Abstract
Background
Hernia repair is one of the most common surgical procedures, and some patients suffer from chronic pain after hernia surgery. The aim of the present study was to evaluate chronic postherniorrhaphy pain in men who underwent Lichtenstein mesh repair or preperitoneal (posterior) repair.
Methods
Our study included 94 male inpatients. Two surgeons experienced in both Lichtenstein and preperitoneal hernia repair performed the procedures. We controlled postoperative pain with systemic analgesic therapy. We evaluated the patients over the subsequent 12 months, using a questionnaire to focus on chronic pain and its limitations to their quality of life.
Results
The overall incidence of chronic pain at 2 months was 5%. About 6% of patients who underwent Lichtenstein repair (n = 70) and 4% of patients who underwent preperitoneal repair (n = 24) experienced chronic pain. All patients with chronic pain rated their pain as slight or moderate. Their pain was present occasionally and was related to physical stress. None of the patients were unable to work. After 12 months of follow-up, the overall incidence of chronic pain decreased to 3%, with 3 patients in Lichtenstein group reporting chronic pain with slight limitations in sports and social activities.
Conclusion
The incidence rates of chronic pain after Lichtenstein and preperitoneal repair were 6% and 4%, respectively. Inpatient status might have resulted in low incidences with both approaches.
Abstract
Contexte
La réparation d'une hernie constitue l'une des interventions chirurgicales les plus courantes. Certains patients souffrent de douleur chronique après cette intervention. La présente étude visait à évaluer la douleur chronique après l'herniorrhaphie chez les hommes qui ont subi une réparation avec treillis par la technique de Lichtenstein ou une réparation par voie prépéritonéale (postérieure).
Méthodes
Notre étude a porté sur 94 patients hospitalisés de sexe masculin. Deux chirurgiens maîtrisant la réparation de hernie par la technique de Lichtenstein et par voie prépéritonéale ont pratiqué les interventions. Nous avons contrôlé la douleur postopératoire au moyen d'une thérapie aux analgésiques systémiques. Nous avons évalué les patients au cours des 12 mois suivants en utilisant un questionnaire pour concentrer les réponses sur la douleur chronique et les limitations qu'elle imposait à leur qualité de vie.
Résultats
L'incidence globale de la douleur chronique à 2 mois s'établissait à 5 %. Environ 6 % des patients qui ont subi l'intervention pratiquée par la technique de Lichtenstein (n = 70) et 4 % de ceux qui ont subi l'intervention par voie prépéritonéale (n = 24) avaient de la douleur chronique. Tous les patients qui avaient de la douleur chronique ont jugé leur douleur bénigne ou modérée. Leur douleur se manifestait à l'occasion et était reliée au stress physique. Aucun des patients n'était incapable de travailler. Après 12 mois de suivi, l'incidence globale de la douleur chronique est tombée à 3 % et 3 patients qui avaient subi l'intervention par la technique de Lichtenstein ont signalé une douleur chronique et de légères limitations des activités sportives et sociales.
Conclusion
Les taux d'incidence de douleur chronique après une réparation par la technique de Lichtenstein et par voie prépéritonéale s'établissaient à 6 % et 4 % respectivement. Le statut de patient hospitalisé peut être lié à une incidence faible avec les 2 méthodes.
Hernia repair is a common surgical procedure, and postoperative recovery is uncomplicated in most patients. However, some patients continue to experience chronic pain and discomfort for months or even years after hernia repair — a complication that is becoming increasingly recognized as an important cause of morbidity after hernia surgery.1 The incidence of chronic pain following hernia repair is not accurately known. A number of studies reported incidence rates of chronic pain varying from 0% to 37%. The overall incidence is reported to be 12%.2 Observational methods vary, prospective studies are few and chronic pain is not a primary outcome parameter in most studies.2,3 Also, the severity of the pain and the social consequences of living with chronic pain following hernia repair have rarely been assessed.4
Several risk factors have been identified for the development of chronic pain after hernia repair: the experience of the surgeon, the presence of preoperative pain, high pain scores in the first week after surgery and day surgery.2,5 The influence of different surgical techniques remains unclear;1,3,6,7 however, a recent meta-analysis of randomized controlled trials concluded that persistent pain occurred less frequently after mesh repair than after sutured repair and that persistent pain was less frequent after laparoscopic repair than after open mesh placement.8
The aim of our study was to evaluate chronic postherniorrhaphy pain in men who underwent Lichtenstein mesh repair or preperitoneal (posterior) mesh repair. We focussed on the development of chronic pain and its influence on patients' physical endurance and their quality of life.
Methods
We included 94 male patients who underwent Lichtenstein mesh repair or preperitoneal mesh repair over a period of 36 months from May 1, 2001, to May 1, 2004. The follow-up period after surgery was 12 months. We obtained informed consent from all patients before surgery. Only patients with recurrent hernia or large hernia underwent preperitoneal hernia repair. Two surgeons experienced in both techniques performed the surgeries (70 Lichtenstein and 24 preperitoneal hernia repairs).
Surgical techniques
Lichtenstein tension-free repair
After opening the aponeurosis of the external oblique muscle, the surgeons mobilized the spermatic cord in the usual way. They inverted and imbricated direct sacs using “0” polypropylene sutures to flatten the posterior wall. They dissected indirect sacs from the spermatic cord up to extraperitoneal fat, and they excised and sutured the sacs. The surgeons covered the posterior wall with a polypropylene mesh of appropriate size and shape. They tacked the mesh medially to the rectus sheath, the internal oblique aponeurosis or muscle above and to the inguinal ligament below with “0” polypropylene continuous sutures. A slit in the mesh at the internal ring allowed the emergence of the spermatic cord and created 2 tails. The surgeons crossed the tails of the mesh, wrapped them around the cord and anchored them to the Poupart ligament lateral to the internal ring. The final position of the mesh was anterior to fascia transversalis (anterior localization).
Posterior preperitoneal mesh repair
After entering the preperitoneal space, the surgeons mobilized the spermatic cord and dissected direct or indirect hernia sacs out of the deep inguinal ring, leaving the sacs in situ after reduction of their contents. They sutured a 15 × 15 cm polypropylene mesh to the medial and inferior Cooper ligament with “00” polypropylene sutures and covered the entire area of direct and indirect herniation. To provide a passage for the cord structures, they closed the inferior longitudinal split with sutures of the same material. They further anchored the mesh with interrupted sutures to the superior and lateral musculoaponeurotic structures. The final position of the mesh was posterior to the fascia transversalis (posterior localization).
All the patients had surgery under spinal or general anesthesia and received one dose of first-generation cephalosporin preoperatively. After surgery, the patients transferred to the postanesthesia care unit. We controlled postoperative pain in the postanesthesia care unit with systemic analgesic therapy, including nonopioids and/or opioids. Postoperatively a surgical resident conducted face-to-face interviews with each patient to evaluate chronic postoperative pain and its limitations to their quality of life. Using a standard questionnaire,9 the resident assessed the localization of pain, the impairment as a consequence of pain, the description of pain, the frequency of pain (seldom, occasionally, always) and the level of pain (no pain, slight pain, moderate pain, severe pain).9 We observed patients who reported chronic postherniorrhaphy pain (pain present 2 months after surgery) for a period of 12 months.
Statistical analysis
We used the Fisher exact test to assess the relation between chronic pain and the type of surgery performed. We deemed results to be significant at p = 0.05.
Results
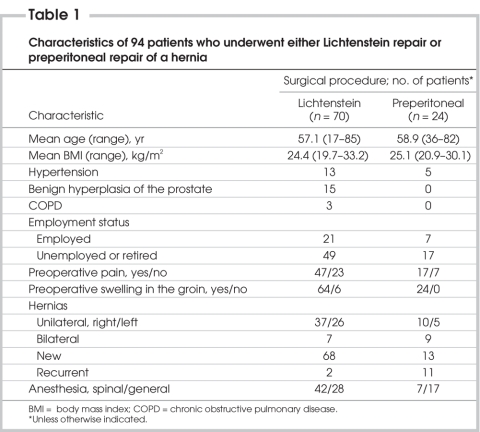
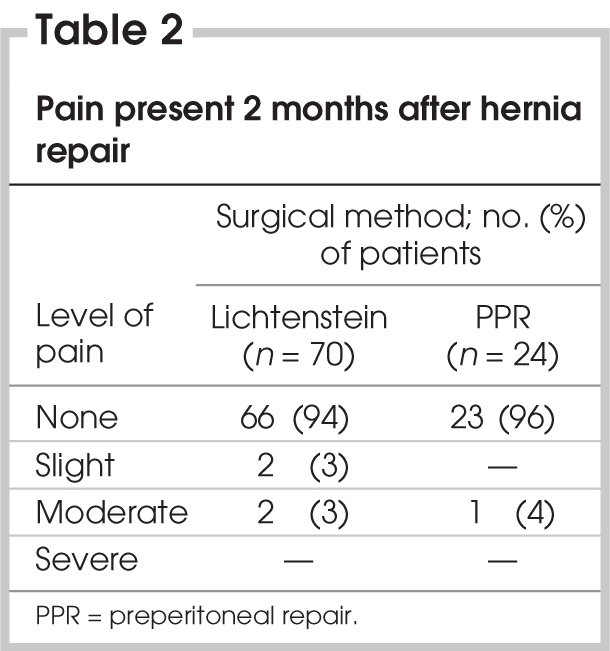
We evaluated 94 patients aged 17–85 years. Patient characteristics are outlined in Table 1. The incidence of preoperative pain was 67% in patients who underwent Lichtenstein mesh repair and 71% in patients who underwent preperitoneal mesh repair. Table 2 shows the results of patient interviews at 2 months after surgery. The overall incidence of chronic pain during this period was 5% (5 patients). About 6% of the patients who underwent Lichtenstein repair (n = 70) and 4% of patients who underwent preperitoneal repair (n = 24) experienced chronic pain, and we observed no significant difference between the outcomes of the surgical techniques (p > 0.05). All of the patients with chronic pain had preoperative pain. Two patients in the Lichtenstein group reported slight pain. Two patients in the Lichtenstein group and 1 patient in the preperitoneal repair group reported moderate pain. No patients reported severe postherniorrhaphy pain. One patient who had chronic pain after preperitoneal hernia repair described the pain as “dull with moderate intensity.” In the Lichtenstein group, 2 patients described “burning” pain, and 2 patients described “pulling” pain. In most patients, the pain was present occasionally and was related to physical stress, including standing for more than 30 minutes, climbing stairs and exercising. All patients were able to work; 3 patients were slightly limited in their participation in sports and social activities. At the 12-month follow-up, only 3 patients in the Lichtenstein group reported chronic pain, therefore, the overall incidence of chronic pain decreased to 3%.
Table 1
Table 2
There was only 1 hernia recurrence in our series, in a patient who underwent preperitoneal repair. There were no complications, including wound infection, and none of the patients reported feeling a foreign body, stiffness or rigidity in the region of the mesh implant.
Discussion
The assessment of postherniorrhaphy has usually focused on recurrence, complications and costs; however, more recently there has been increased attention on other outcomes such as acute or chronic pain and convalescence.10 Defining the point at which pain becomes chronic is always difficult. It has been suggested that chronic pain is pain that is unlikely to resolve or that lasts longer than the usual healing time; various time scales have been suggested, usually 3 or 6 months.11 The definition of chronic pain was not explicit in most reviews evaluating chronic postherniorrhaphy pain. Only a small number of studies included a definition of chronic pain within their methods section. In studies that provided definitions, these varied. Some defined chronic pain as pain that persisted for 1 year postoperatively.12–14 A Dutch study defined chronic pain as pain in the groin or scrotum lasting more than 1 month after surgery.15 Another study defined chronic pain as pain lasting beyond 3 months,7 whereas the criterion for inclusion in a recent review was pain lasting 6 months or longer after hernia repair.16
Macrae17,18 reported that for pain to be classified as chronic postoperative pain, the following criteria should be satisfied: the pain developed after a surgical procedure, it persisted for at least 2 months, other causes for the pain (e.g., continuing infection) were excluded and the pain was deemed to be unrelated to a preexisting problem. In our study we defined chronic postoperative pain as pain that persisted for at least 2 months after surgery, and we found that the overall incidence of chronic postherniorrhaphy pain at the second month was 5%. About 6% of patients who underwent Lichtenstein repair and 4% of patients who underwent preperitoneal repair experienced chronic pain, and we observed no significant difference between the outcomes of both techniques. All patients with chronic pain rated their pain as slight or moderate, and pain was present occasionally and was related to physical stress. None of the patients were unable to work. At the end of the 12-month follow-up period, only 3 patients in the Lichtenstein group reported chronic pain with slight limitations in their ability to participate in sports and social activities. The overall incidence of chronic pain at 12-month follow-up decreased to 3%, and these patients rated their pain as slight or moderate.
Poobalan and colleagues5 studied chronic pain after inguinal herniorrhaphy and observed chronic moderate to severe pain in 10% of the patients undergoing inguinal herniorrhaphy. They also found that chronic pain restricted daily activities in up to 25% of the patients. More recently, Aasvang and Kehlet16 studied chronic pain after inguinal herniorrhaphy and found that the overall incidence of chronic pain after inguinal herniorrhaphy was 12% (18% of patients who had open surgery [range 0%–75.5%] and 6% of patients treated laparoscopically [range 1%–16%]). O'Dwyer and colleagues19 found that 3% of patients reported severe or very severe chronic postherniorrhaphy pain, which had a profound effect on physical and social activities and generally limited the patients' ability to work. The incidence and severity of chronic pain after inguinal herniorrhaphy observed in our study are low compared with these reports.
Several risk factors have been identified to play a crucial role in the development of chronic pain, including the intensity of early postoperative pain, the degree of specialization and experience of the surgeon, and day surgery.5,9 The studies by Poobalan and colleagues and by Aasvang and Kehlet suggested that the severity of early postoperative pain correlated with the risk of chronic pain developing.5,16 In one study, patients who had day surgery reported chronic pain more often than inpatients (54% v. 24%).7 These risk factors were eliminated in our study because the 2 surgeons were experienced in both techniques, the patients did not have day surgery and we treated the acute postoperative pain with nonopioids and/or opioids during the patients' stays in hospital.
The problem of postoperative pain after discharge has generally been poorly studied in patients who had day surgery.20 Contrary to the common belief that day surgery is followed by mild pain, recent studies have shown that the undertreatment of pain is common. About 30%–40% of discharged outpatients may experience moderate to severe pain during the first 24–48 hours.21,22 Lengthy surgical procedures and certain types of surgery, including hernia repair, tend to be associated with severe postoperative pain and require more analgesia.20–22 The intensity of early postoperative pain may be an important predictor of the development of chronic pain after hernia repair.5,16 Therefore, effective control of acute postoperative pain is needed to decrease the incidence of chronic pain after hernia repair in both outpatients and inpatients. Optimal control of postoperative pain in patients who have day surgery should be effective and safe, produce minimal side-effects, facilitate recovery and be easily managed by patients at home. Patients should be informed about the need to treat pain at home, and the information about analgesic use should be given verbally and in writing. Once at home, outpatients with severe pain do not always take their medication as prescribed and may even use alternative analgesics of their choosing. Inpatient status may offer the opportunity for better control of postoperative pain than outpatient status. Therefore, dispensing appropriate analgesia with clear instructions for the outpatients is crucial. Rescue analgesia should be provided if the prescribed analgesic is ineffective. Providing patients prepacked analgesics for anticipated mild, moderate or severe pain along with clear instructions has the potential to better control postoperative pain at home. After discharge, patient follow-up is essential to monitor the effectiveness of pain treatment.20
Our study had some limitations. First, we did not randomly assign equal numbers of patients to the Lichtenstein mesh repair or preperitoneal mesh repair groups to compare the incidence of chronic postherniorrhaphy pain between both groups. However, Lichtenstein mesh repairs are practical for small direct and indirect hernias, whereas the preperitoneal approach can be used to repair the most difficult hernias, including the repairs of recurrent, prevascular, sliding, enormous and bilateral hernias.23 We have performed groin hernia repairs using the above-mentioned indications, so randomization was not used in our study. Second, our study limited the repair of complex hernias to the use of the preperitoneal approach and our sample of patients was small. Future studies involving a greater number of patients are warranted to evaluate chronic pain after the repair of complex hernias using the preperitoneal approach.
In conclusion, the incidence of chronic postherniorrhaphy pain in men who underwent the Lichtenstein repair or preperitoneal repair was low in our study. Inpatient status might have resulted in low incidences with both surgical techniques. Prospective studies are needed to determine whether the lower incidence of chronic pain in inpatients is related to specific anesthetic, analgesic or organizational factors.
Contributors: Drs. Y. and E. Erhan designed the study, acquired and analyzed the data and wrote and reviewed the article. Drs. Mercan and Tok acquired the data, which Drs. Aydede and Mercan analyzed. Dr. Tok wrote the article, which Drs. Aydede and Mercan reviewed. All authors gave final approval for publication.
Competing interests: None declared.
Accepted for publication Apr. 23, 2007
Correspondence to: Associate Prof. Y. Erhan, 113 sokak No: 41, Kat:1, Daire:1, Evren apt., Profesörler Sitesi, Bornova, 35040, Izmir, Turkey; fax 90 232 3757321; yamacerhan@yahoo.com
References
- 1.Kumar S, Wilson RG, Nixon SJ, et al. Chronic pain after laparosopic and open mesh repair of groin hernia. Br J Surg 2002;89:1476-9. [DOI] [PubMed]
- 2.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000;93:1123-33. [DOI] [PubMed]
- 3.Koninger J, Redecke J, Butters M. Chronic pain after hernia repair: a randomized trial comparing Shouldice, Lichtenstein and TAPP. Langenbecks Arch Surg 2004;389:361-5. [DOI] [PubMed]
- 4.Bay-Nielsen M, Nilsson E, Nordin P, et al.; Swedish Hernia Data Base the Danish Hernia Data Base. Chronic pain after open mesh and sutured repair of indirect inguinal hernia in young males. Br J Surg 2004;91:1372-6. [DOI] [PubMed]
- 5.Poobalan AS, Bruce J, Smith WC, et al. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain 2003;19:48-54. [DOI] [PubMed]
- 6.Callesen T, Bech K, Kehlet H. Prospective study of chronic pain after groin hernia repair. Br J Surg 1999;86:1528-31. [DOI] [PubMed]
- 7.Poobalan AS, Bruce J, King PM, et al. Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 2001;88:1122-6. [DOI] [PubMed]
- 8.EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg 2002;235:322-32. [DOI] [PMC free article] [PubMed]
- 9.Bay-Nielsen M, Perkins FM, Kehlet H. Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg 2001;233:1-7. [DOI] [PMC free article] [PubMed]
- 10.Kehlet H, Bay-Nielsen M, Kingsnorth A. Chronic postherniorrhaphy pain — a call for uniform assessment. Hernia 2002;6:178-81. [DOI] [PubMed]
- 11.Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: IASP Press; 1994.
- 12.Laparoscopic versus open repair of groin hernia: a randomized comparison. MRC Laparoscopic Groin Hernia Trial Group. Lancet 1999;354:185-90. [PubMed]
- 13.McGillicuddy JE. Prospective randomized comparison of the Shouldice and Lichtenstein hernia repair procedures. Arch Surg 1998;133:974-8. [DOI] [PubMed]
- 14.Callesen T, Bech K, Thorup J, et al. Cryoanalgesia: effect on postherniorrhaphy pain. Anesth Analg 1998;87:896-9. [DOI] [PubMed]
- 15.Juul P, Christensen K. Randomized clinical trial of laparoscopic versus open inguinal hernia repair. Br J Surg 1999;86:316-9. [DOI] [PubMed]
- 16.Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguial herniorrhaphy. Br J Anaesth 2005;95:69-76. [DOI] [PubMed]
- 17.Macrae WA, Davies HTO. Chronic postoperative pain. In: Crombie IK, editor. Epidemiology of pain. Seattle: IASP Press; 1999. p. 125-42.
- 18.Macrae WA. Chronic pain after surgery. Br J Anaesth 2001;87:88-98. [DOI] [PubMed]
- 19.O'Dwyer PJ, Serpell MG, Millar K, et al. Local or general anaesthesia for open hernia repair: a randomized trial. Ann Surg 2003;237:574-9. [DOI] [PMC free article] [PubMed]
- 20.Rawal N. Analgesia for day-case surgery. Br J Anaesth 2001;87:73-87. [DOI] [PubMed]
- 21.Beauregard L, Pomp A, Choinière M. Severity and impact of pain after day-surgery. Can J Anaesth 1998;45:304-11. [DOI] [PubMed]
- 22.Rawal N, Hylander J, Nydahl P-A, et al. Survey of postoperative analgesia following ambulatory surgery. Acta Anaesthesiol Scand 1997;41:1017-22. [DOI] [PubMed]
- 23.Stoppa RE, Warlaumont CR. The preperitoneal approach and prosthetic repair of groin hernia. In: Nyhus LM, Condon RE, editors. Hernia. 3rd ed. Philadelphia: JB Lippincott Company; 1989. p.199-225.