Abstract
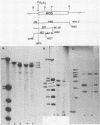
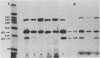
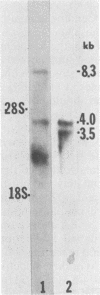
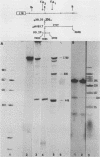
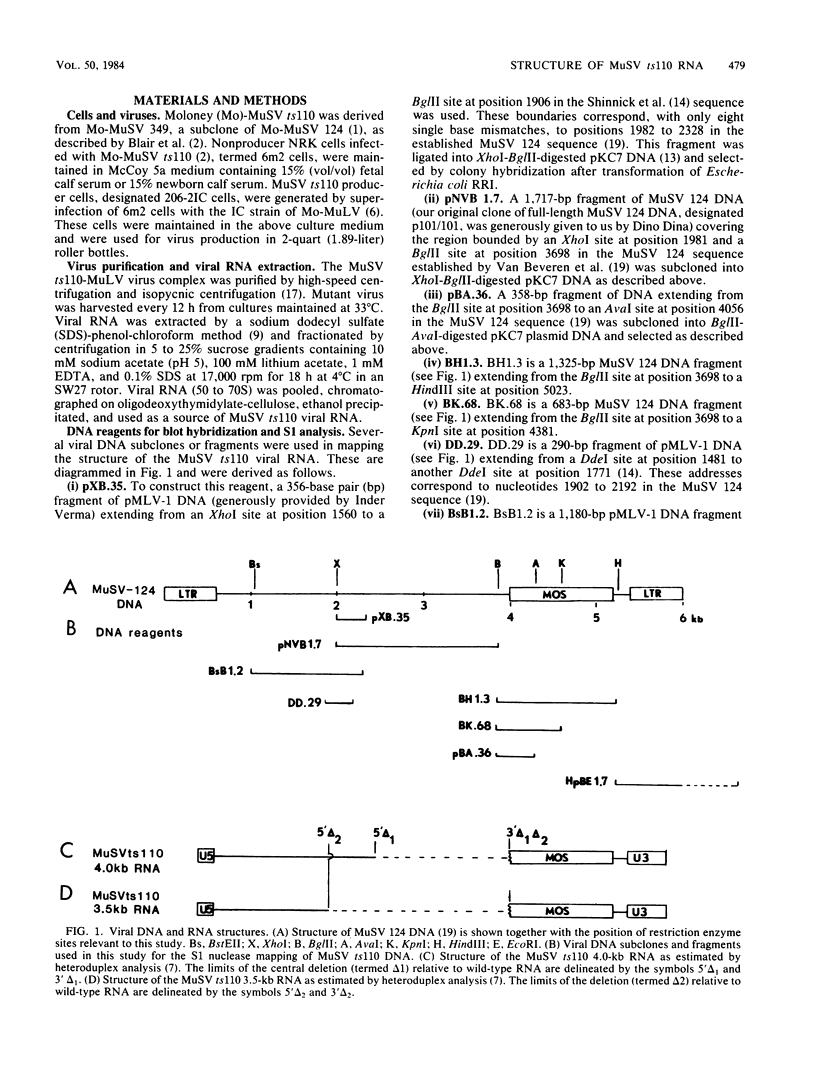
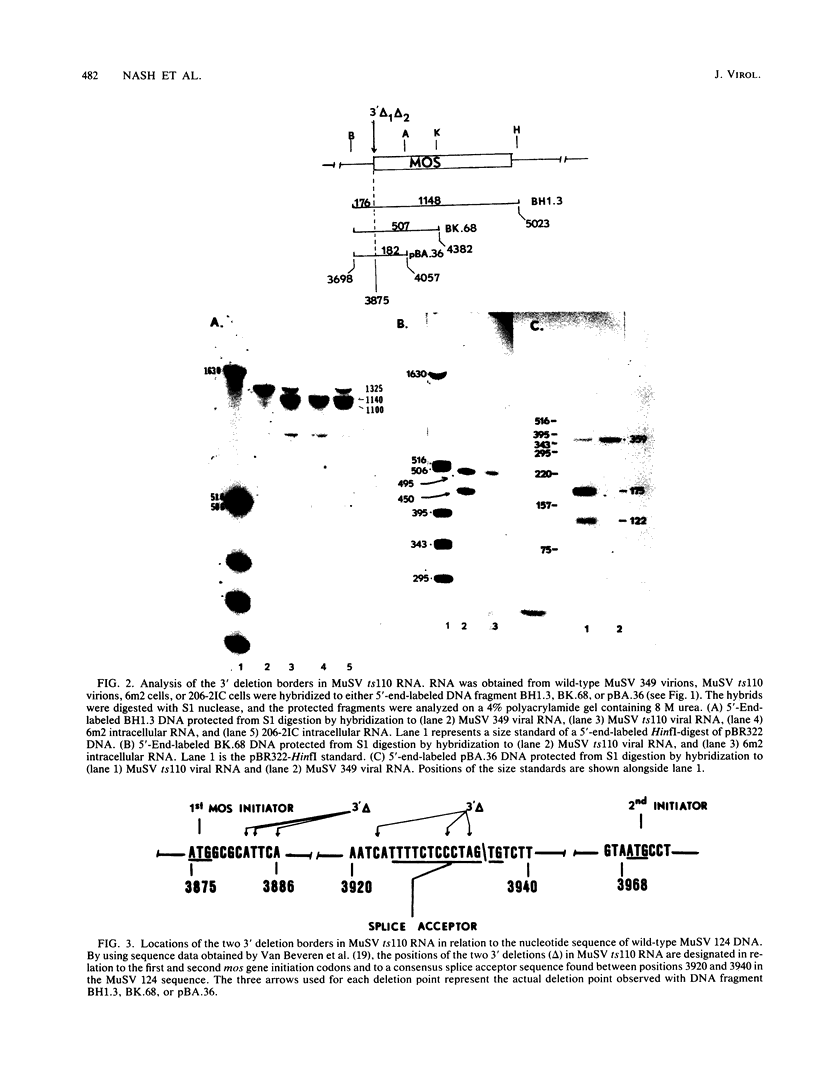
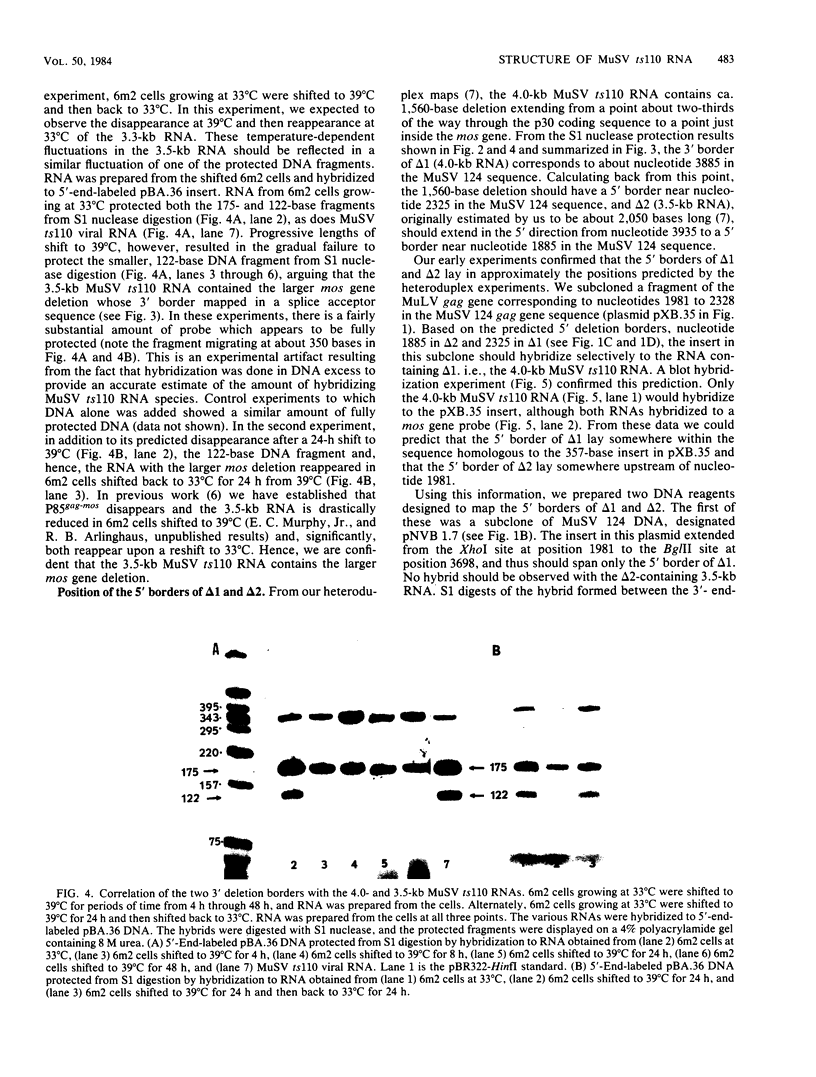
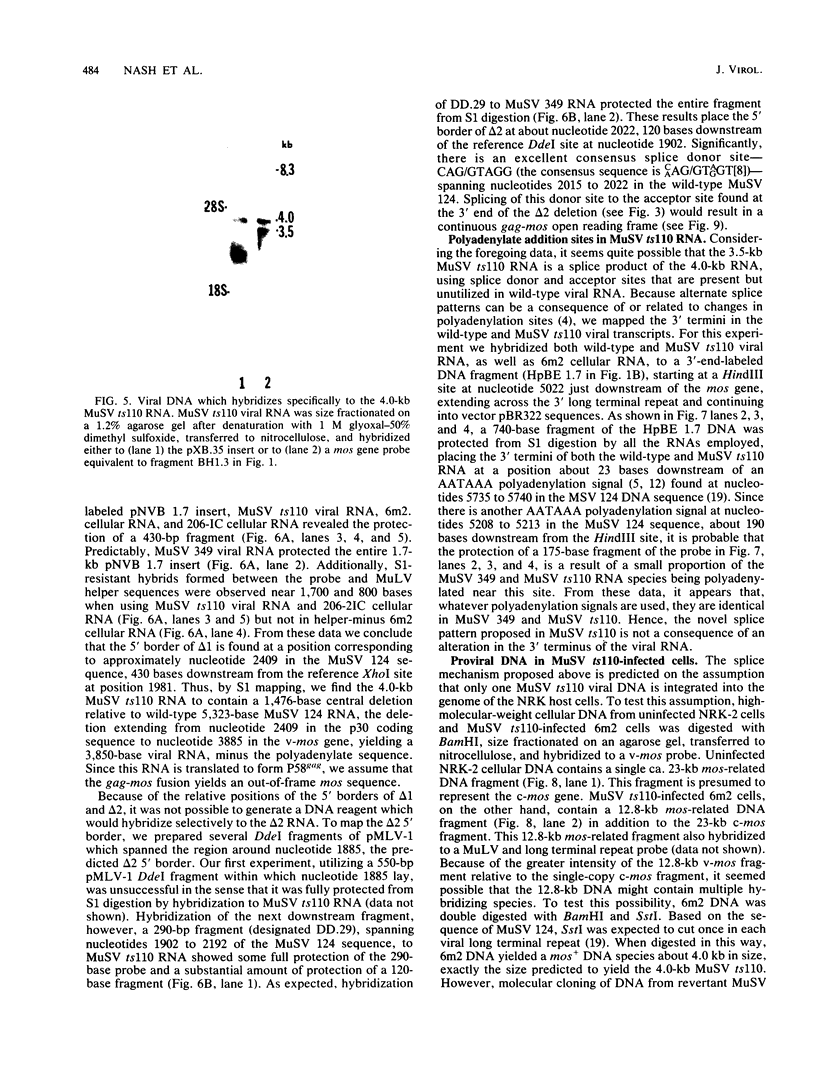
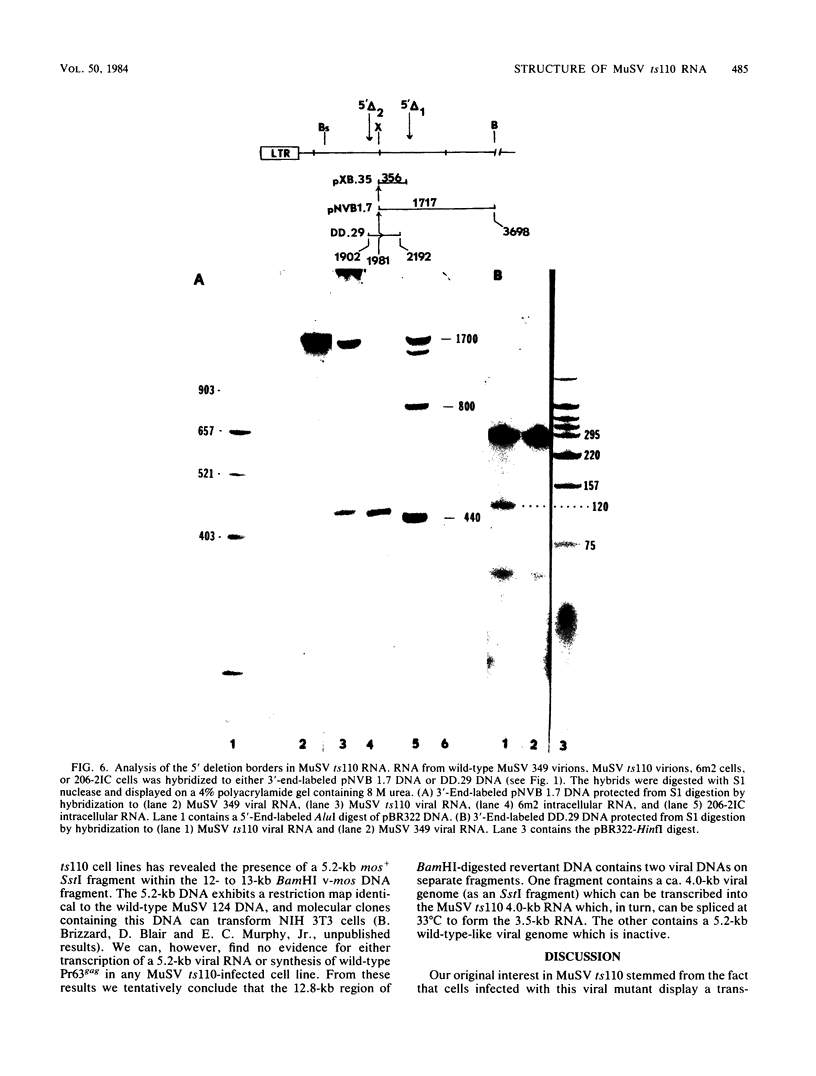
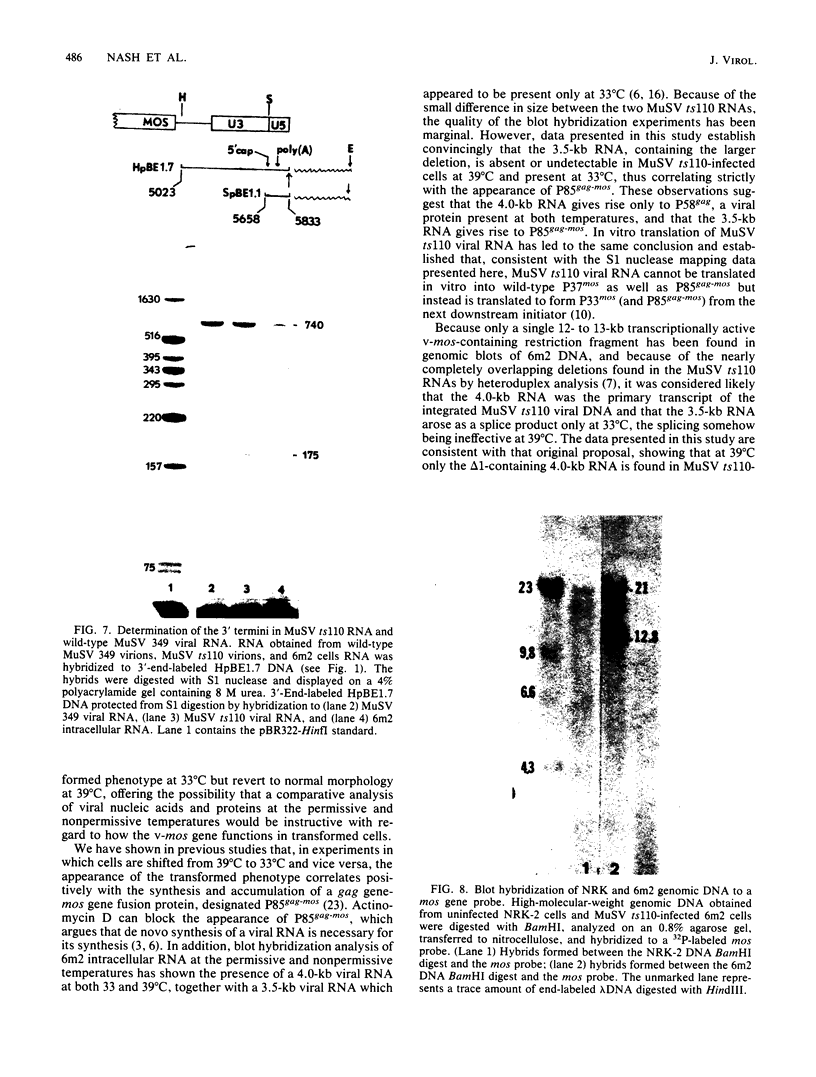
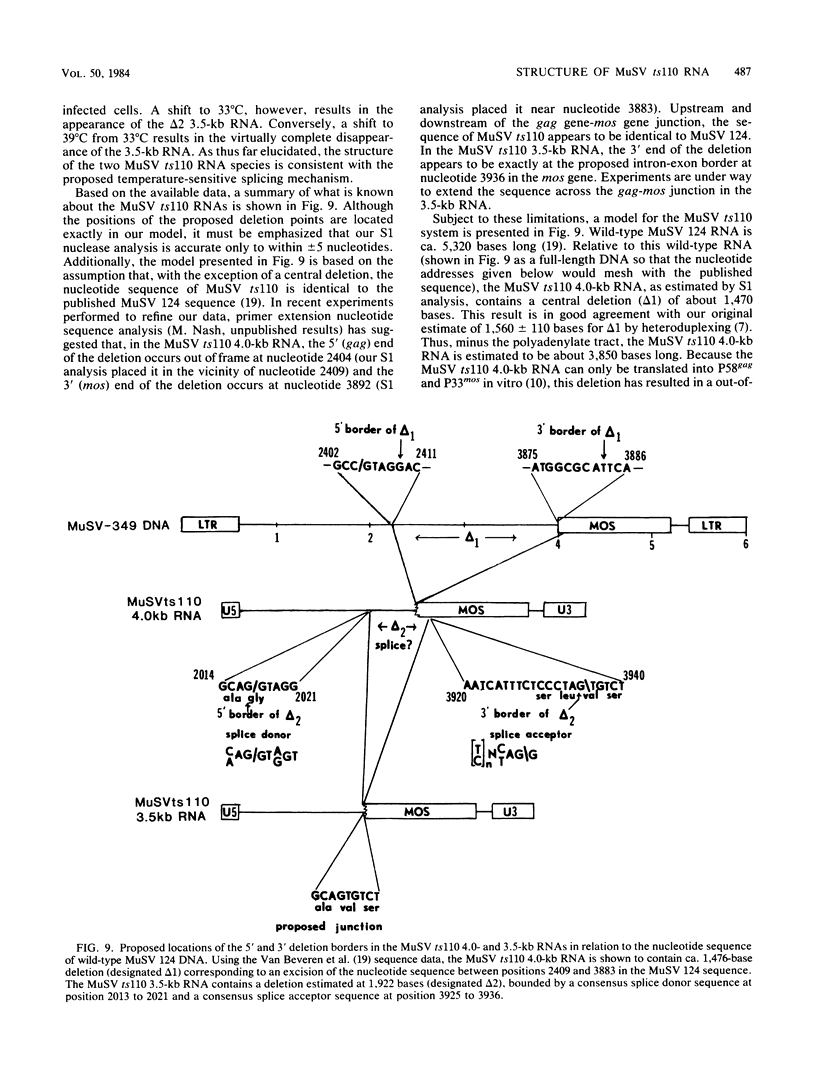
The structures of murine sarcoma virus (MuSV) ts110 viral RNA and intracellular RNA present in MuSV ts110-infected cells (6m2 cells) have been examined by S1 nuclease analysis. A previous study involving heteroduplex analysis of MuSV ts110 viral RNAs hybridized to wild-type DNA revealed the presence of two MuSV ts110 RNAs, 4.0 and 3.5 kilobases (kb) in length, containing overlapping central deletions relative to wild-type MuSV 124 viral RNA (Junghans et al., J. Mol. Biol. 161:229-255, 1982). Here we show that the deletion (termed delta 1) in the 4.0-kb RNA has a 5' border located at about nucleotide 2409 (using the numbering system of Van Beveren et al., Cell 27:97-108, 1981), a position 63 bases upstream of the junction of the p30 and p10 coding sequences. The 3' border of the delta 1 deletion is found 1,473 bases downstream at approximately nucleotide 3883, 10 nucleotides downstream of the first mos gene initiation codon. In the 3.5-kb MuSV ts110 RNA, the 5' border of the deleted central region (termed delta 2) is located in a splice consensus donor site at approximately nucleotide 2017, 330 bases downstream from the junction of the p12 and p30 coding sequences, and extends about 1,915 bases in the downstream direction to nucleotide 3935, found in a splice consensus acceptor site about 55 nucleotides downstream of the first mos gene initiation codon and 30 bases upstream of the second initiation codon. No alteration of polyadenylate addition sites was observed in either MuSV ts110 RNA species, as compared with MuSV 349 RNA. The observation that the 5' and 3' borders of the deletion in the 3.5-kb RNA are within in-frame splice donor and acceptor sites suggests strongly that the 3.5-kb RNA is derived from the 4.0-kb RNA by a temperature-sensitive splice mechanism. Data presented here show unequivocally that formation of the 3.5-kb MuSV ts110 RNA from which the P85gag-mos polypeptide is translated is temperature sensitive. At 33 degrees C, with S1 analysis, the 3.5-kb RNA is found readily in 6m2 cells. Within 4 h of a shift to 39 degrees C, however, only trace amounts of this RNA can be found. Moreover, reshifting 6m2 cells to 33 degrees C permits the reappearance of the 3.5-kb RNA at its original level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Horn J. P., Wible L., Arlinghaus R. B., Brinkley B. R. Sequence of events in the transformation process in cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5593–5597. doi: 10.1073/pnas.78.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Murphy E. C., Jr, Arlinghaus R. B. Electron microscopic analysis of ts1 10 Moloney mouse sarcoma virus, a variant of wild-type virus with two RNAs containing large deletions. J Mol Biol. 1982 Oct 25;161(2):229–250. doi: 10.1016/0022-2836(82)90150-4. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Cell-free synthesis of Rauscher murine leukemia virus "gag" and "gag-pol" precursor polyproteins from virion 35 S RNA in a mRNA-dependent translation system derived from mouse tissue culture cells. Virology. 1978 May 15;86(2):329–343. doi: 10.1016/0042-6822(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Comparative tryptic peptide analysis of candidate P85gag-mos of ts110 Moloney murine sarcoma virus and P38-P23 mos gene-related proteins of wild-type virus. Virology. 1982 Sep;121(2):372–383. doi: 10.1016/0042-6822(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Horn J. P., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. Gag-mos Polyproteins encoded by variants of the Moloney strain of mouse sarcoma virus. Virology. 1983 Apr 15;126(1):336–347. doi: 10.1016/0042-6822(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., Peltier-Horn J., Robey W. G., Blair D. G., Arlinghaus R. B. Characterization of virus-specified proteins present in NRK cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):747–754. doi: 10.1101/sqb.1980.044.01.080. [DOI] [PubMed] [Google Scholar]