Abstract
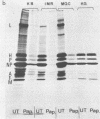
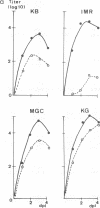
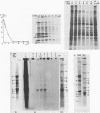
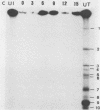
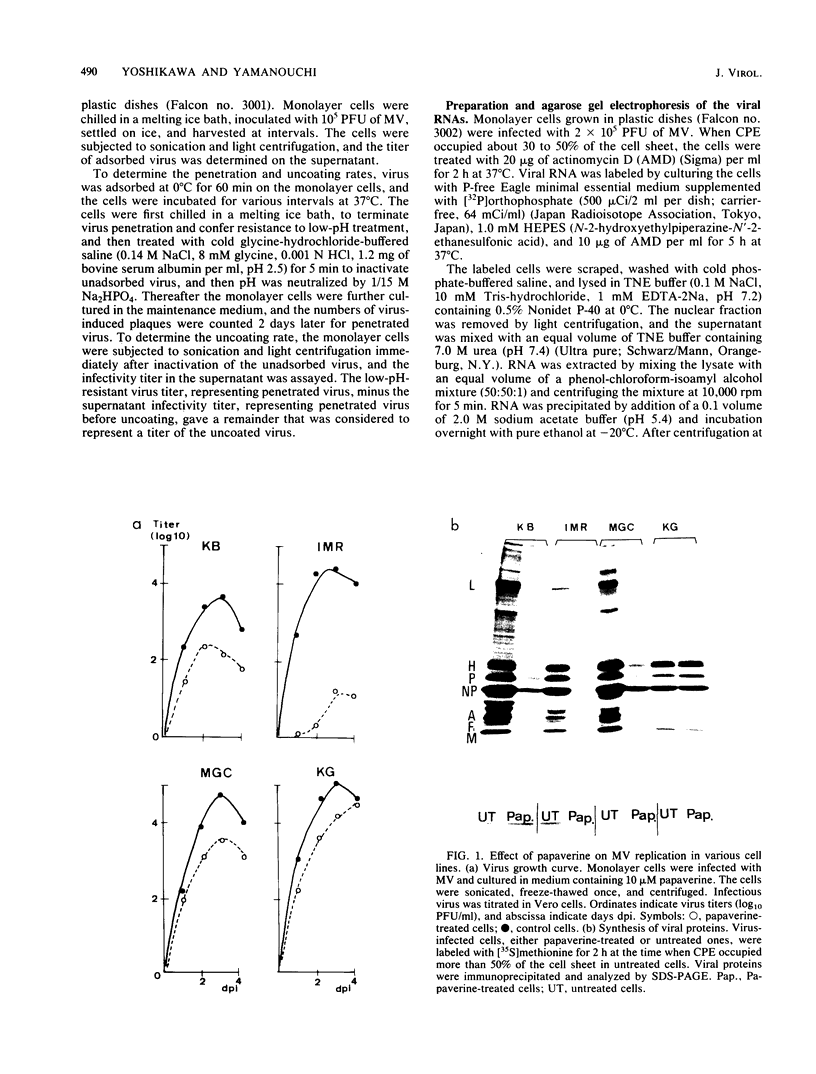
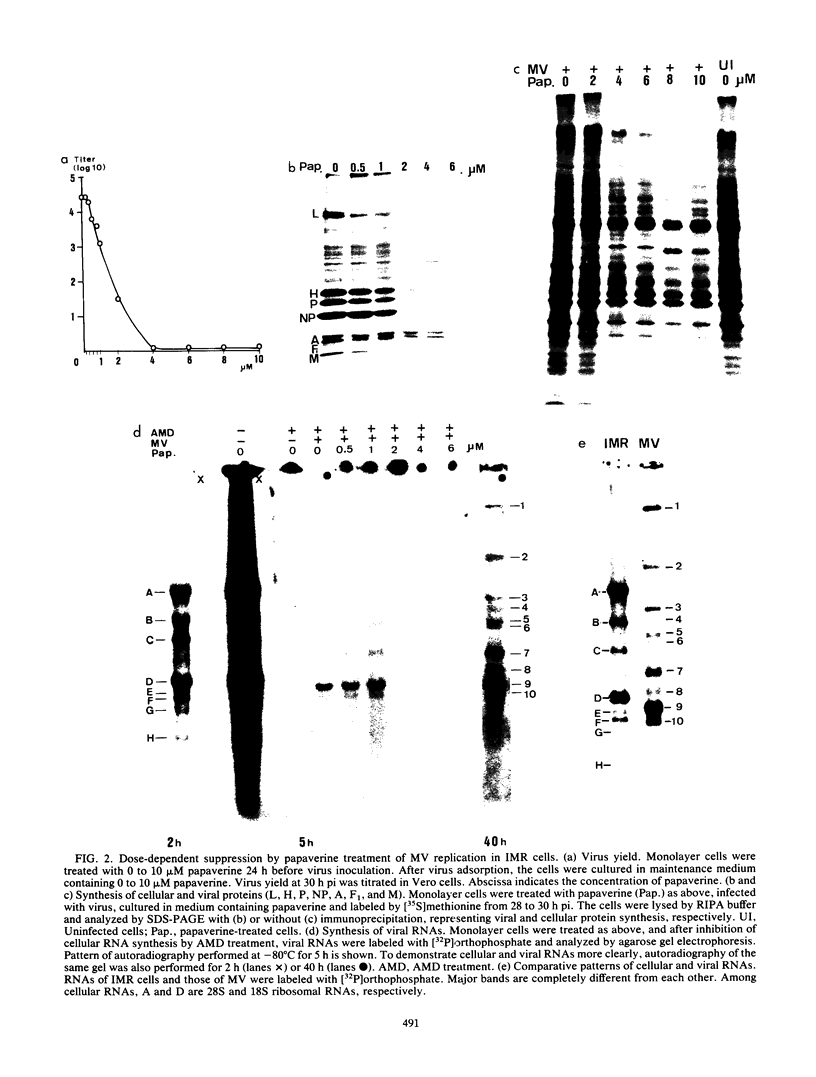
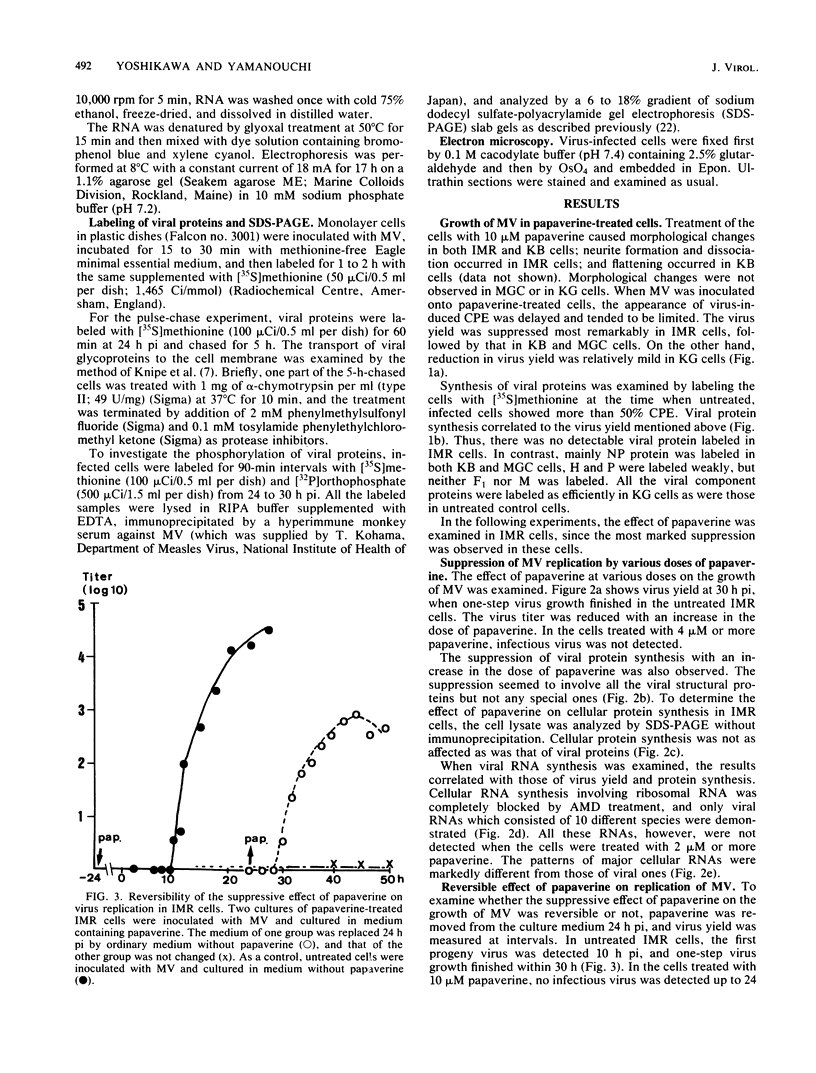
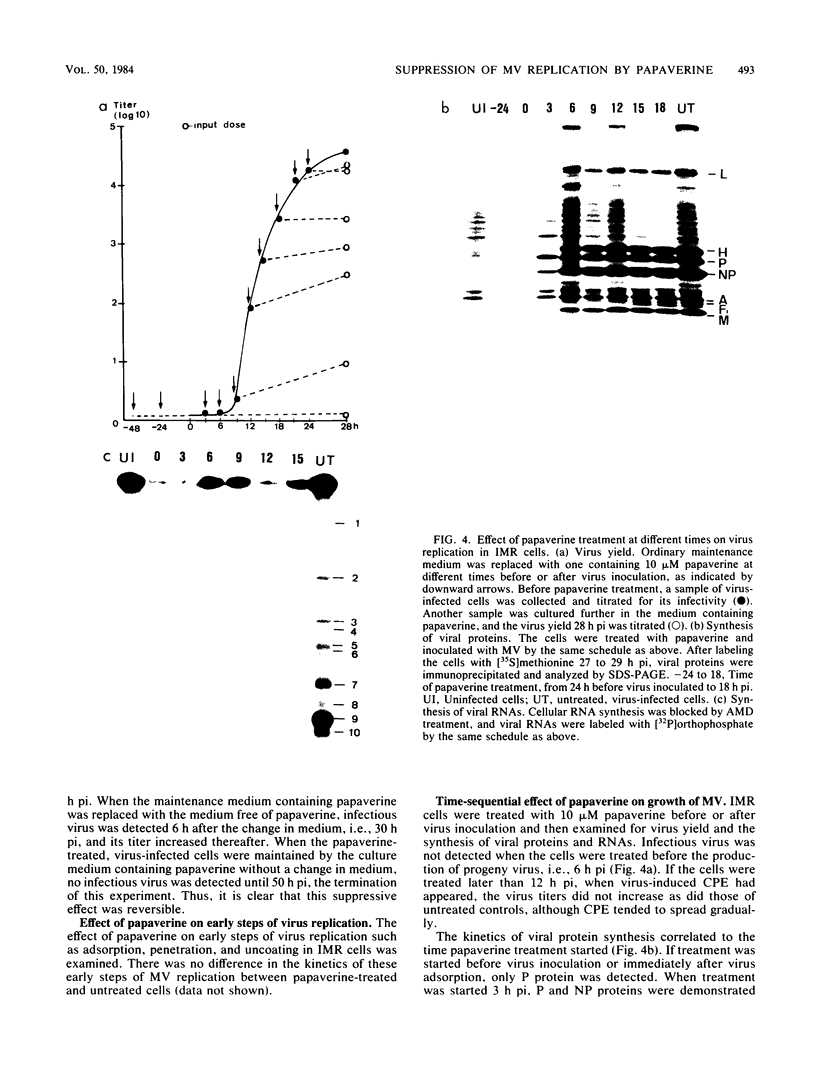
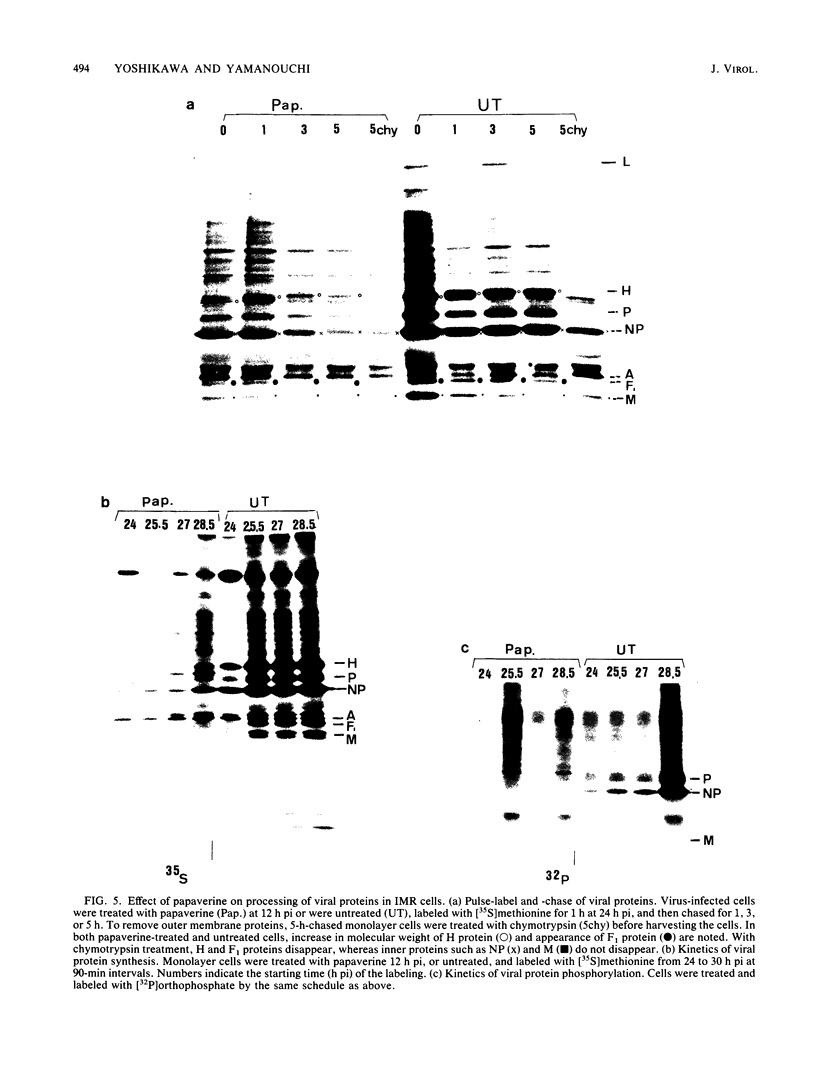
The replication of measles virus in human neural and nonneural cell lines in terms of growth and cytopathic effect was affected by treatment of the cells with papaverine, which increases endogenous cyclic AMP. Suppression of virus growth was most prominent in neuroblastoma cells, followed by that in epidermoid carcinoma and glioblastoma cells, whereas the suppressive effect was relatively weak in oligodendroglioma cells. The papaverine-induced suppression of virus growth in neuroblastoma cells was studied in detail. The suppression that occurred was dependent on the dose of papaverine and was reversible. By treatment with 10 microM papaverine, virus-cell interactions were modified as follows: (i) early replication steps such as adsorption, penetration, and uncoating of the virus were not affected; (ii) synthesis of viral RNAs, including genomic RNA and mRNA, was inhibited; (iii) translation of viral proteins from mRNA was not blocked; and (iv) glycosylation and transport of viral glycoproteins to the cell membrane were not inhibited, but phosphorylation was blocked. The significance of suppressed virus replication in neural cells is discussed in relation to the persistence mechanisms of measles virus in the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Fleury H., Pasquier P. D. Replication of measles virus in a cell culture from a glioblastoma of human origin. J Neuropathol Exp Neurol. 1977 Sep-Oct;36(5):842–845. doi: 10.1097/00005072-197709000-00007. [DOI] [PubMed] [Google Scholar]
- Gorecki M., Rozenblatt S. Cloning of DNA complementary to the measles virus mRNA encoding nucleocapsid protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3686–3690. doi: 10.1073/pnas.77.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Ventura P., Gibbs C. J., Jr, Tourtellotte W. W. Measles virus nucleotide sequences: detection by hybridization in situ. Science. 1981 May 8;212(4495):672–675. doi: 10.1126/science.7221554. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Lodish H. F., Baltimore D. Localization of two cellular forms of the vesicular stomatitis viral glycoprotein. J Virol. 1977 Mar;21(3):1121–1127. doi: 10.1128/jvi.21.3.1121-1127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune K., Yamanouchi K., Yoshikawa Y., Hayami M., Shishido A. Growth of measles virus in continuous cell lines derived from the nervous tissues of human and rat. Arch Virol. 1979;61(1-2):115–125. doi: 10.1007/BF01320596. [DOI] [PubMed] [Google Scholar]
- Kono S., Kohase M., Suganuma M. Growth of measles virus in a mouse derived established cell line of L cells. Jpn J Med Sci Biol. 1968 Oct;21(5):301–311. doi: 10.7883/yoken1952.21.301. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J. C., Rezee A., Galama J. M. Activation of measles virus from silently infected human lymphocytes. J Exp Med. 1978 Oct 1;148(4):940–952. doi: 10.1084/jem.148.4.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A., Carrigan D. R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake E. Establishment of a human oligodendroglial cell line. Acta Neuropathol. 1979 Apr 12;46(1-2):51–55. doi: 10.1007/BF00684804. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Rapp F. Measles virus and its associated diseases. Bacteriol Rev. 1977 Sep;41(3):636–666. doi: 10.1128/br.41.3.636-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J., Macintyre E. H. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74(4):465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Robbins S. J., Rapp F. Inhibition of measles virus replication by cyclic AMP. Virology. 1980 Oct 30;106(2):317–326. doi: 10.1016/0042-6822(80)90255-x. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Gesang C., Lavie V., Neumann F. S. Cloning and characterization of DNA complementary to the measles virus mRNA encoding hemagglutinin and matrix protein. J Virol. 1982 Jun;42(3):790–797. doi: 10.1128/jvi.42.3.790-797.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop W. G., Baringer J. R. Persistent, slow and latent viral infections. Prog Med Virol. 1982;28:1–43. [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Udem S. A., Cook K. A. Isolation and characterization of measles virus intracellular nucleocapsid RNA. J Virol. 1984 Jan;49(1):57–65. doi: 10.1128/jvi.49.1.57-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Meissner H. C. Measles and SSPE viruses: similarities and differences. Prog Med Virol. 1982;28:65–95. [PubMed] [Google Scholar]
- West G. J., Uki J., Herschman H. R., Seeger R. C. Adrenergic, cholinergic, and inactive human neuroblastoma cell lines with the action-potential Na+ ionophore. Cancer Res. 1977 May;37(5):1372–1376. [PubMed] [Google Scholar]
- Yoshikawa Y., Yamanouchi K., Morikawa Y., Sakaguchi M. Characterization of canine distemper viruses adapted to neural cells and their neurovirulence in mice. Microbiol Immunol. 1983;27(6):503–518. doi: 10.1111/j.1348-0421.1983.tb00612.x. [DOI] [PubMed] [Google Scholar]