Abstract
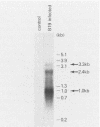
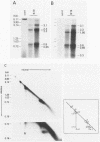
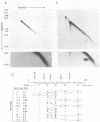
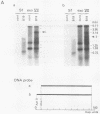
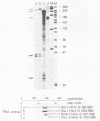
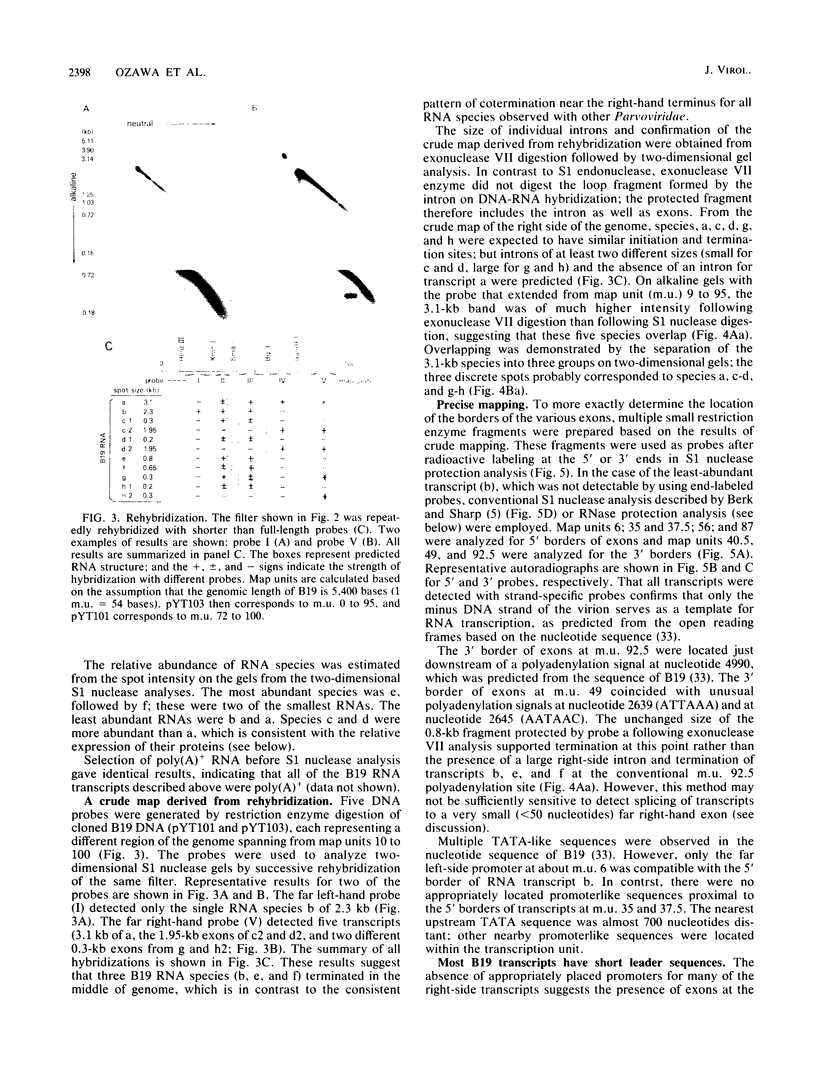
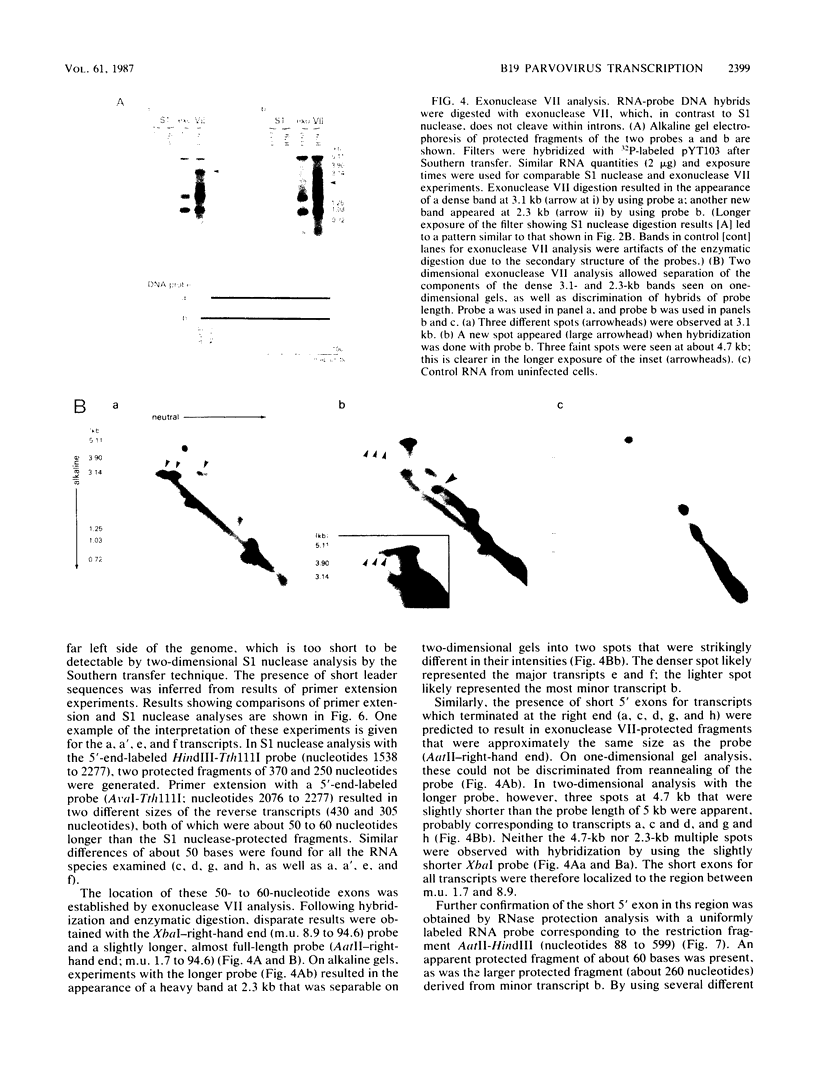
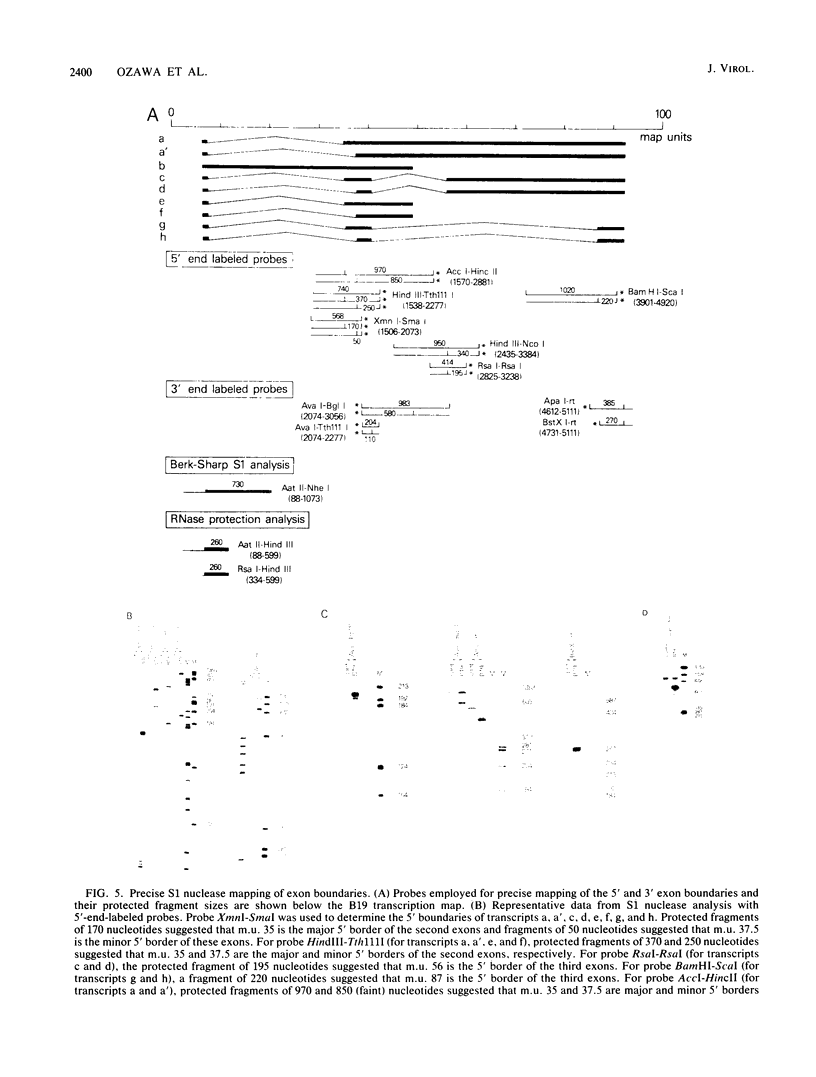
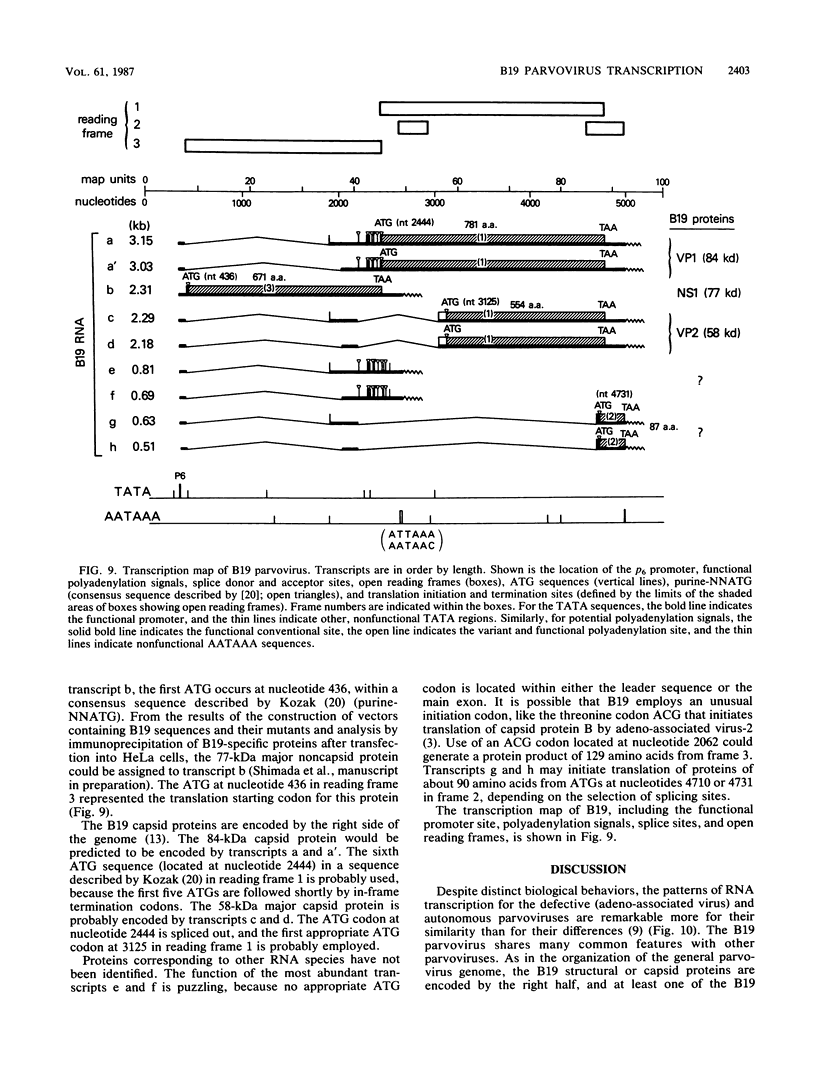
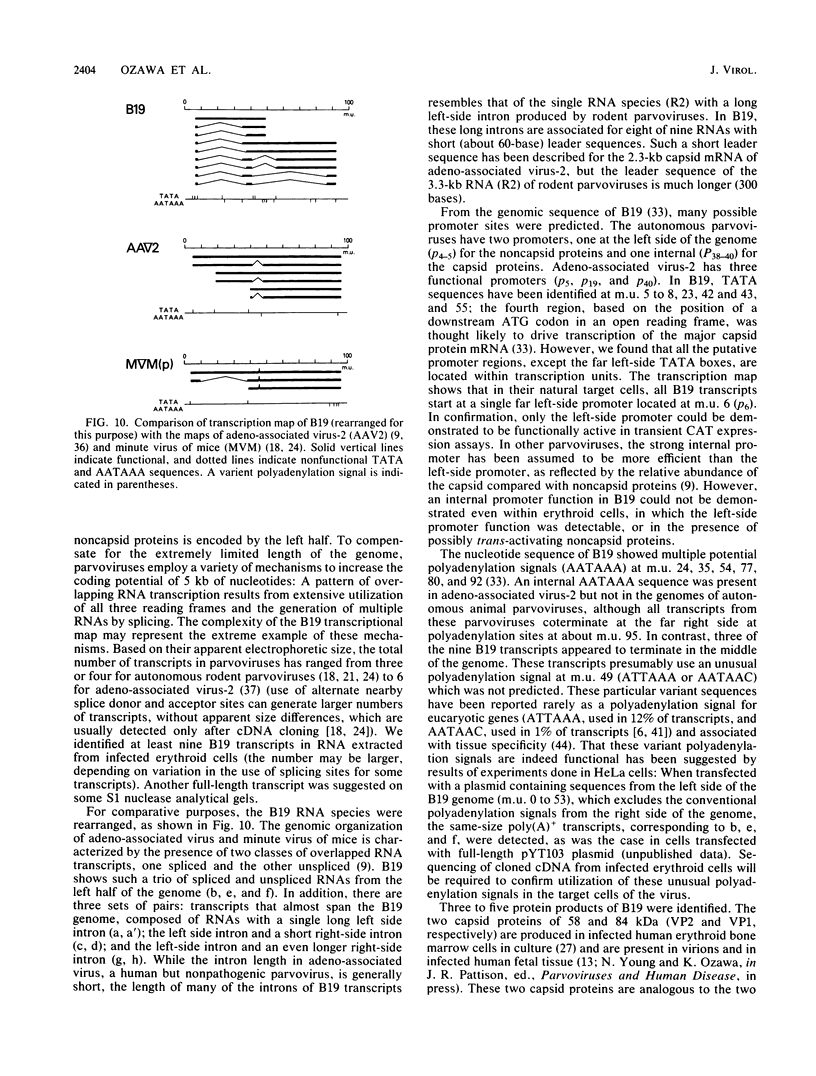
The B19 parvovirus, a small single-stranded DNA virus of 5.4 kilobases, is pathogenic in humans. B19 has remarkable specificity for erythroid progenitor cells and has been propagated in vitro only with human erythroid bone marrow. Replication of viral DNA and the viral protein products of B19 appear similar to those of other animal parvoviruses. However, B19 transcription had unusual features in comparison with that in other animal parvoviruses. At least nine overlapping poly(A)+ transcripts were identified in infected cells; all but one contained large introns. B19 differed from other parvoviruses in the initiation of all transcripts at a strong left side promoter (p6) and the absence of a functional internal promoter; the presence of short 5' leader sequences of about 60 bases and very large introns for RNAs encoded by the right side of the genome; two separate transcription termination sites, in contrast to cotermination at the far right side of the genome for other parvoviruses; the probable utilization by three transcripts of a variant polyadenylation signal (ATTAAA or AATAAC) in the middle of the genome; and the abundance of two unique transcripts from the middle of the genome which did not code for capsid proteins. The unusual transcription map of B19 suggests that regulation of the relative abundance of transcripts occurs by splicing and termination-polyadenylation events rather than by promoter strength. In combination with the published nucleotide sequence, the novel transcription map separated the pathogenic B19 virus at a molecular level from other animal parvoviruses and human adeno-associated virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Higgins P. G., Davis L. R., Willman J. S., Jones S. E., Kidd I. M., Pattison J. R., Tyrrell D. A. Experimental parvoviral infection in humans. J Infect Dis. 1985 Aug;152(2):257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Lewis E., Kidd I. M., Hall S. M., Cohen B. J. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984 Aug;93(1):85–93. doi: 10.1017/s0022172400060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M., Robberson B. L. U1, U2, and U4/U6 small nuclear ribonucleoproteins are required for in vitro splicing but not polyadenylation. Cell. 1986 Aug 29;46(5):691–696. doi: 10.1016/0092-8674(86)90344-2. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., McKie V. C., Anderson L. J., Astell C. R., Tattersall P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol. 1986 Nov;60(2):548–557. doi: 10.1128/jvi.60.2.548-557.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott P. D., Welply G. A., Anderson M. J. Serologically proved intrauterine infection with parvovirus. Br Med J (Clin Res Ed) 1984 Dec 15;289(6459):1660–1660. doi: 10.1136/bmj.289.6459.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Roeder R. G. Parvovirus H-1 expression: mapping of the abundant cytoplasmic transcripts and identification of promoter sites and overlapping transcription units. J Virol. 1986 May;58(2):271–280. doi: 10.1128/jvi.58.2.271-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Young N. S., Mortimer P. P., Moore J. G., Humphries R. K. Characterization of a virus that causes transient aplastic crisis. J Clin Invest. 1984 Jan;73(1):224–230. doi: 10.1172/JCI111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N., Harrison M., Moore J., Mortimer P., Humphries R. K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest. 1984 Dec;74(6):2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]