greater maximal STD in leads V5 or V6 than participants who did not develop heart failure, but were similar with respect to history of coronary heart disease, smoking status, body mass index, and C-reactive protein, low and high density lipoprotein cholesterol levels.
Table 1.
Demographic and Clinical Characteristics in Relation to Development of New-Onset Heart Failure
| Variables | Heart Failure | p value | |
|---|---|---|---|
| No (n=1982) | Yes (n=77) | ||
| Age (years) | 59±8 | 63±8 | <0.001 |
| Women | 63% | 75% | 0.030 |
| Diabetes | 51% | 87% | <0.001 |
| History of coronary heart disease | 3.3% | 7.8% | 0.076 |
| Body mass index (kg/m2) | 30.9±6.0 | 30.8±6.3 | 0.919 |
| Fasting glucose (mg/dL) | 153±80 | 206±95 | <0.001 |
| Hemoglobin A1C (%) | 6.9±2.5 | 8.5±2.3 | <0.001 |
| Fibrinogen (mg/dL) | 361±79 | 408±88 | <0.001 |
| C-reactive protein (mg/dL) | 6.5±9.5 | 7.4±10.1 | 0.436 |
| Low density lipoprotein cholesterol (mg/dL) | 120±34 | 120±40 | 0.923 |
| High density lipoprotein cholesterol (mg/dL) | 42±14 | 39±12 | 0.056 |
| Triglycerides (mg/dL) | 154±109 | 190±127 | 0.018 |
| Urine albumin/creatinine ratio (log mg/g) | 3.2±1.9 | 5.0±2.3 | <0.001 |
| Smoking status | 0.401 | ||
| Never | 29% | 25% | |
| Previous | 39% | 47% | |
| Current | 31% | 28% | |
| Systolic blood pressure (mm Hg) | 129±19 | 136±23 | 0.001 |
| Diastolic blood pressure (mm Hg) | 75±10 | 74±12 | 0.481 |
| Cornell voltage-duration product (mm•msec) | 1438±619 | 1731±846 | 0.003 |
| Sokolow-Lyon voltage (mm) | 20.3±6.1 | 23.7±9.0 | 0.001 |
| Maximal ST depression V5/V6 (μV) | 12±27 | −11±35 | <0.001 |
STD was a strong univariate predictor of new-onset heart failure, whether examined as a continuous variable or in increasing quartiles (Table 2 and Figure 1). In univariate Cox analyses, each 10 μV greater magnitude of STD in the lateral precordial leads was associated with a 31% greater risk of developing heart failure (Table 2). When participants were grouped in quartiles of STD, increasing magnitude of STD in the lateral precordial leads was associated with stepwise increasing incidence and risk of new heart failure (Table 2 and Figure 1). The 7-year actuarial incidence of heart failure was only 1.1% in participants in the first quartile of STD, 2.9% in those in the second quartile, 4.5% in participants in the third quartile, and increased to 10.7% among participants in the fourth quartile with STD of ≤ −5 μV (p<0.0001).
Table 2.
Univariate and Multivariable Cox Regression Analyses to Assess the Predictive Value of Electrocardiographic ST Segment Depression for the Development of New-Onset Heart Failure
| Predictor Variable | X2 | p value | Hazard Ratio | 95% CI |
|---|---|---|---|---|
| Univariate | ||||
| ST depression (per 10 μV) | 73.35 | <0.001 | 1.31 | 1.24 – 1.39 |
| ST depression quartiles | 36.76 | <0.001 | ||
| ≥ 25 μV | ---- | ---- | 1 | ---- |
| 10 to 24 μV | 2.27 | 0.132 | 2.39 | 0.77 – 7.40 |
| −4 to 9 μV | 3.84 | 0.050 | 3.01 | 1.00 – 9.08 |
| ≤−5 μV | 17.86 | <0.001 | 9.06 | 3.26 – 25.16 |
| Multivariable* | ||||
| ST depression (per 10 μV) | 31.06 | <0.001 | 1.22 | 1.13 – 1.32 |
| ST depression quartiles | 18.83 | <0.001 | ||
| ≥ 25 μV | ---- | ---- | 1 | ---- |
| 10 to 24 μV | 0.96 | 0.326 | 1.79 | 0.56 – 5.71 |
| −4 to 9 μV | 3.24 | 0.072 | 2.76 | 0.91 – 8.34 |
| ≤−5 μV | 10.39 | 0.001 | 5.55 | 1.96 – 15.74 |
age, prevalent diabetes and urine albumin/creatinine ratio also entered the multivariable Cox models
Figure 1.
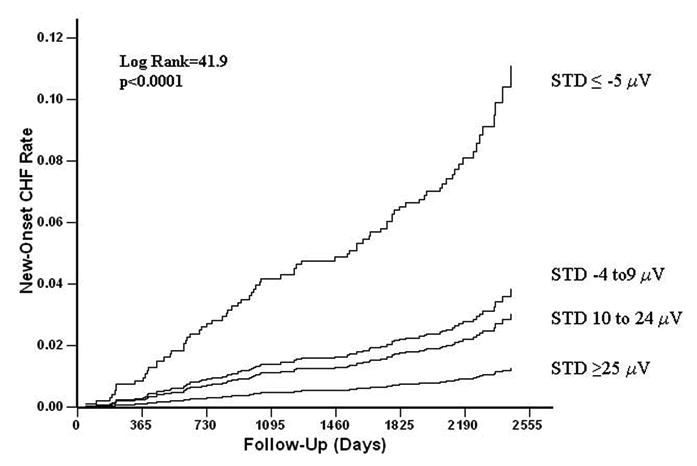
Kaplan-Meier curves comparing event rates between participants grouped according to maximal ST segment deviation in lead V5 or V6 for the development of heart failure. (CHF=congestive heart failure; STD=ST deviation)
Because patients who developed heart failure differed significantly from those who did not develop heart failure with respect to demographic and clinical variables which could affect outcome (Table 1), the independent relation of new-onset heart failure to the magnitude of STD was examined after adjusting for these variables. In step-wise multivariable Cox models (Table 2) in which age, diabetes and urine albumin/creatinine ratio were also significant predictors of heart failure, STD remained associated with new heart failure, whether STD was included as a continuous variable, with each 10 μV increment of STD associated with a 22% greater risk of developing heart failure, or examined as increasing quartiles, with a significantly increased risk of heart failure confined to participants in the highest quartile of STD who had a 5.6-fold greater risk of new heart failure.
The predictive value of lateral precordial STD for new-onset heart failure in relevant subsets of the population is examined in Table 3. The association between strain and new-onset heart failure was similar in participants grouped according to gender, age above and below 65 years, presence of diabetes, obesity, Cornell product LV hypertrophy on the ECG and according to the presence or absence of albuminuria, with nonsignificant interaction terms between STD and these variables. Of note, Sokolow-Lyon voltage LV hypertrophy was present in too small a number of the population (n=30, 1.5%) to allow for meaningful subgroup analysis.
Table 3.
Univariate Cox Analyses to Assess the Predictive Value of Electrocardiographic ST Depression for New-Onset Heart Failure in Relevant Subgroups of the Study Population
| Predictor Variable | New-Onset Heart Failure (n) | X2 | p value | Hazard Ratio* | 95% CI | P value for interaction |
|---|---|---|---|---|---|---|
| Sex | 0.774 | |||||
| Male (n=747) | 19 | 15.16 | <0.001 | 1.34 | 1.15 – 1.54 | |
| Female (n=1312) | 58 | 54.13 | <0.001 | 1.30 | 1.21 – 1.40 | |
| Body Mass Index (kg/m2) | 0.183 | |||||
| ≤ 30 (n=983) | 35 | 36.66 | <0.001 | 1.39 | 1.25 – 1.55 | |
| >30 (n=1076) | 42 | 33.07 | <0.001 | 1.27 | 1.18 – 1.38 | |
| Age (years) | 0.928 | |||||
| < 65 (n=1548) | 46 | 21.07 | <0.001 | 1.28 | 1.15 – 1.43 | |
| ≥ 65 (n=511) | 31 | 37.92 | <0.001 | 1.28 | 1.19 – 1.40 | |
| Diabetes | 0.978 | |||||
| No (n=979) | 10 | 4.90 | 0.027 | 1.28 | 1.03 – 1.60 | |
| Yes (n=1080) | 67 | 56.06 | <0.001 | 1.27 | 1.20 – 1.36 | |
| Cornell Product left ventricular hypertrophy | 0.429 | |||||
| No (n=1761) | 60 | 45.42 | <0.001 | 1.28 | 1.20 – 1.38 | |
| Yes (n=298) | 17 | 20.45 | <0.001 | 1.35 | 1.19 – 1.54 | |
| Albuminuria (mg/g) | 0.754 | |||||
| < 30 (n=1328) | 23 | 11.00 | 0.001 | 1.28 | 1.11 – 1.49 | |
| ≥ 30 (n=693) | 54 | 40.27 | <0.001 | 1.26 | 1.16 – 1.34 |
per 10 μV ST depression
Discussion
This study demonstrates that the presence of small but measurable increases in the magnitude of STD in the lateral leads of the standard resting ECG is associated with increased risk of developing new heart failure and that the increased risk of heart failure associated with STD persists after adjusting for other clinical predictors of new heart failure in this population. The present findings using a quantitative measure of STD that can be applied using standard computerized electrocardiography are supported by previous evidence of an association of the more qualitative ECG strain pattern with the development of heart failure (7).
The relation of a more qualitative assessment of lateral repolarization abnormalities on the ECG to the development of heart failure has not been extensively examined. Although a number of previous studies have demonstrated the predictive value of ECG LV hypertrophy criteria which include some measure of the qualitative ECG strain pattern in their determination for the development of heart failure (8-12), these studies did not examine the predictive value of strain alone. In addition, among studies that examined ECG strain alone for Cardiovascular outcomes (7,13-18), the relationship of ECG strain to developing heart failure was evaluated in only one (7). In a previous report on hypertensive patients with ECG LV hypertrophy in the LIFE study (7), typical strain on the baseline ECG was a significant predictor of new-onset heart failure and heart failure mortality. The predictive value of strain for heart failure outcomes in LIFE was independent of the possible impact of standard Cardiovascular risk factors, baseline and in-treatment severity of ECG LV hypertrophy by both Cornell product and Sokolow-Lyon criteria, baseline and in-treatment blood pressures, baseline demographic differences, and of randomized study treatment (7). In addition, the association between strain and new heart failure was similar in all relevant subsets of the LIFE Study population with the exception of patients with and without diabetes or microalbuminuria (7). However, quantitative measures of STD were not available in the LIFE study, precluding evaluation of the possible predictive value of lesser degrees of STD for new heart failure.
The present study extends these observations to a large, prospectively-studied population-based sample without preselection for LV hypertrophy or hypertension, confirming the strong association between lateral repolarization abnormalities and new heart failure and further demonstrating that gradual decreases in the amplitude of the ST segment in leads V5 and/or V6, even when above the isoelectric baseline, increase the risk of developing heart failure, independent of the predictive value of other risk factors for heart failure. In this context, it is important to note that the association between STD and heart failure in the current study does not reflect the impact of the typical strain pattern on the magnitude of STD, as only four participants had >0.1 mV (100 μV) of STD, which would be considered to reflect strain on the ECG. However, the greatest risk of developing heart failure was seen in those participants with true, although mostly minimal, STD (quartile 4), with STD of ≤ −5 μV associated with a 10.7% incidence of heart failure over 7 years, a more than 5.5-fold greater risk compared to the lowest quartile of STD after controlling for other risk factors. Moreover, STD remained a strong predictor of new heart failure when considered as a continuous variable, further demonstrating the predictive value of STD over the range of measured values. In addition, the association between STD and new heart failure was similar in all subgroups of the population (Table 3).
Several potential limitations should be considered with respect to these findings. First, it is unclear to what degree these findings in American Indians can be extrapolated to other ethnic populations. However, the demonstrated predictive value of ECG strain and minor degrees of STD for Cardiovascular morbidity and mortality in other populations (7,8,14-19) suggest that quantitative analysis of lateral STD will stratify the risk of developing heart failure in other populations as well. Second, the absence of information from serial ECGs precludes analysis of the impact of changes in STD on risk. The demonstrated relationship of changes in the strain pattern to Cardiovascular morbidity and mortality (16) suggest that the present findings may underestimate the true prognostic value of STD because it is likely that a proportion of participants will have new or worsening repolarization abnormalities over time. Further analysis of changing levels of lateral STD considered as time-varying covariates should provide useful insights into the value of serial analysis of STD for more accurately stratifying heart failure risk. Lastly, the relatively small number of incident heart failure events limits the power to detect significant interactions in this population.
Acknowledgments
Supported in part by grants HL-41642, HL-41652, HL-41654, and HL-65521 from the National Heart, Lung, and Blood Institute, Bethesda, MD and by grant M10RR0047-34 (GCRC) from the National Institutes of Health, Bethesda, MD.
The authors thank the Strong Heart Study participants, staff and coordinators. The views expressed in this article are those of the authors and do not necessarily reflect those of the Indian Health Service.
Footnotes
CONFLICTS OF INTEREST
Drs. Roman, Lee, Galloway and Best have no financial disclosures or conflicts relevant to this manuscript. Dr. Okin has received grant support from Merck & Co., Inc. Dr. Devereux has received grant support and honoraria from Merck & Co., Inc and is on an advisory committee for Merck. Dr. Howard is a consultant for Merck, the Egg Nutritional Council and General Mills, has received research support from donation of drugs from Pfizer, Merck and Schering-Plough, and has received honoraria for lectures for Schering-Plough.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter M. Okin, The Greenberg Division of Cardiology, Department of Medicine, Weill Medical College of Cornell University, New York, NY.
Mary J. Roman, The Greenberg Division of Cardiology, Department of Medicine, Weill Medical College of Cornell University, New York, NY.
Elisa T. Lee, College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
James M. Galloway, University of Arizona, Tucson, AZ.
Lyle G. Best, Missouri Breaks Industries Research Inc., Timber Lake, SD.
Barbara V. Howard, Medstar Research Institute, Washington, DC.
Richard B. Devereux, The Greenberg Division of Cardiology, Department of Medicine, Weill Medical College of Cornell University, New York, NY.
References
- 1.Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV. Quantitative assessment of electrocardiographic left ventricular strain predicts increased left ventricular mass: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1395–1400. doi: 10.1016/s0735-1097(02)02171-x. [DOI] [PubMed] [Google Scholar]
- 2.Okin PM, Roman MJ, Lee ET, Galloway JM, Howard BV, Devereux RB. Combined echocardiographic left ventricular hypertrophy and electrocardiographic ST depression improve prediction of mortality in American Indians: The Strong Heart Study. Hypertension. 2004;43:769–774. doi: 10.1161/01.HYP.0000118585.73688.c6. [DOI] [PubMed] [Google Scholar]
- 3.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le N, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study: a study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 4.Okin PM, Roman MJ, Devereux RB, Kligfield P. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25:417–423. doi: 10.1016/0735-1097(94)00371-v. [DOI] [PubMed] [Google Scholar]
- 5.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 6.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 7.Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Dahlöf B. Electrocardiographic strain pattern and prediction of new-onset congestive heart failure in hypertensive patients: The LIFE Study. Circulation. 2006;113:67–73. doi: 10.1161/CIRCULATIONAHA.105.569491. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB. Prevalence and natural history of electrocardiographic left ventricular hypertrophy. Am J Med. 1983;75 I:4–11. doi: 10.1016/0002-9343(83)90111-0. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 10.Aronow WS, Ahn C. Association of electrocardiographic left ventricular hypertrophy with the incidence of new congestive heart failure. J Am Geriatrics Soc. 1998;46:1280–1281. doi: 10.1111/j.1532-5415.1998.tb04546.x. [DOI] [PubMed] [Google Scholar]
- 11.Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991;67:295–299. doi: 10.1016/0002-9149(91)90562-y. [DOI] [PubMed] [Google Scholar]
- 12.Pope JH, Ruthazer R, Kontos M, Beshansky JR, Griffith JL, Selker HP. The impact of electrocardiographic left ventricular hypertrophy and bundle branch block on the triage and outcome of ED patients with a suspected acute coronary syndrome: a multicenter study. Am J Emer Med. 2004;22:156–163. doi: 10.1016/j.ajem.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow M, Perloff D. The prognosis of essential hypertension treated conservatively. Circulation. 1961;33:697–713. [Google Scholar]
- 14.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampo I, Porcellati C. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 1998;31:383–390. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 15.Okin PM, Devereux RB, Nieminen MS, et Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S, Snapinn S, Dahlöf B. Electrocardiographic strain pattern and prediction of cardiovascular morbidity and mortality in hypertensive patients. Hypertension. 2004;44:48–54. doi: 10.1161/01.HYP.0000132556.91792.6a. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 17.Sundström J, Lind L, Ärnlöv J, Zethelius B, Andrén B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CT, Dahlin J, Blackburn H, Scharling H, Appleyard M, Sigurd B, Schnohr P. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave: The Copenhagen City Heart Study. Eur Heart J. 2002;23:315–324. doi: 10.1053/euhj.2001.2774. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB. Prevalence and natural history of electrocardiographic left ventricular hypertrophy. Am J Med. 1970;72:813–822. doi: 10.1016/0002-9343(83)90111-0. [DOI] [PubMed] [Google Scholar]


