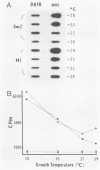
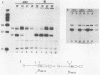
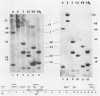
Abstract
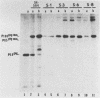
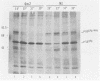
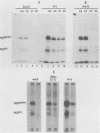
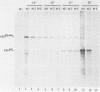
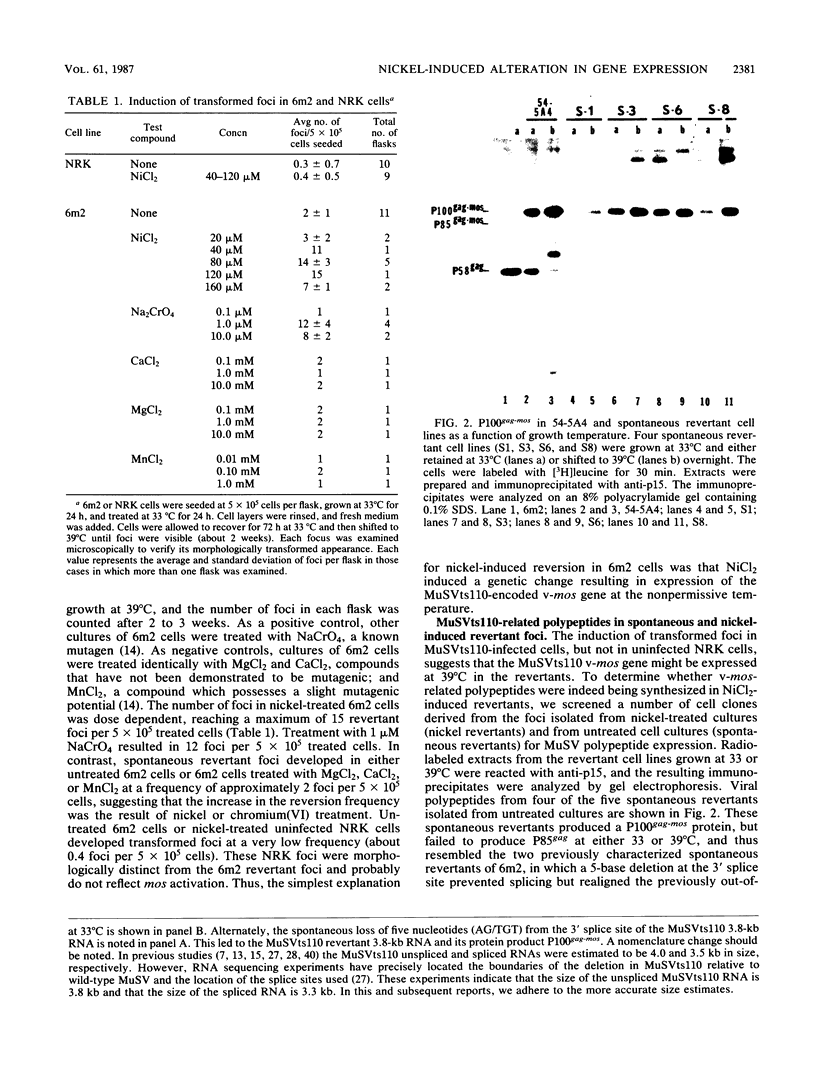
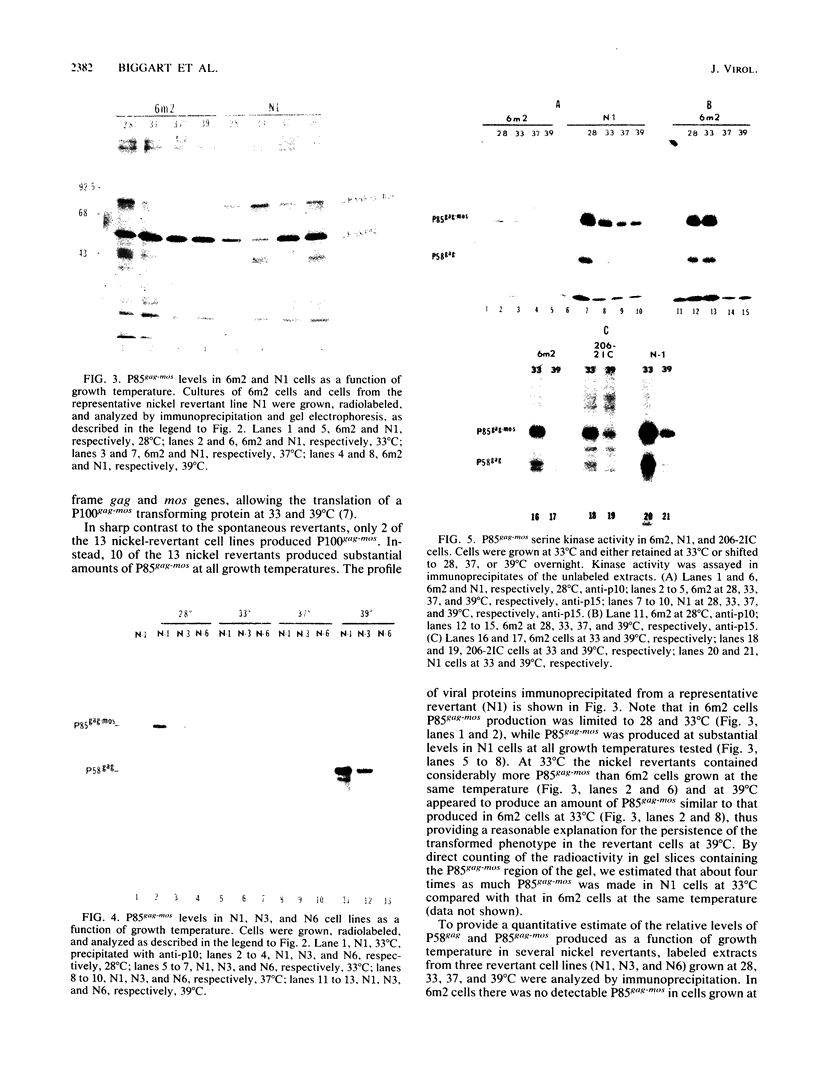
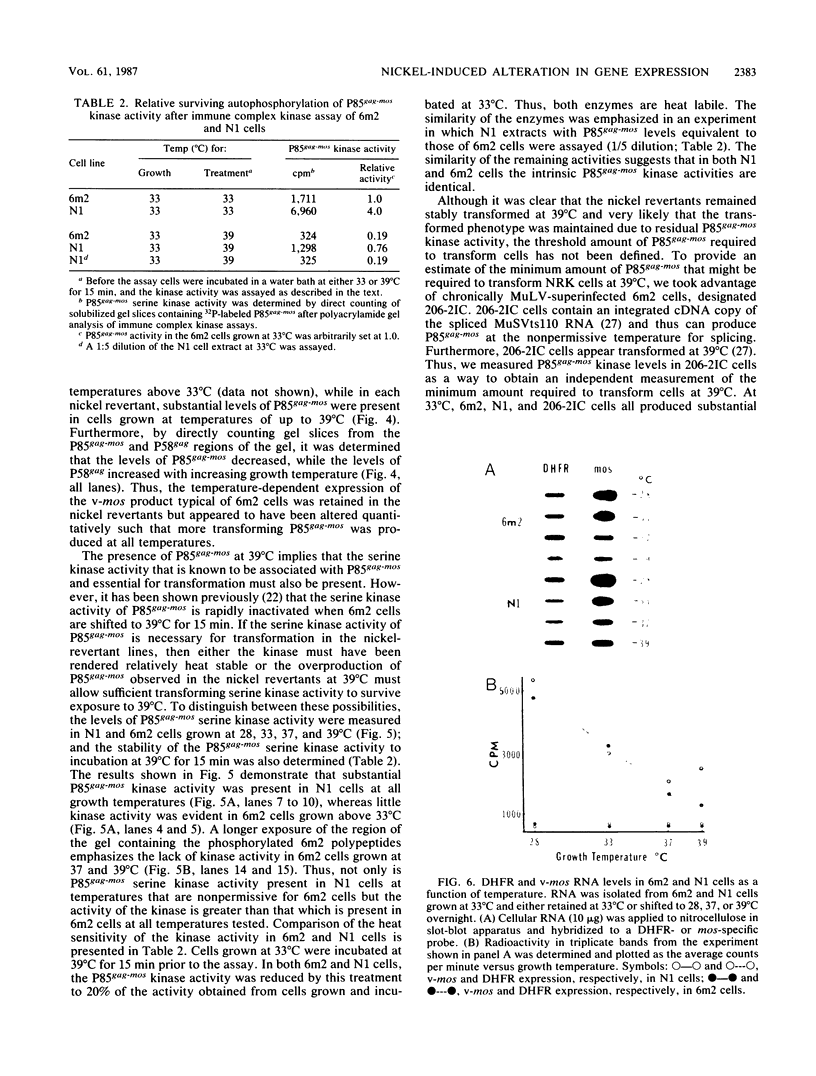
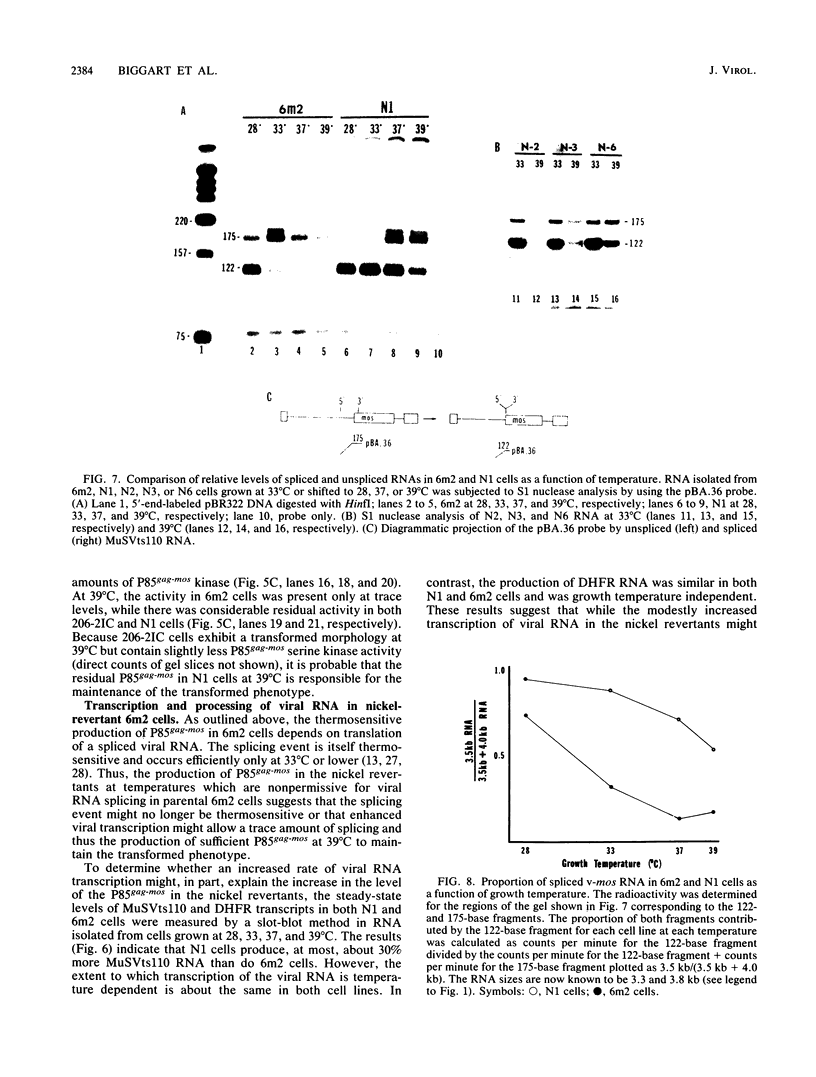
Determination of the mutagenic effects of carcinogenic nickel compounds has been difficult because, like many metals, nickel is poorly or nonmutagenic in procaryotic mutagenicity assays. We attempted to characterize nickel-induced genetic lesions by assessing the effect of nickel chloride on the conditionally defective expression of the v-mos transforming gene in normal rat kidney cells infected with the Murine sarcoma virus mutant ts110 (MuSVts110) retrovirus. MuSVts110 contains an out-of-frame gag gene-mos gene junction that prevents the expression of the v-mos gene at the nonpermissive temperature (39 degrees C). In MuSVts110-infected cells (6m2 cells) grown at 33 degrees C, however, this defect can be suppressed by a splicing event that restores the mos reading frame, allowing the expression of a gag-mos fusion protein which induces the transformed phenotype. The capacity to splice the viral transcript at 33 degrees C, but not at 39 degrees C, is an intrinsic property of the viral RNA. This property allowed us to target the MuSVts110 genome using a positive selection scheme whereby nickel was used to induce genetic changes which resulted in expression of the transformed phenotype at 39 degrees C. We treated 6m2 cells with NiCl2 and isolated foci consisting of cells which had reverted to the transformed phenotype at 39 degrees C. We found that brief nickel treatment increased the reversion frequency of 6m2 cells grown at 39 degrees C sevenfold over the spontaneous reversion frequency. The nickel-induced revertants displayed the following heritable characteristics: They stably maintained the transformed phenotype at 39 degrees C; unlike the MuSVts110 RNA in 6m2 cells, the nickel-induced revertant viral RNA could be spliced efficiently at 39 degrees C; as a consequence of the enhanced accumulation of spliced viral RNA, the nickel-induced revertants produced substantial amounts of the transforming v-mos protein P85gag-mos at 39 degrees C; the nickel-induced revertant P85gag-mos serine kinase, like the parental 6m2 P85gag-mos kinase, was found to be rapidly inactivated at 39 degrees C; however, in the nickel-induced revertants, overproduction of P85gag-mos allowed the transformed state to be maintained; and even though viral RNA processing was much changed, no rearrangements of the viral DNA in the nickel-induced revertant cells were detected by partial restriction analysis.
Full text
PDF

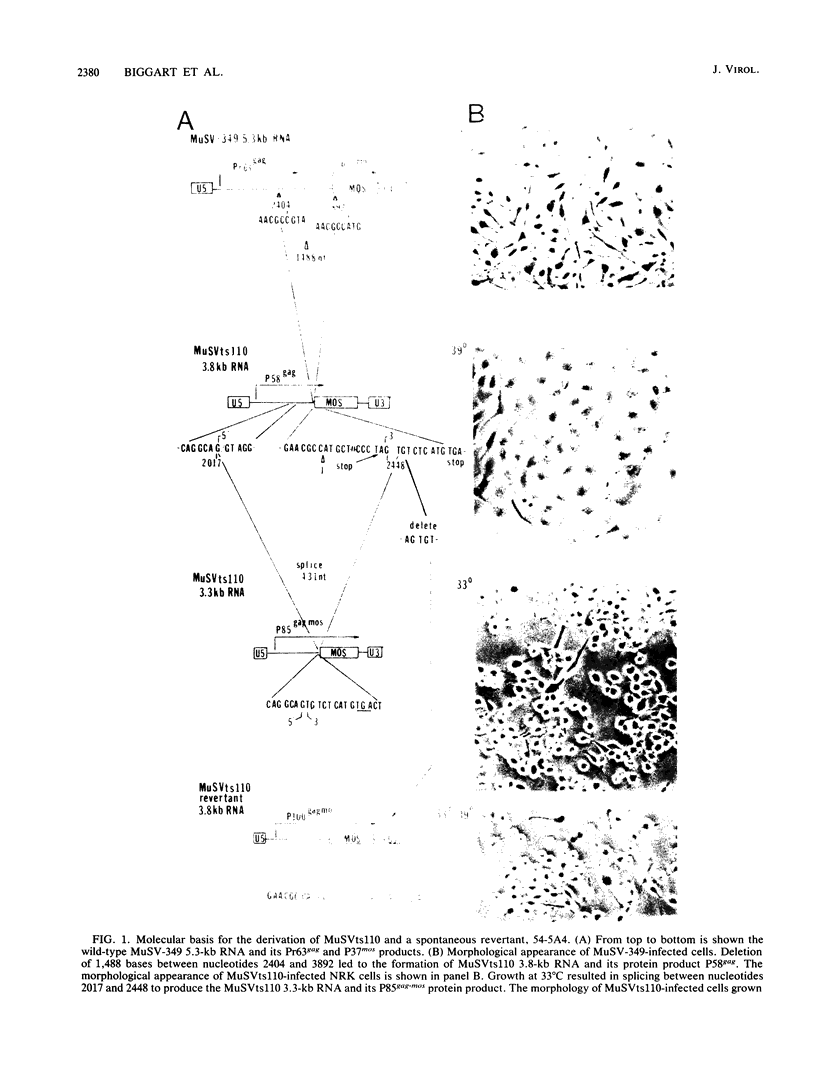



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Amacher D. E., Paillet S. C. Induction of trifluorothymidine-resistant mutants by metal ions in L5178Y/TK+/- cells. Mutat Res. 1980 Jul;78(3):279–288. doi: 10.1016/0165-1218(80)90110-x. [DOI] [PubMed] [Google Scholar]
- Arlauskas A., Baker R. S., Bonin A. M., Tandon R. K., Crisp P. T., Ellis J. Mutagenicity of metal ions in bacteria. Environ Res. 1985 Apr;36(2):379–388. doi: 10.1016/0013-9351(85)90032-5. [DOI] [PubMed] [Google Scholar]
- Biggart N. W., Costa M. Assessment of the uptake and mutagenicity of nickel chloride in salmonella tester strains. Mutat Res. 1986 Dec;175(4):209–215. doi: 10.1016/0165-7992(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R. B., Wetterhahn K. E. Nickel-bound chromatin, nucleic acids, and nuclear proteins from kidney and liver of rats treated with nickel carbonate in vivo. Cancer Res. 1984 Sep;44(9):3892–3897. [PubMed] [Google Scholar]
- Cizdziel P. E., Nash M. A., Blair D. G., Murphy E. C., Jr Molecular basis underlying phenotypic revertants of Moloney murine sarcoma virus MuSVts110. J Virol. 1986 Jan;57(1):310–317. doi: 10.1128/jvi.57.1.310-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986 Dec;6(12):4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Muriel W. J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat Res. 1976 Feb;38(1):3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Brizzard B. L., Nash M. A., Murphy E. C., Jr, Arlinghaus R. B. Temperature-sensitive viral RNA expression in Moloney murine sarcoma virus ts110-infected cells. J Virol. 1985 Feb;53(2):616–623. doi: 10.1128/jvi.53.2.616-623.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Hwang L. S., Park J., Gilboa E. Role of intron-contained sequences in formation of moloney murine leukemia virus env mRNA. Mol Cell Biol. 1984 Nov;4(11):2289–2297. doi: 10.1128/mcb.4.11.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D. J., Willis R. C., Friedmann T. Variable stability of a selectable provirus after retroviral vector gene transfer into human cells. Mol Cell Biol. 1986 Apr;6(4):1141–1147. doi: 10.1128/mcb.6.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu N., Hara M., Kada T. Rec assay and mutagenicity studies on metal compounds. Mutat Res. 1980 Feb;77(2):109–116. doi: 10.1016/0165-1218(80)90127-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kloetzer W. S., Arlinghaus R. B. Binding of retrovirus-associated protein kinase and proteins to Staphylococcus aureus. J Gen Virol. 1982 Jun;60(Pt 2):365–370. doi: 10.1099/0022-1317-60-2-365. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. Further characterization of the P85gag-mos -associated protein kinase activity. Virology. 1984 Oct 15;138(1):143–155. doi: 10.1016/0042-6822(84)90154-5. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S. A., Arlinghaus R. B. Use of site-specific antipeptide antibodies to perturb the serine kinase catalytic activity of p37mos. J Virol. 1985 Sep;55(3):874–876. doi: 10.1128/jvi.55.3.874-876.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. Insertion of several different DNAs in reticuloendotheliosis virus strain T suppresses transformation by reducing the amount of subgenomic mRNA. J Virol. 1986 Apr;58(1):75–80. doi: 10.1128/jvi.58.1.75-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki M., Akamatsu N., Ono T., Koyama H. Mutagenicity of metal cations in cultured cells from Chinese hamster. Mutat Res. 1979 Nov;68(3):259–263. doi: 10.1016/0165-1218(79)90157-5. [DOI] [PubMed] [Google Scholar]
- Nash M. A., Brizzard B. L., Wong J. L., Murphy E. C., Jr Murine sarcoma virus ts110 RNA transcripts: origin from a single proviral DNA and sequence of the gag-mos junctions in both the precursor and spliced viral RNAs. J Virol. 1985 Feb;53(2):624–633. doi: 10.1128/jvi.53.2.624-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975 Jun;31(3):185–189. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Sugiyama M., Basilion J. P., Costa M. Preferential DNA-protein cross-linking by NiCl2 in magnesium-insoluble regions of fractionated Chinese hamster ovary cell chromatin. Cancer Res. 1985 Nov;45(11 Pt 2):5787–5794. [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Sen P., Costa M. Incidence and localization of sister chromatid exchanges induced by nickel and chromium compounds. Carcinogenesis. 1986 Sep;7(9):1527–1533. doi: 10.1093/carcin/7.9.1527. [DOI] [PubMed] [Google Scholar]
- Sen P., Costa M. Induction of chromosomal damage in Chinese hamster ovary cells by soluble and particulate nickel compounds: preferential fragmentation of the heterochromatic long arm of the X-chromosome by carcinogenic crystalline NiS particles. Cancer Res. 1985 May;45(5):2320–2325. [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. Alternative splicing caused by RNA secondary structure. Cell. 1985 Dec;43(3 Pt 2):667–676. doi: 10.1016/0092-8674(85)90239-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Horn J. P., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. Gag-mos Polyproteins encoded by variants of the Moloney strain of mouse sarcoma virus. Virology. 1983 Apr 15;126(1):336–347. doi: 10.1016/0042-6822(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr Recent research on nickel carcinogenesis. Environ Health Perspect. 1981 Aug;40:131–141. doi: 10.1289/ehp.8140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso W. W., Fung W. P. Mutagenicity of metallic cations. Toxicol Lett. 1981 Jun-Jul;8(4-5):195–200. doi: 10.1016/0378-4274(81)90100-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]