Abstract
Oxidative damage to proteins such as apolipoprotein B100 increases the atherogenicity of low density lipoproteins (LDL). However, little is known about the potential oxidative damage to apolipoprotein E (apoE), an exchangeable anti-atherogenic apolipoprotein. ApoE plays an integral role in lipoprotein metabolism by regulating the plasma cholesterol and triglyceride levels. Hepatic uptake of lipoproteins is facilitated by apoE's ability to bind with cell surface heparan sulfate proteoglycans and to lipoprotein receptors via basic residues in its 22 kDa N-terminal domain (NT). We investigated the effect of acrolein, an aldehydic product of endogenous lipid peroxidation and a tobacco smoke component, on the conformation and function of recombinant human apoE3-NT. Acrolein caused oxidative modification of apoE3-NT as detected by Western blot with acrolein-lysine-specific antibodies, and tertiary conformational alterations. Acrolein-modification impairs the ability of apoE3-NT to interact with heparin and the LDL receptor. Furthermore, acrolein-modified apoE3-NT displayed a 5-fold decrease in its ability to interact with lipid surfaces. Our data indicate that acrolein disrupts the functional integrity of apoE3, which likely interferes with its role in regulating plasma cholesterol homeostasis. These observations have implications regarding the role of apoE in the pathogenesis of smoking- and oxidative stress-mediated cardiovascular and cerebrovascular diseases.
Keywords: Cardiovascular disease, apolipoprotein E, acrolein, tobacco smoke, oxidative stress, aging, LDL receptor, heparan sulfate proteoglycan, lysine modification
Oxidative stress is recognized as a major factor in the onset of atherosclerosis, cardiovascular disease and stroke in humans; of several factors that lead to increased oxidative stress, aging and life style (diet, smoking) play crucial roles. Atherosclerosis is characterized by the intracellular and extra cellular deposition of low density lipoproteins (LDL) in macrophages (1). Oxidative modification of plasma LDL leads to their uptake by scavenger receptors located on cell surfaces of macrophages; subsequent accumulation of oxidized LDL converts the macrophages to lipid-loaded foam cells that are deposited as fatty streaks in the vasculature, a signature feature of atherosclerotic plaques (2). While oxidized LDL is a significant contributor to the etiology of plaque formation, little is known about the role of oxidative modification of apolipoprotein E (apoE), an exchangeable amphipathic apolipoprotein that is considered a major anti-atherogenic protein. ApoE plays a crucial role in regulating plasma and cellular cholesterol and triglyceride homeostasis (3).
The role of apoE in cholesterol metabolism emerged from studies with transgenic mice over expressing apoE, which manifested decreased plasma cholesterol levels on a chow diet and a marked resistance to hypercholesterolemia when fed a cholesterol rich diet (4). On the other hand, apoE-deficient subjects develop symptoms of type III hyperlipoproteinemia (5), with elevated plasma cholesterol levels and accumulation of particles bearing size in the very low density lipoprotein (VLDL) and intermediate density lipoprotein (IDL) range (6,7). Further, inactivation of apoE by gene targeting leads to accumulation of cholesterol-rich remnant lipoproteins and spontaneous atherosclerotic lesions in mice (8,9). Taken together, these early studies have established the key role of apoE in regulating plasma cholesterol and triglyceride levels, which are recognized as indicators of heart disease. The focus of this study is to investigate the role of age- and lifestyle-related oxidative stress on the structural and functional integrity of apoE at a molecular level and the potential physiological implications of the process.
ApoE is located on a sub-class of high density lipoproteins (HDL), VLDL, IDL and chylomicron remnants (10). It belongs to the family of exchangeable amphipathic apolipoproteins that display the ability to exist in lipid-free and lipid-bound states. Human apoE is composed of 299 amino acids, folded into two domains: a 22 kDa N-terminal domain (NT) comprising residues 1-191, and a 10 kDa C-terminal domain (CT), comprising residues 210-299 (11,12). The two domains are linked via a protease sensitive loop. The main functions of apoE are: (i) to serve as a ligand for the cell surface localized lipoprotein receptors, which facilitate the uptake and clearance of lipoprotein particles via endocytosis (reviewed in 10), and, (ii) to interact with heparan sulfate proteoglycans (HSPG) on the cell surface, which also leads to the uptake of the lipoproteins (13). The binding of apoE to HSPG represents a crucial step in the transfer and internalization of lipoproteins by the hepatocytes, either for the direct uptake mechanism or for optimal presentation to the lipoprotein receptors (13-16). These pathways constitute a critical role for apoE in the clearance of VLDL, IDL and chylomicron remnants, thereby regulating the plasma lipoprotein homeostasis. Uptake of apoE-containing lipoproteins by the LDL receptor (LDLr) involves an initial binding interaction that requires the presentation of positively charged residues (17) in an optimal arrangement in the N-terminal domain of apoE3 (10,18). Thus, processes that affect the chemistry of the basic residues can be expected to alter the specific binding interaction between apoE/HSPG, and apoE/LDLr.
In the present study, we investigated the effect of acrolein, a highly reactive aldehyde present in cigarette smoke, heated oils and generated as a metabolite of age-related oxidative stress, on apoE3. Since the high resolution structural information is available only for the isolated human apoE NT domain (18), we employed apoE3 (residues 1-191), which encapsulates the entire NT domain (apoE3-NT). Importantly, the NT domain recapitulates the LDLr and the high-affinity HSPG binding activity of the intact protein, is well characterized (10,19-22) and exists as a stable independently folded unit. Employing recombinant human protein, we demonstrate that acrolein severely compromises the functional integrity of apoE3-NT in terms of heparin binding, lipid binding and LDLr binding interaction.
Experimental Procedures
Materials
1-anilinonaphthalene-8-sulfonic acid (ANS), fluorescein 5(6)-isothiocyanate, 2-propenal (acrolein) and anti-c-Myc-agarose affinity gel were obtained from Sigma-Aldrich. 5-iodoacetamidofluorescein was from Invitrogen (Molecular Probes). Anti-acrolein-lysine antibody mAb5F6 was generated as previously described (23). Anti-human apoE monoclonal antibodies (1D7 and 6C5) were obtained from the Ottawa Heart Institute Research Corporation (Ottawa, Canada). Dimyristoylphosphatidylcholine (DMPC) was obtained from Avanti-Polar Lipids (Alabaster, AL). All chemicals and solvents were of analytical grade.
Expression, isolation, and purification of apoE3-NT
Wild type (WT) human recombinant apoE3-NT, apoE2-NT and Lys143Ala/Lys146Ala/apoE3-NT bearing a hexa-His tag at the N-terminal end, was expressed in Escherichia coli as described earlier (22, 24). Following induction with 0.5 mM isopropyl 1-thio-β-D-galactoside, apoE3-NT was isolated and purified under denaturing conditions from cell lysates using a His-Trap HP column (Amersham). A Detergent Compatible protein assay (BioRad) was carried out to estimate the protein concentration.
Modification of apoE3-NT
One mg of apoE3-NT was incubated with acrolein (1:10 molar ratio, apoE3-NT: acrolein) in 20 mM sodium phosphate, pH 7.4 at 37 °C for 4 h. In control reactions, apoE3-NT was incubated as such, with no added acrolein. Excess un-reacted acrolein was removed by extensive dialysis against 20 mM sodium phosphate pH 7.4 for 48 h with 3 changes.
Western Blot
Western blot analysis of acrolein-modified apoE was carried out following SDS-PAGE on a 4-20% acrylamide gradient gel with anti-apoE antibody mAb1D7 or mAb6C5, and an antibody specific for acrolein-lysine adducts, mAb5F6 (24,25) and horseradish peroxidase-conjugated antimouse IgG (Chemicon). ECL western blot detection system was used to detect chemiluminescence (GE Healthcare).
Secondary structure determination
Prior to secondary structure measurement by circular dichroism (CD) or infrared spectroscopy, the samples were dialyzed against 20 mM sodium phosphate, pH 7.4. The CD spectra of unmodified and acrolein-modified apoE3-NT were recorded in a 0.05 cm path length cuvette on a Jasco-710 spectropolarimeter maintained at 24 °C. The average of four scans were obtained from spectra recorded between 185 and 260 nm at 50 nm/min with a 1 nm slit width and a time constant of 0.5 s for a nominal resolution of 1.7 nm. Spectra were analyzed for secondary structure using the CDPRO analysis software (26). The secondary structure of unmodified and acrolein-modified apoE3-NT was independently confirmed by infrared spectroscopy (27).
Fluorescence spectroscopy
To assess if acrolein modification causes tertiary conformational changes in apoE3-NT, two independent fluorescence spectroscopic approaches were employed using a LS50B Perking Elmer fluorimeter: (i) monitor changes in the intrinsic fluorescence emission intensity of the protein contributed by Trp residues, and, (ii) evaluate changes in the fluorescence emission of an extrinsic probe that localizes to hydrophobic “patches” on proteins (28,29). In the first approach, Trp fluorescence emission spectra of unmodified or acrolein-modified apoE3-NT were recorded between 300 and 500 nm following excitation of 35 μg protein in 20 mM Tris-HCl, pH 7.4 containing 150 mM NaCl (Tris-buffered saline, TBS) at 295 nm. The excitation and emission slit widths were set at 3nm. In the second approach, 250 μM 1-anilinonaphthalene-8-sulfonic acid (ANS) was mixed with 6 μM unmodified or acrolein-modified apoE3-NT in TBS and the fluorescence emission spectra recorded between 400 and 600 nm at an excitation wavelength at 395 nm.
Heparin binding assay
The heparin binding ability of acrolein-modified apoE3-NT was assessed as previously described using 1 ml heparin-Sepharose columns (Amersham) in the ÄKTA FPLC system (29). A solution of 300 μg of the unmodified or acrolein-modified apoE3-NT in 20 mM phosphate buffer, pH 7.4 was injected onto a 1 ml Hi-Trap heparin-Sepharose column, at a flow rate of 0.5 ml/min. The bound samples were eluted via an increasing salt gradient (0-1 M NaCl).
Lipid binding assay
A hallmark feature of apolipoproteins is their ability to convert large phospholipid vesicles into small bilayer discoidal complexes. This ability has been used as a reliable and sensitive assay to evaluate lipid binding ability of apolipoproteins (30,31) in terms of the initial kinetics of transformation. Briefly, 1 ml TBS was added to a thin film of 5 mg DMPC, followed by vigorous vortexing to generate multi lamellar vesicles (MLVs). DMPC vesicles (100 μg) were equilibrated at 24 °C, and then mixed with unmodified or acrolein-modified apoE3-NT (200 μg). The change in absorbance due to the transformation of MLVs to discoidal complexes was followed as a decrease in turbidity at 325 nm in a Perkin Elmer UV/VIS spectrophotometer maintained at 24 °C in a Peltier controlled (PTP-6) cell holder.
DMPC/apoE3-NT complex preparation
DMPC/apoE-NT complexes were prepared as described earlier (20). Following incubation of 1 mg apoE3-NT with 2.5 mg DMPC small unilamellar vesicles (prepared by sonication of MLVs) at 24 °C for 16 h, lipid-bound protein was separated from the unbound protein by density gradient ultracentrifugation using a KBr gradient. The DMPC/apoE3-NT complexes were characterized in terms of particle size and homogeneity, lipid and protein composition as described earlier (20,32).
LDLr binding assay
To assess whether acrolein modification affected the LDLr binding activity of apoE3-NT, we performed an immunoprecipitation (IP) analysis, as described earlier (33). A construct bearing the LDLr ligand binding domains 3-6 (residues Lys86 to Asn251 of the mature LDLr) with a c-Myc epitope was employed (construct provided by Drs Stephen Blacklow and Carl Fisher, Brigham and Women's Hospital, Boston). This construct represents the essential ligand binding elements of the extra cellular soluble portion of the LDLr (designated as sLDLr/LA3-LA6/Myc). sLDLr/LA3-LA6/Myc was expressed, isolated and purified as described previously (33) and the folding behavior of the purified protein was optimized. DMPC/acrolein-modified apoE3-NT (1 and 3 μg protein) was incubated with 1 μg of sLDLr/LA3-LA6, followed by IP with an anti-c-Myc agarose affinity gel. LDLr-bound apoE was detected by Western blot using anti-apoE monoclonal antibody 1D7. In addition, DMPC/unmodified apoE3-NT was used as a positive control, while DMPC/Lys143Ala/Lys146Ala/apoE3-NT, and DMPC/apoE2, were used as negative controls. Duplicate gels were analyzed with an anti-c-Myc antibody (9E10) to confirm the presence of similar levels of LDLr in each reaction mixture.
Results
The objective of this study was to investigate if acrolein modifies apoE3 and, if acrolein modification causes functional impairment of apoE3. Acrolein is an aldehydic compound that is a major component of tobacco smoke and a product of age-related lipid peroxidation. We studied acrolein modification of apoE3-NT, the structural organization of which is represented in Figure 1.
Figure 1. Ribbon structure of apoE3-NT.
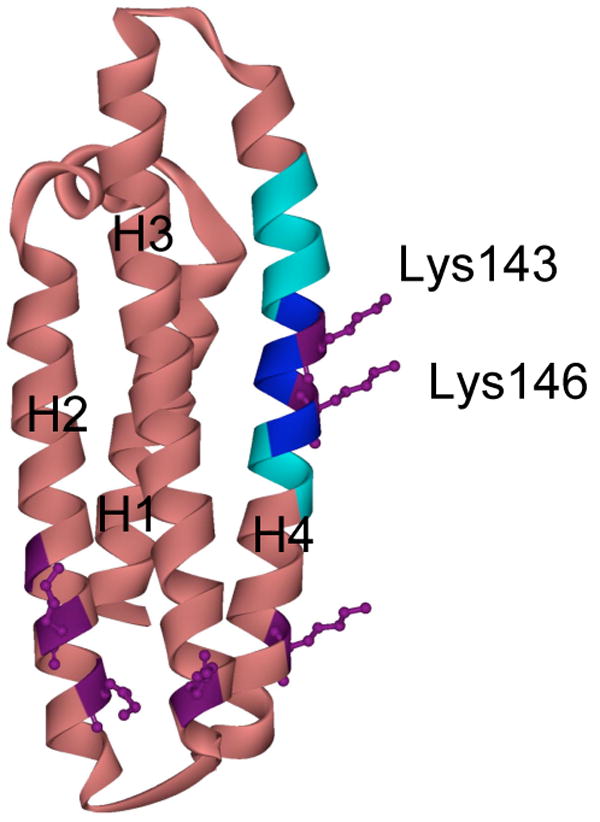
ApoE3-NT is comprised of 4 long amphipathic α-helices (labeled H1 to H4) that are folded into a helix bundle structure (rose) (18). The heparin binding site (dark blue) (residues 142-147, including Lys143 and Lys146 in purple) and the lipoprotein receptor binding site (light blue) (residues 136-150) are located on helix 4. The side chains of the other lysine residues visible in the crystal structure are indicated in purple.
ApoE3-NT was incubated with a 10-fold molar excess of acrolein followed by extensive dialysis against TBS to remove unbound acrolein. ApoE3-NT bears 8 Lys and 1 Cys residue: thus we carried out all our studies with a 1:10 apoE3-NT:acrolein molar ratio (thereby providing an acrolein molecule for every potential modification site). SDS-PAGE analysis under reducing conditions indicated the presence of monomers, cross-linked dimers and some oligomers in acrolein-modified apoE3-NT, Figure 2, Panel A. Western blot analysis was carried out using anti-apoE antibody (mAb1D7), Panel B, which indicates that the 1D7 epitope on apoE3-NT (which overlaps with the LDLr and heparin binding sites on helix 4) was unaffected by acrolein modification. In other studies, use of anti-apoE antibody mAb6C5 (epitope located at the amino terminus of apoE) as an alternative indicated that the 6C5 epitope was unaffected as well by acrolein modification (data not shown). Further, acrolein modification of apoE3-NT generated epitopes that are recognized by mAb5F6, the anti-acrolein-lysine antibody, Panel C. In control experiments, unmodified apoE3-NT was not detected by mAb5F6. We conclude that acrolein modification leads to intra- and inter-molecular crosslinking in apoE3, and that the lysine residues are likely targets.
Figure 2. SDS-PAGE and Western blot analyses of unmodified and acrolein-modified apoE3-NT.
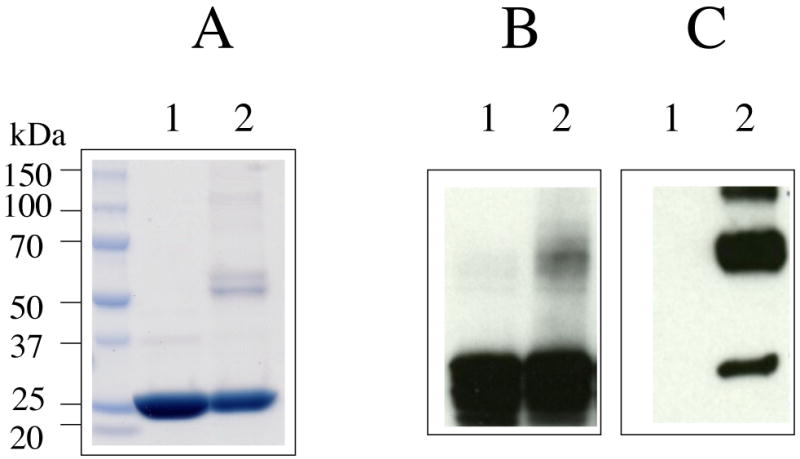
ApoE3-NT (50 μM) was incubated without or with 0.5 mM acrolein for 4 h at 37 °C. Unmodified and acrolein-modified apoE3-NT (lanes 1 and 2, respectively) were analyzed by SDS-PAGE (Panel A) and Western blot using anti-apoE antibody mAb1D7 (Panel B) or anti-acrolein-lysine antibody mAb5F6 (Panel C).
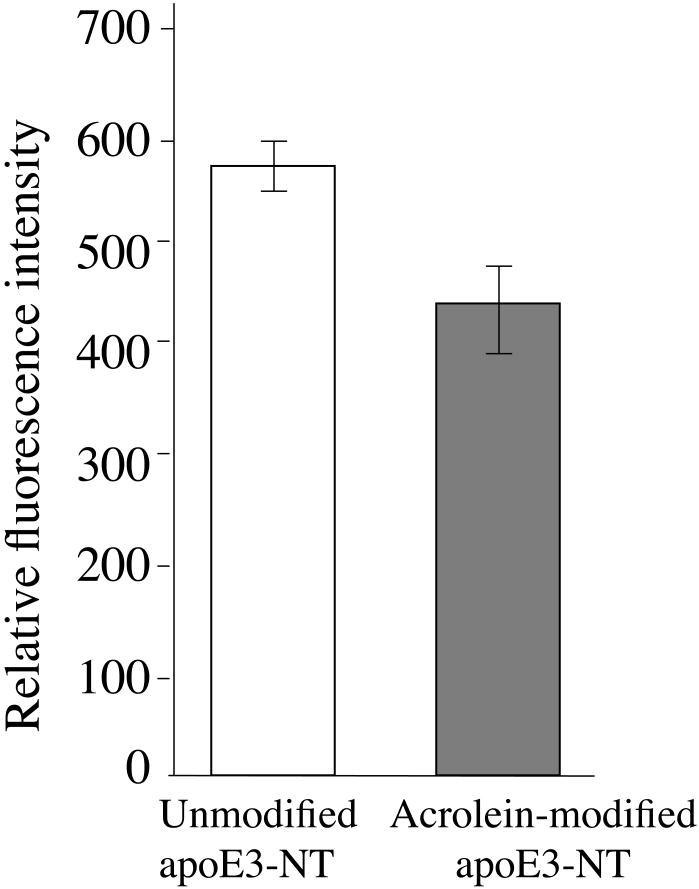
To independently confirm that the lysine residues are affected, we compared unmodified and acrolein-modified apoE3-NT for the availability of lysine residues for subsequent modification by a fluorescent probe bearing amine-reactive functional group, Figure 3 (34). Unmodified or acrolein-modified apoE3-NT were incubated with fluorescein isothiocyanate at 37 °C for 10 min, followed by extensive dialysis to remove the unbound probe. The fluorescence emission spectra of the labeled samples were recorded and their fluorescence emission intensities at 517 nm were compared. A 25% decrease in the relative fluorescence emission intensity of acrolein-modified apoE3-NT compared to unmodified apoE3-NT was noted. This suggests lesser availability of the ε-amino groups of lysine residues for interaction with the isothiocyanate functional group in acrolein-modified apoE3-NT.
Figure 3. Modification of unmodified and acrolein-modified apoE3-NT by fluorescein isothiocyanate.
Unmodified (white bar) and acrolein-modified (grey) apoE3-NT (100 μg protein) were modified with 10-fold molar excess of fluorescein 5(6)-isothiocyanate. The reaction was carried out in 20 mM Tris-HCl, pH 8.0 for 10 min at 37 °C to label accessible lysine residues. Following modification, the samples were dialyzed against TBS to remove excess probe. Fluorescein fluorescence emission intensity was recorded at 517 nm following excitation at 495 nm. Values are average ± SD (n=3). Statistical analysis was carried out by Student's t test (P<0.01).
In the next step, we investigated if acrolein modification affected the secondary structure of apoE3-NT by CD and infrared analyses. CD analysis (Figure 4) revealed that both unmodified and acrolein-modified apoE3-NT are highly structured with the minima at 222 and 209 nm indicative of an alpha-helical structure. The spectra were analyzed for secondary structure content with the CDPRO software (Selcon3, Continll and Cdsstr with the protein reference set 7 having 48 proteins), which revealed the presence of 60% α-helix, 4% β-strand, 12% β-turn and 23% random coil structures for unmodified apoE3-NT, and 54% α-helix, 7% β-strand, 13% turns, and 26% random coil for acrolein-modified apoE3-NT. These percentages do not reveal any significant secondary structural change of apoE3 NT-upon acrolein modification. Infrared analysis confirmed these observations (data not shown).
Figure 4. Effect of acrolein modification on secondary structural characteristics of apoE3-NT.
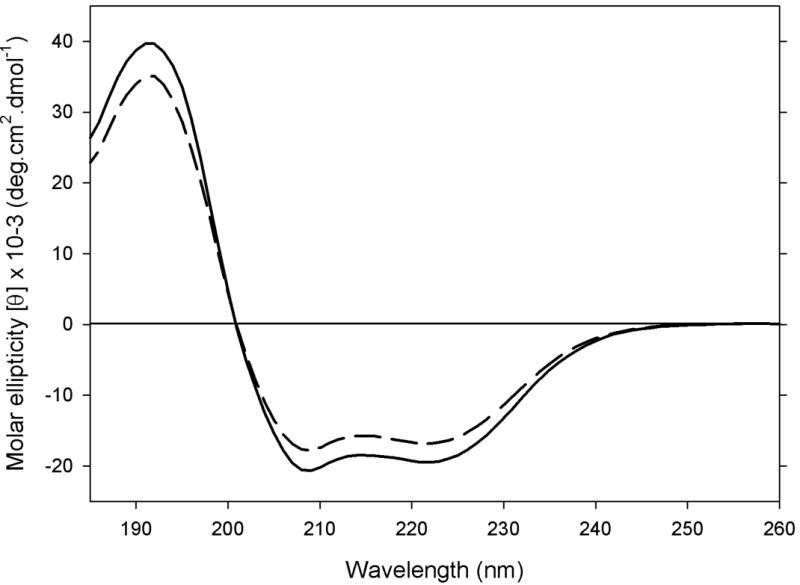
CD spectra of 0.2 mg/ml unmodified (solid) and acrolein-modified (dashed) apoE3-NT were recorded in 20 mM sodium phosphate, pH 7.4 in a 0.05 cm cuvette. 4 scans were acquired at a scan speed of 50 nm/min.
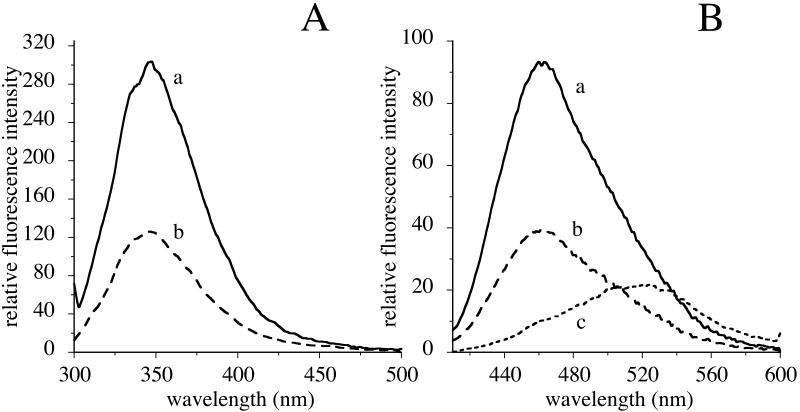
Subsequently, we evaluated if acrolein modification leads to tertiary structural changes in apoE3-NT by fluorescence spectroscopic analysis. The intrinsic Trp fluorescence emission spectrum of acrolein-modified apoE3-NT was compared with that of apoE3-NT, Figure 5, Panel A. Upon excitation at 295 nm, unmodified apoE3-NT displays fluorescence emission with maximal intensity at 350 nm (λmax). Acrolein-modified apoE3-NT elicited a similar λmax, but with a 50% decrease in fluorescence emission intensity. In a complementary approach, we employed ANS, an extrinsic fluorophore that interacts with “hydrophobic patches” on the protein to assess possible acrolein-modification induced changes in the tertiary fold, Figure 5, Panel B. ANS is routinely employed to evaluate surface hydrophobicity on proteins (28,29,35), which is directly correlated with the fluorescence intensity. In the absence of protein, an aqueous solution of ANS in TBS elicits a very low fluorescence with a spectrum centered ∼520 nm. Upon binding to unmodified apoE, ANS displayed an enhanced fluorescence emission intensity with a shift in the λmax from ∼520 to 460 nm; however, in the presence of acrolein-modified apoE3-NT, ANS displayed a substantially lowered (50%) fluorescence emission intensity compared to that noted for the unmodified protein, indicative of an altered tertiary conformation.
Figure 5. Effect of acrolein modification on tertiary structural features of apoE3-NT.
Panel A. Trp fluorescence emission. Trp fluorescence emission spectra of 35 μg unmodified (solid line, a) or acrolein-modified (broken line, b) apoE3-NT were recorded between 300 and 500 nm following excitation at 295 nm. Panel B. ANS fluorescence emission. Unmodified (solid line, a) or acrolein-modified (broken line, b) apoE3-NT (35 μg protein) were treated with 250 μM ANS in 20 mM sodium phosphate, pH 7.4 for 10 min at 24 °C. The fluorescence emission spectrum of the incubation mixtures was monitored between 400 and 600 nm following excitation at 395 nm. Fluorescence emission spectrum of 250 μM ANS alone in 20 mM sodium phosphate, pH 7.4 (dotted line, c) is shown for comparison.
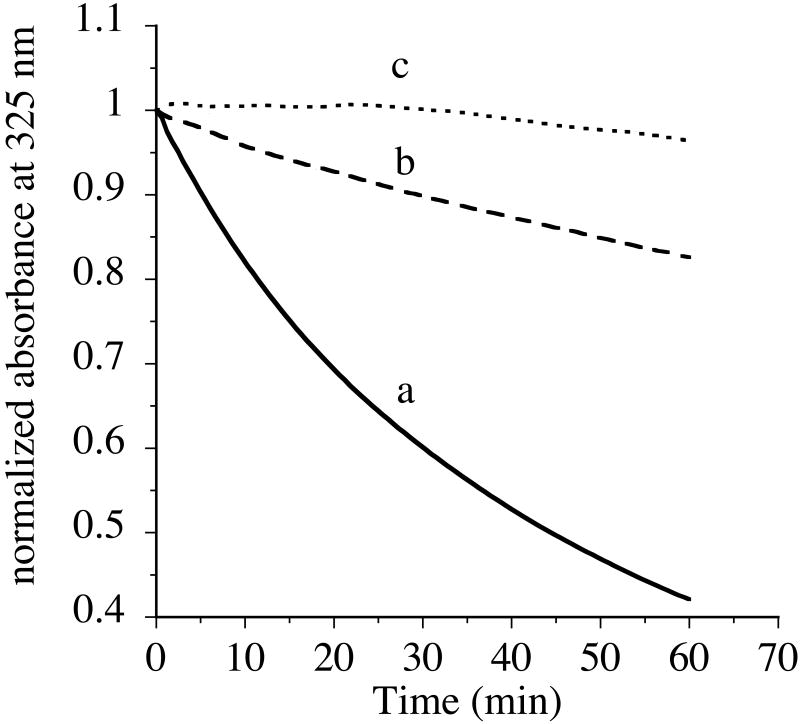
To assess if acrolein modification alters the functional features of apoE3-NT, we performed three different assays to determine its ability to bind: (i) lipids, (ii) heparin, and (iii) the LDLr. Apolipoproteins bear the ability to transform phospholipid bilayer vesicles to discoidal bilayer complexes at the transition temperature of the lipid. This transformation is routinely employed to evaluate the initial binding rates of apolipoproteins and lipids and can be followed in a spectrophotometer as a decrease in sample turbidity at 325 nm. The effect of acrolein modification on the ability of apoE3-NT (200 μg protein) to interact with DMPC MLVs (100 μg DMPC) was investigated (Figure 6) at 24 °C (lipid/protein molar ratio of ∼10:1). Upon addition of unmodified apoE3-NT, a decrease in the absorbance was noted, with an end point after about 70 min, curve a. However, upon addition of acrolein-modified apoE3-NT, a significant decrease in the ability to transform MLVs into discoidal complexes was observed, curve b. In the absence of added protein, the absorbance of MLVs at 325 nm remains unchanged, curve c. The T1/2 (time required for a 50% decrease in the initial absorbance) and the rate constant, K (calculated as reciprocal of T1/2), were calculated for the vesicles to discs transformation process. The T1/2 for the acrolein-modified apoE3-NT (247 min) was about 5 times longer compared to the unmodified protein (49 min) (K = 0.02 and 0.004 min-1, respectively), suggesting impairment in the lipid binding ability. However, prolonged incubation of modified apoE3-NT with phospholipids leads to the formation of lipoprotein complexes, which are similar to those formed with unmodified apoE3-NT in terms of molecular mass and lipoprotein particle diameter (data not shown).
Figure 6. Effect of acrolein modification on the ability of apoE3-NT to bind phospholipid vesicles.
Unmodified (solid line, curve a) or acrolein-modified (dashed line, curve b) apoE3-NT (200 μg) were treated with DMPC MLVs (100 μg DMPC) (∼10:1 lipid:protein molar ratio) in TBS. The mixture was maintained at 24 °C, the transition temperature of DMPC. The change in absorbance at 325 nm was followed as a function of time and shown as normalized absorbance units. In the absence of protein, there was no change in the absorbance of DMPC vesicles (dotted line, curve c). Representative curves from 5 different experiments are shown.
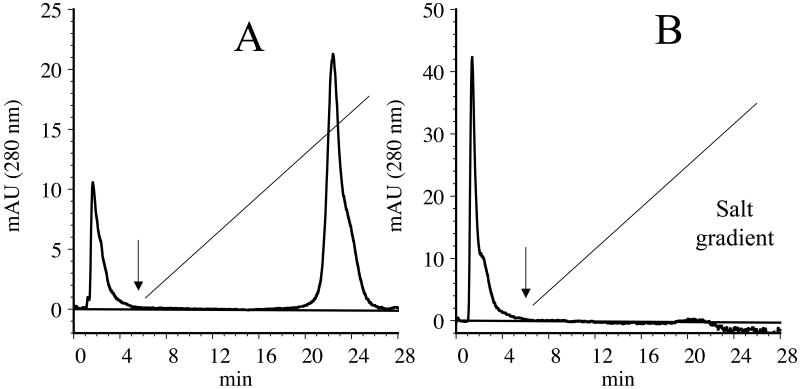
In the next step, the heparin binding ability of acrolein-modified apoE3-NT was compared with that of unmodified apoE3-NT, Figure 7. Loading of unmodified apoE3-NT (100 μg) onto a heparin-Sepharose column resulted in binding of the protein; the bound protein could be eluted with ∼ 400 mM NaCl on a 0 – 1 M gradient (Panel A). When unmodified apoE3-NT was present in the lipid-bound state (DMPC/apoE3-NT complex), it displayed a similar behavior as lipid-free protein (not shown). On the other hand, acrolein-modified apoE3-NT displayed a decreased ability to bind heparin, with a significant portion of the loaded protein appearing in the flow-through fractions prior to initiation of salt gradient (Panel B). Similarly, lipid-bound acrolein-modified apoE3-NT displayed poor heparin binding (not shown).
Figure 7. Heparin binding activity of unmodified and acrolein-modified apoE3-NT.
Unmodified (Panel A) or acrolein-modified (Panel B) apoE3-NT (100 μg) was loaded onto a heparin-Sepharose column in 20 mM sodium phosphate pH 7.4 in a ÄKTA FPLC system. The flow rate was maintained at 0.5 ml/min. A salt gradient consisting of 0-1 M NaCl was applied (black straight line) and the elution of the protein monitored at 280 nm. The black arrow represents the time at which the gradient was initiated.
Finally, the LDLr receptor binding ability of acrolein-modified apoE3-NT was examined by an IP assay using sLDLr/LA3-LA6/Myc bound to Protein G Agarose, Figure 8. ApoE displays the ability to interact with the LDLr only in the lipid (or lipoprotein)-bound state. Lipid-bound complexes of unmodified and acrolein-modified apoE3-NT were prepared by overnight incubation of the protein and lipids, followed by separation of the bound from the unbound protein by density gradient ultracentrifugation. DMPC/unmodified apoE3-NT bears the ability to interact with the LDLr in a concentration dependent manner (1 and 3 μg protein, lanes 1 and 3, respectively); Lys143Ala/Lys146Ala/apoE3-NT, an apoE variant with a targeted disruption of LDLr binding activity (33) (lane 5) and DMPC/apoE2, an apoE isoform (Cys at position 158) with poor LDLr binding activity (lane 6), were used as negative controls. DMPC/acrolein-modified apoE3-NT was unable to bind the LDLr at both the low (1 μg protein, lane 2) and high (3 μg protein, lane 4) concentrations. In summary, acrolein modification affects the ability of apoE3-NT to bind lipids, heparin and the LDLr.
Figure 8. Effect of acrolein modification on the LDLr binding ability of apoE3-NT.
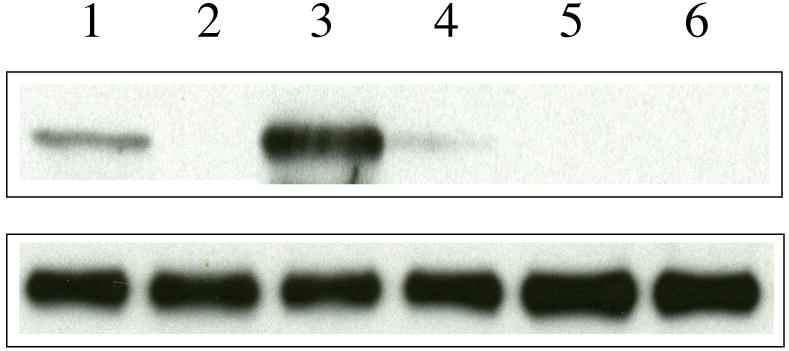
DMPC/apoE complexes were incubated with 1 μg of sLDLr/LA3-LA6/Myc in 25 mM Tris-HCl, pH 7.4, 140 mM NaCl, 27 mM KCl, 2 mM CaCl2 for 2 h at 4 °C, followed by IP with anti-c-Myc antibody. LDLr-bound apoE was detected by Western blot using anti-apoE monoclonal antibody 1D7 (top panel). The lane assignments are as follows: lane 1, unmodified apoE3-NT (1 μg); lane 2, acrolein-modified apoE3-NT (1 μg); lane 3, unmodified apoE3-NT (3 μg); lane 4, acrolein-modified apoE3-NT (3 μg); lane 5, Lys143Ala/Lys146Ala/apoE3-NT (3 μg); lane 6, apoE2 (3 μg). A duplicate analysis was carried out in parallel with anti-c-Myc antibody (bottom panel) to confirm the presence of similar levels of LDLr in each reaction mixture.
Discussion
Acrolein (CH2=CH-CHO) is an acrid smelling environmental pollutant that is formed during combustion of organic and plastic substances (36). It is also generated as a natural metabolite during oxidative stress mediated lipid peroxidation. Acrolein is present as one of the major components in the gaseous phase of tobacco smoke (up to 140 μg/cigarette) (37). Smoking and the associated oxidative stress with cellular organelle dysfunction (38,39) may be considered as a model of aging. Acrolein is the most reactive of all α,β-unsaturated aldehydes and causes oxidative modification of proteins by reacting towards the sulfhydryl side chain of cysteines, ε-amino groups of lysines and imidazole group of histidines (37, 40). Acrolein is about 100 fold more reactive than 4-hydroxy-2-nonenal (4-HNE), another endogenously generated lipid peroxidation product (41) and constituent of tobacco smoke. Both the lipid and protein components of plasma lipoproteins are major targets for oxidative damage by acrolein and other pro-oxidants. The relevance of LDL oxidation in atherosclerosis and heart disease is well established. Our objective is to establish possible links between smoking-related oxidative stress and apoE function. The focus of the present study was to examine if oxidative modification by acrolein causes functional alterations in recombinant apoE.
A major role of apoE is to serve as a ligand for the cell surface localized HSPG (13,14) and the LDLr, both interactions involving basic residues in the protein (Figure 1). An optimal arrangement of residues 136 to 150 (located on helix 4) and Arg172 is required for the interaction of these basic residues with negatively charged residues in the ligand-binding domain of LDLr (18,22). Employing heparin, the interaction sites on apoE were found to localize to residues 142-147 in the N-terminal domain and between 243 and 272 in the C-terminal domain. Heparin is a highly sulfated in vitro model of HSPG, which is isolated from bovine intestinal mucosa; it serves as an excellent surrogate of cell surface HSPG. The heparin binding site in the N-terminal domain is a high affinity site (42,43) that is available in both lipid-free and lipoprotein-associated states (44) and involves ionic interaction between the basic residues and the acidic sugar moieties located on the HSPG (43). Recently, it was proposed that apoE/heparin interaction possibly occurs as a two-step process, the first step involving a fast association with electrostatic interactions, and the second step involving hydrophobic interactions (45). Taken together with our data and other researchers reports, we predicted that the ε-amino groups of the lysine side chains in apoE are potential targets in vivo for interaction with acrolein in tobacco smoke. Indeed, we obtained physiological relevance for our in vitro biochemical analysis from observations of the occurrence of acrolein-modified apoE in the plasma of smokers and in rats exposed to passive or second hand smoke, also known as environmental tobacco smoke1. While the plasma content of free and protein-bound acrolein are found to be ∼0.5 and 30 nmol/ml plasma, respectively, in normal subjects (46), we anticipate the levels to be higher in smokers and subjects exposed to passive smoke.
We noted that acrolein modification of apoE does not alter the overall secondary structure characteristics and the helical content of apoE3-NT (Figure 4). Both CD and IR analysis independently validated these observations. On the other hand, fluorescence analysis indicated substantial tertiary structural changes in acrolein-modified apoE3-NT. Comparing the Trp fluorescence emission spectra (Figure 5, Panel A), it was noted that the fluorescence intensity of acrolein-modified apoE3-NT was ∼50% lower than that of unmodified apoE3-NT, indicative of fluorescence quenching, possibly due to exposure to the aqueous environment. However, the wavelength of maximal fluorescence emission remained unaltered. Although it is generally expected that a lower quantum yield (or decreased emission intensity) is accompanied by a red-shifted spectrum, we did not note such a correlation, as also described in several instances (47). Our observations from ANS experiments (Figure 5, Panel B) suggest that there is a decreased extent of surface hydrophobicity in acrolein-modified protein. Taken together, the fluorescence data may be interpreted as reflecting a substantially altered tertiary conformation in acrolein-modified apE3-NT.
Use of the monoclonal antibody (5F6) directed against acrolein-lysine adducts in proteins (23,25) allowed us to evaluate acrolein modification of apoE3. In the case of apoE3-NT, acrolein modification appears to lead to formation of cross-linked dimers not involving disulfide bond formation. In addition, this antibody also detects monomeric, and to a lesser extent, oligomeric apoE3-NT (Figure 2). It is possible that the monomeric form includes intra-molecular cross-linked species. The chemical nature of acrolein cross-linking or adduct formation with apoE3 is not known at present. In other studies, we noted that acrolein modified apoE3-NT without the His-tag as well (data not shown). Since Nε-(3-methylpyridinium)lysine appears to be the major epitope for mAb5F6 in other acrolein-modified proteins (48-50), it is likely that this may be the case for acrolein-modified apoE as well. The formation of other types of acrolein-lysine-adducts of apoE such as Nε-(3-formy-3,4-dehydropiperidino)lysine, for which mAb5F6 also shows some immunoreactivity (48), cannot be excluded at this point. Nevertheless, probing with a fluorophore bearing a functional group reactive with lysine residues (isothiocyanate) reveals labeling of all accessible lysine side chains for unmodified apoE3-NT (Figure 3). However, in the case of acrolein-modified apoE3-NT, the labeling was lowered to ∼75%, indicating that some of the accessible lysine sites have been modified by acrolein. We estimate that at least 2 lysine residues are modified by acrolein. In other studies, cysteine residues have been indicated as potential targets in proteins that are oxidized by acrolein (37) to form disulfide bonded homo-dimers or cross-linked adducts. We cannot exclude the possibility of acrolein modification of the single Cys at position 112 in apoE3-NT. This Cys residue is partially buried at the helix-helix interface (18) and we anticipate that it is less accessible to modification compared to the Lys residues.
In a related study, 4-HNE, also an aldehydic product of lipid peroxidation (40), has been shown to cause greater cross-linking of apoE3 than of apoE4, the isoform lacking cysteine residue (51). In addition, 4-HNE also caused cross-linking of apoE not involving disulfide bonding, an observation also noted with apoE in the cerebrospinal fluid, which has implications in the role of oxidative damage in neurodegenerative diseases such as Alzheimer's disease (51). On the other hand, other researchers suggest that the apoE2 isoform, bearing two Cys residues, may serve as an antioxidant affording protection against neurotoxicity due to peroxidation by 4-HNE (52).
The impairment of heparin binding activity (Figure 7) is consistent with our interpretation that lysine residue(s) may be modified by acrolein in apoE3-NT. It is possible that acrolein interacts with the lysine residues 143 and 146, which are directly involved in heparin binding. NMR studies suggest that these lysine residues have a lower pKa values compared to the other lysine residues in DMPC-bound state, possibly due to a highly positive electrostatic potential in their microenvironment (42,53). An alternative possibility is that acrolein modification at lysines located elsewhere leads to conformational changes that indirectly alter the heparin binding ability. Other researchers report that oxidative modification of apoB-100 lysines on LDL by reagents such as Cu2+ decreased its ability to bind to proteoglycans (54). Further studies are currently underway to define the residues modified by acrolein and the mechanisms underlying the functional consequence of acrolein modification of apoE3-NT.
An intriguing aspect of the effect of acrolein on apoE function was the decreased rate of lipid binding of acrolein-modified apoE3-NT compared to that noted for unmodified protein (Figure 6). We exclude the possibility that the observed lipid binding is due to the presence of unmodified protein in the mixture based on our observations in the heparin binding assay (Figure 7): a majority of the protein appeared in the flow through fraction prior to start of the salt gradient; we conclude that the mixture has negligible amounts of unmodified protein. Previous studies indicate that upon lipid association, the 4-helix bundle structure of apoE3-NT undergoes a conformational change involving movement of helices 1 and 2 away from helices 3 and 4, possibly followed by further movement of the helices to present an extended helical conformation to the lipid surface (55). It is possible that the acrolein-mediated covalent (intra- and inter-molecular) cross-linking restricts the helix bundle opening of apoE3-NT and decreases the rate of lipid binding, analogous to the restraint caused by a intra molecular disulfide-bond formation demonstrated earlier for apoE3 (55) and structurally related apolipoproteins (56). Under normal physiological conditions, apoE3 exists predominantly as a dimeric protein (both homodimeric and heterodimeric with apolipoprotein AII) (57). In addition, it is believed that a significant amount of apoE is in the lipoprotein-associated state, which is also the LDLr-binding competent state. However, a prototypical feature of apoE and other related exchangeable apolipoproteins is their ability to exist in a dynamic equilibrium between lipid-free and lipid-bound states. Thus, it may be anticipated that there will be more lipid-free apoE in the plasma under conditions of oxidative stress, such as aging and smoking. In a recent study, it was suggested that endogenously generated acrolein may form covalent adducts with apoAI, a related exchangeable apolipoprotein that is considered anti-atherogenic (49,50). It was noted that acrolein modification correlated with a decreased cellular cholesterol efflux capability of apoAI mediated by the ATP-binding cassette transporter AI. Further, the authors suggested co-localization of apoAI with acrolein adducts in atherosclerotic plaques in humans.
Not surprisingly, in addition to impairing the heparin binding ability, acrolein modification also alters the LDLr binding ability of apoE3-NT (Figure 8). However, possible changes in the receptor binding affinity cannot be excluded at this point based on the IP assay. LDLr binding involves participation of multiple sites on apoE3-NT, with several apoE molecules on the lipoprotein particle involved in co-operative binding. The presence of positively charged residues and their proper alignment is an essential aspect of the role of apoE as a ligand for the LDLr; on the other hand, the heparin binding activity relies more on the positive charges at these sites as noted by studies involving reductive methylation (58). Our proposal that Lys143 and Lys146 may be modified by acrolein is supported by the loss of LDLr binding capability of Lys143Ala/Lys146Ala/apoE3-NT variant, noted by others (33) and used by us as a control. A recent study reported poor uptake of acrolein-modified VLDL by human hepatoma cells and rat hepatocytes (59), suggesting a mechanism similar to that suggested in our study and by that noted in the case of Cu2+ mediated VLDL oxidation (60).
Since lipid or lipoprotein binding is an essential prerequisite for apoE to elicit lipoprotein receptor binding capability, it is expected that under physiological conditions, the clearance of the apoE-containing lipoprotein particles will be affected by acrolein modification. Taken together with the observation that HSPG mediated cellular lipoprotein uptake represents a significant pathway for clearance of plasma lipoproteins (13), our results provide a possible mechanism for the documented reports of increased plasma levels of LDL cholesterol and triglycerides, and, decreased HDL cholesterol levels in smokers (61,62), recognized as risk factors in atherogenesis. These implications may be more significant in the brain (which lacks triglyceride-rich lipoproteins and apoB-100), where apoE is the major apolipoprotein involved in the delivery of cholesterol to the neurons. Further studies are in progress to investigate the implications of the role of oxidative modification of apoE in neurodegenerative diseases (63,64) such as Alzheimer's disease.
Acknowledgments
This work was supported by funding from an American Heart Association Postdoctoral Fellowship (0625168Y) (SK), an Alzheimer's Association grant (TLL-03-5281) (VN), a KFP Foundation (Japan) grant (HK), and a NHLBI Comprehensive Sickle Cell Center Training grant (P60 MD000222). VR is a Research Associate at the National Fund for Scientific Research (Belgium).
The abbreviations used are
- ANS
1-anilinonaphthalene-8-sulfonic acid
- apoE
apolipoprotein E
- DMPC
1,2-dimyristoyl-snglycero-3-phosphocholine
- HSPG
heparan sulfate proteoglycans
- 4-HNE
4-hydroxy-2-nonenal
- IDL
intermediate density lipoprotein
- LDL
low density lipoproteins
- MLV
multi lamellar vesicles
- NT
N-terminal domain
- LDLr
low density lipoprotein receptor
- VLDL
very low density lipoproteins
Footnotes
S. Tamamizu-Kato, O. Akintunde, K. Pinkerton, & V. Narayanaswami, unpublished observations
References
- 1.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2003;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Ann Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 3.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Ann Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 4.Shimano H, Yamada N, Katsuki M, Yamamoto K, Gotoda T, Harada K, Shimada M, Yazaki Y. Plasma lipoprotein metabolism in transgenic mice overexpressing apolipoprotein E. Accelerated clearance of lipoproteins containing apolipoprotein B. J Clin Invest. 1992;90:2084–2091. doi: 10.1172/JCI116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB., Jr Familial apolipoprotein deficiency. J Clin Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiselli G, Gregg RE, Zech LA, Schaefer EJ, Brewer HB., Jr Phenotype study of apolipoprotein E isoforms in hyperlipoproteinemic patients. Lancet. 1982;214:405–409. doi: 10.1016/s0140-6736(82)90439-1. [DOI] [PubMed] [Google Scholar]
- 7.Kurosaka D, Teramoto T, Matsuhima T, Yokoyama T, Yamada A, Aikawa T, Miyamoto Y, Kurokawa K. Apolipoprotein E deficiency with a depressed mRNA of normal size. Atherosclerosis. 1991;88:15–20. doi: 10.1016/0021-9150(91)90252-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 9.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 10.Weisgraber KH. Apolipoprotein E: Structure-Function relationships. Adv Protein Chem. 1994;45:250–295. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- 11.Aggerbeck LP, Wetterau JR, Weisgraber KH, Wu CS, Lindgren FT. Human apolipoprotein E3 in aqueous solution. II. Properties of the amino- and carboxyl-terminal domains. J Biol Chem. 1988;263:6249–6258. [PubMed] [Google Scholar]
- 12.Wetterau JR, Aggerbeck LP, Rall SC, Jr, Weisgraber KH. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J Biol Chem. 1988;263:6240–6248. [PubMed] [Google Scholar]
- 13.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparin sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 14.Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TI, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–10167. [PubMed] [Google Scholar]
- 15.Ji ZS, Fazio S, Lee YL, Mahley RW. Secretion-capture role for apolipoprotein E in remnant lipoprotein metabolism involving cell surface heparan sulfate proteoglycans. J Biol Chem. 1994;269:2764–277. [PubMed] [Google Scholar]
- 16.Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, apoE(130-149) and apoE(141-155)2 bind to LRP1. Biochemistry. 2004;43:7328–7338. doi: 10.1021/bi036208p. [DOI] [PubMed] [Google Scholar]
- 17.Lalazar A, Weisgraber KH, Rall SCJ, Giliad H, Innerarity TL, Levanon AZ, Boyles JK, Amit B, Gorecki M, Mahley RW, Vogel T. Site-specific mutagenesis of human apolipoprotein E. Receptor binding activity of variants with single amino acid substitutions. J Biol Chem. 1988;263:3542–3545. [PubMed] [Google Scholar]
- 18.Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- 19.Fisher CA, Wang J, Francis GA, Sykes BD, Kay CM, Ryan RO. Bacterial overexpression, isotope enrichment, and NMR analysis of the N-terminal domain of human apolipoprotein E. Biochem Cell Biol. 1997;75:45–53. [PubMed] [Google Scholar]
- 20.Fisher CA, Narayanaswami V, Ryan RO. The lipid-associated conformation of the low density lipoprotein receptor binding domain of human apolipoprotein E. J Biol Chem. 2000;275:33601–33606. doi: 10.1074/jbc.M002643200. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CA, Ryan RO. Lipid binding-induced conformational changes in the N-terminal domain of human apolipoprotein E. J Lipid Res. 1999;40:93–99. [PubMed] [Google Scholar]
- 22.Gupta V, Narayanaswami V, Budamagunta MS, Yamamoto T, Voss JC, Ryan RO. Lipid-induced extension of apolipoprotein E helix 4 correlates with low density lipoprotein receptor binding ability. J Biol Chem. 2006;281:39294–39299. doi: 10.1074/jbc.M608085200. [DOI] [PubMed] [Google Scholar]
- 23.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuna Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: Potential markers for oxidative stress. Proc Natl Acad Sci (USA) 1998;95:4882–4897. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choy N, Raussens V, Narayanaswami V. Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J Mol Biol. 2003;334:527–539. doi: 10.1016/j.jmb.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 26.Sreerama N, Venyaminov SY, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: inclusion of denatured proteins with native proteins in the analysis. Anal Biochem. 2000;287:243–251. doi: 10.1006/abio.2000.4879. [DOI] [PubMed] [Google Scholar]
- 27.Goormaghtigh E, Raussens V, Ruysschaert JM. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim Biophys Acta. 1999;1422:105–185. doi: 10.1016/s0304-4157(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 28.Weers PMM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–3735. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- 29.Kiss RS, Weers PMM, Narayanaswami V, Cohen J, Kay CM, Ryan RO. Structure-guided protein engineering modulates helix bundle exchangeable apolipoprotein properties. J Biol Chem. 2003;278:21952–21959. doi: 10.1074/jbc.M302676200. [DOI] [PubMed] [Google Scholar]
- 30.Detloff M, Niere M, Ryan RO, Kay CM, Wiesner A, Weers PMM. Differential lipid binding of truncation mutants of Galleria mellonella apolipophorin III. Biochemistry. 2002;41:9688–9695. doi: 10.1021/bi0200108. [DOI] [PubMed] [Google Scholar]
- 31.Weers PMM, Abdullahi WE, Cabrera JM, Hsu TC. Role of buried polar residues in helix bundle stability and lipid binding of apolipophorin III: Destabilization by threonine 31. Biochemistry. 2005;44:8810–8816. doi: 10.1021/bi050502v. [DOI] [PubMed] [Google Scholar]
- 32.Narayanaswami V, Szeto S, Ryan RO. Lipid-association induced N- and C-terminal domain reorganization in human apolipoprotein E3. J Biol Chem. 2001;276:37853–37860. doi: 10.1074/jbc.M102953200. [DOI] [PubMed] [Google Scholar]
- 33.Fisher C, Abdul-Aziz D, Blacklow SC. A two-module region of the low-density lipoprotein receptor sufficient for formation of complexes with apolipoprotein E ligands. Biochemistry. 2004;43:1037–1044. doi: 10.1021/bi035529y. [DOI] [PubMed] [Google Scholar]
- 34.Tuls J, Geren L, Millett F. Fluorescein isothiocyanate specifically modifies lysine 338 of cytochrome P-450scc and inhibits adrenodoxin binding. J Biol Chem. 1989;264:16421–16425. [PubMed] [Google Scholar]
- 35.Cardamone M, Puri NK. Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem J. 1992;282:589–593. doi: 10.1042/bj2820589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauchamp RO, Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck H. A critical review of the literature on acrolein toxicity. Crit Rev Toxicol. 1985;14:309–380. doi: 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- 37.Witz G. Biological interactions of alpha, beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 38.Ames BN. Delaying the mitochondrial decay of aging -- a metabolic tune-up. Alzheimer Dis Assoc Disord. 2003;17(Suppl 2):S54–S57. [PubMed] [Google Scholar]
- 39.Sun L, Luo C, Long J, Wei D, Liu J. Acrolein is a mitochondrial toxin: Effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion. 2006;6:136–142. doi: 10.1016/j.mito.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 41.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 42.Saito H, Dhanasekaran P, Nguyen D, Baldwin F, Weisgraber KH, Wehrli S, Phillips MC, Lund-Katz S. Characterization of the heparin binding sites in human apolipoprotein E. J Biol Chem. 2003;278:14782–14787. doi: 10.1074/jbc.M213207200. [DOI] [PubMed] [Google Scholar]
- 43.Libeu CP, Lund-Katz S, Phillips MC, Wehrli S, Hernáiz MJ, Capila I, Linhardt RJ, Raffai RL, Newhouse YM, Zhou F, Weisgraber KH. New insights into the heparan sulfate proteoglycan-binding activity of apolipoprotein E. J Biol Chem. 2001;276:39138–39144. doi: 10.1074/jbc.M104746200. [DOI] [PubMed] [Google Scholar]
- 44.Weisgraber KH, Rall SC, Jr, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein E. Determination of the heparin binding sites of apolipoprotein E3. J Biol Chem. 1986;261:2068–2076. [PubMed] [Google Scholar]
- 45.Futamura M, Dhanasekaran P, Handa T, Phillips MC, Lund-Katz S, Saito H. Two-step mechanism of binding of apolipoprotein E. Implications for the kinetics of apolipoprotein E-heparan sulfate proteoglycan complex formation on cell surfaces. J Biol Chem. 2005;280:5414–5422. doi: 10.1074/jbc.M411719200. [DOI] [PubMed] [Google Scholar]
- 46.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31:371–374. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 47.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd. Kluwer Academic/Plenum Publishers; New York, NY: [Google Scholar]
- 48.Furuhata A, Ishi T, Kumazawa S, Yamada T, Nakayama T, Uchida K. N(epsilon)-(3-Methylpyridinium)lysine, a major antigenic adduct generated in acrolein-modified protein. J Biol Chem. 2003;278:48658–48665. doi: 10.1074/jbc.M309401200. [DOI] [PubMed] [Google Scholar]
- 49.Shao B, O'Brien KD, McDonald TO, Fu X, Oram J, Uchida K, Heinecke JW. Acrolein modifies apolipoprotein A-I in the human artery wall. Ann N Y Acad Sci. 2005;1043:396–403. doi: 10.1196/annals.1333.046. [DOI] [PubMed] [Google Scholar]
- 50.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O'Brien KD, Oram J, Heinecke JW. Acrolein impairs ATP binding cassette transporter AI-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 2005;280:36386–36396. doi: 10.1074/jbc.M508169200. [DOI] [PubMed] [Google Scholar]
- 51.Montine TJ, Huang DY, Valentine WM, Amarnath V, Saunders A, Weisgraber KH, Graham DG, Strittmatter WJ. Crosslinking of apolipoprotein E by products of lipid peroxidation. J Neuropath Exp Neurology. 1996;55:202–210. doi: 10.1097/00005072-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- 53.Lund-Katz S, Zaiou M, Wehrli S, Dhanasekaran P, Baldwin F, Weisgraber KH, Phillips MC. Effects of lipid interaction on the lysine microenvironments in apolipoprotein E. J Biol Chem. 2000;275:34459–34464. doi: 10.1074/jbc.M005265200. [DOI] [PubMed] [Google Scholar]
- 54.Oorni K, Pentikainen MO, Annila A, Kovanen PT. Oxidation of low density lipoprotein particles decreases their ability to bind to human aortic proteoglycans. J Biol Chem. 1997;272:21303–21311. doi: 10.1074/jbc.272.34.21303. [DOI] [PubMed] [Google Scholar]
- 55.Lu B, Morrow JA, Weisgraber KH. Conformational reorganization of the four-helix bundle of human apolipoprotein E in binding to phospholipid. J Biol Chem. 2000;275:20775–20781. doi: 10.1074/jbc.M003508200. [DOI] [PubMed] [Google Scholar]
- 56.Narayanaswami V, Wang J, Kay CM, Scraba DG, Ryan RO. Disulfide bond engineering to monitor conformational opening of apolipophorin III during lipid binding. J Biol Chem. 1996;271:26855–26862. doi: 10.1074/jbc.271.43.26855. [DOI] [PubMed] [Google Scholar]
- 57.Weisgraber KH, Shinto LH. Identification of the disulfide-linked homodimer of apolipoprotein E3 in plasma. Impact on receptor binding activity. J Biol Chem. 1991;266:12029–12034. [PubMed] [Google Scholar]
- 58.Weisgraber KH, Innerarity TI, Mahley RW. Role of the lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978;253:9053–9062. [PubMed] [Google Scholar]
- 59.Arai H, Uchida K, Nakamura K. Effect of ascorbate on acrolein modification of very low density lipoprotein and uptake of oxidized apolipoprotein E by hepatocytes. Biosci Biotechnol Biochem. 2005;69:1760–1762. doi: 10.1271/bbb.69.1760. [DOI] [PubMed] [Google Scholar]
- 60.Arai H, Kashiwagi S, Nagasaka Y, Uchida K, Hoshii Y, Nakamura K. Oxidative modification of apolipoprotein E in human very low density lipoprotein and its inhibition by glycosaminoglycans. Arch Biochem Biophys. 1999;367:1–8. doi: 10.1006/abbi.1999.1222. [DOI] [PubMed] [Google Scholar]
- 61.Freeman DJ, Griffin B, Murray E, Lindsay GM, Gaffney D, Packard CJ, Shepherd J. Smoking and plasma lipoproteins in man: effects on low density lipoprotein cholesterol levels and high density lipoprotein subfraction distribution. Eur J Clin Invest. 1993;23:630–640. doi: 10.1111/j.1365-2362.1993.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 62.Brischetto CS, Connor WE, Connor SL, Matarazzo JD. Plasma lipid and lipoprotein profiles of cigarette smokers from randomly selected families: enhancement of hyperlipidemia and depression of high-density lipoprotein. Am J Cardiol. 1983;52:675–680. doi: 10.1016/0002-9149(83)90396-x. [DOI] [PubMed] [Google Scholar]
- 63.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 64.Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer's amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]