Abstract
For malaria transmission, Plasmodium parasites must successfully complete gametocytogenesis in the vertebrate host. Differentiation into mature male or female P. falciparum gametocytes takes 9–12 days as the parasites pass through 5 distinct morphologic stages (I – V). To evaluate the signals controlling the initiation of stage and/or sex-specific expression, reporter constructs containing the 5’-flanking regions (FR) of seven genes with distinct expression patterns through gametogenesis were developed. The regulatory information present in the 5’-FR of each selected gene was found to be sufficient to drive the appropriate sex- and stage-specific pattern reporter gene expression. The transformed parasite lines also provide in vivo markers to identify gametocytes at specific stages, including a subpopulation of schizonts that express early gametocyte markers.
In P. falciparum, the parasite that causes the most virulent form of malaria in 30 humans, differentiation into a mature male or female gametocyte takes 9–12 days during which time the parasite passes through 5 distinct morphologic stages (I – V). Once taken up in a blood meal by a mosquito the gametocytes are stimulated to round up and emerge from the red blood cell (RBC)2. Male gametocytes undergo 3 rounds of DNA replication and exflagellate, producing microgametes that can fertilize emerged female gametes and continue sporogonic development. The time course and morphologic changes associated with gametocytogenesis have been known for many years [1] and recently, the P. falciparum transcriptome through gametocytogenesis [2,3] and proteomic analysis of stage V gametocytes and gametes have been reported [4]. However, the regulatory mechanisms responsible for stage- and sex-specific expression in P. falciparum gametocytes remain unknown. It was recently demonstrated in P. berghei, which causes malaria in rodents, that mRNA transcripts for 370 proteins produced once the parasite enters the mosquito midgut are generated in late stage gametocytes and stored until the parasite is taken up in a blood meal [5].
To evaluate sex- and stage-specific expression through gametocytogenesis in P. falciparum, reporter constructs were produced to determine the regulatory regions required to drive the distinct expression patterns of seven selected genes. The selected genes include two genes that are expressed in both asexual parasites and gametocytes, alpha-tubulin (PFI0180w), and calmodulin (PF14_0323), three gametocyte-specific genes, Pfs16 (PFD0310w), Pfs230 (PFB0405w), and Pfs28 (PF10_0302) and two male gametocyte-specific genes, alpha-tubulin II (PFD1050w) and PfsMR5 (PFB0400w) [6,7]. Microarray analysis indicates that of the selected gametocyte-specific genes, Pfs16, is expressed earliest. Pfs16 transcript levels reach a maximum in stage II gametocytes, followed by alpha-tubulin II (stage II/III gametocytes), then Pfs230 (stage III gametocytes), PfsMR5 (stage IV gametocytes) and Pfs28 (stage V gametocytes) [2]. The P. berghe ortholog of Pfs28 (P28) is one of the genes mentioned previously that is transcribed in stage IV gametocytes, but translation is repressed until gametogenesis is stimulated by uptake into the mosquito midgut [5]. This translational repression has been shown to be mediated by an RNA helicase, DOZI.
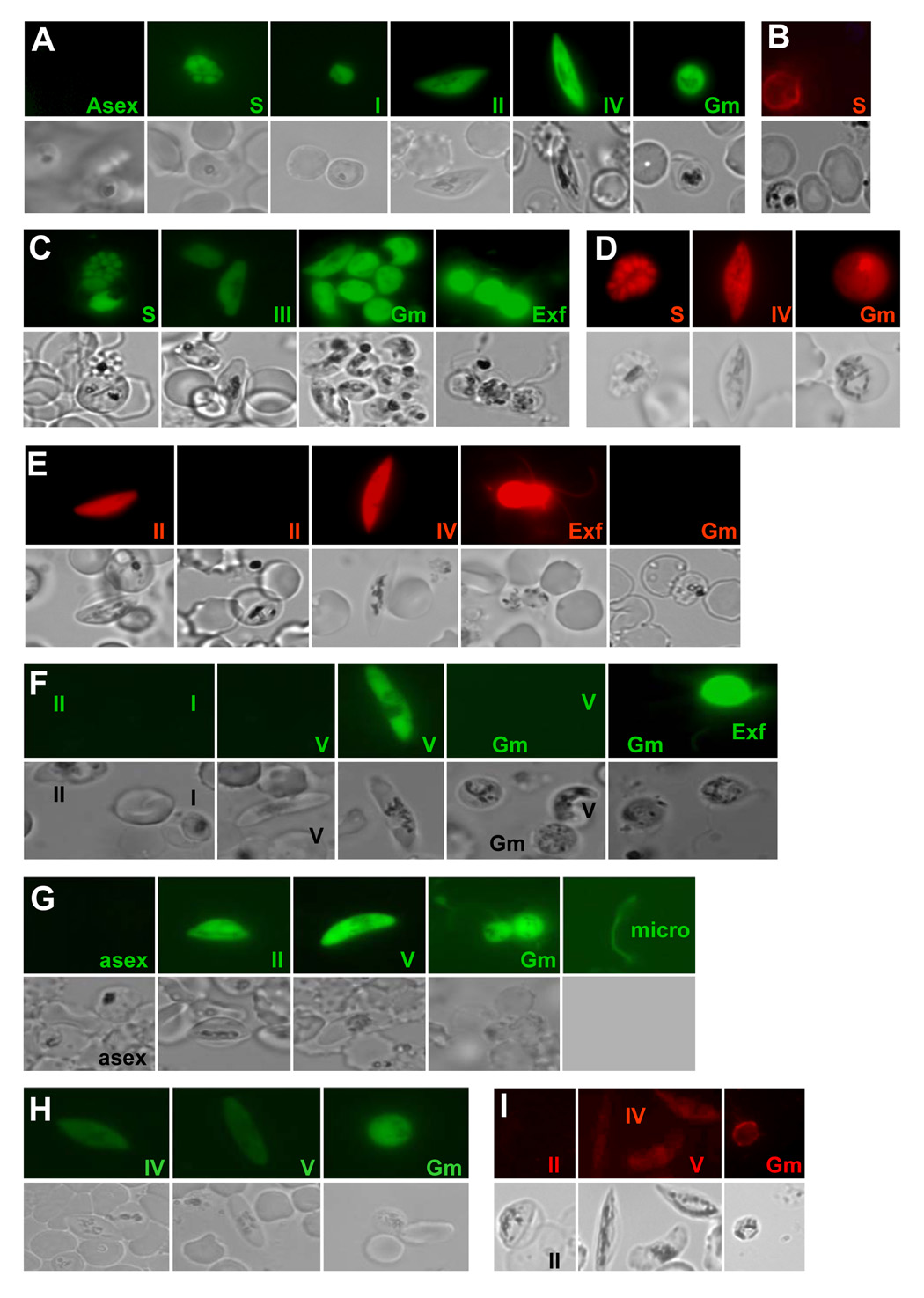
Transformation of wt 3D7 parasites with a reporter construct containing −783 bp 5’ to the Pfs16 start codon inserted directly upstream from GFP (pCBM.BSD.5’16.GFP) resulted in the production of a parasite line (5’16.GFP) that expressed GFP in a majority of the morphologically distinct gametocytes (stages I –V), as well as a small subpopulation of late stage schizonts and ring stage parasites (Fig. 1A). Pfs16 protein expression has previously been associated with early gametocytes but has not been reported in schizonts [8–12]. Subsequent immunofluorescence assay (IFA) also identified a small population of schizonts that expressed Pfs16 protein (Fig. 1B). These findings would be consistent with the merozoites released from the Pfs16-positive schizonts being committed to gametocyte formation. It has recently been suggested that Pfs16 mRNA increases in the “asexual” cycle before invading merozoites develop into stage I gametocytes, but that translation is delayed until stage I gametocytes [12]. The identification of Pfs16 protein positive schizonts suggests that both transcription and translation may begin prior to invasion of a committed merozoite and development into a stage I gametocyte. The precise timing and quantification of Pfs16 mRNA and protein in a specific cell as it becomes committed to gametocytogenesis is difficult to assay directly. Only a subpopulation of the replicating cells in a culture will become schizonts with merozoites committed to sexual differentiation and they are morphologically identical to those that will continue asexual reproduction. The 5’16.GFP parasites described here provide a useful tool to continue to evaluate this important transition in the parasite’s life cycle as additional early “pre”-gametocyte markers are identified.
Figure 1. Reporter gene expression in P. falciparum parasites.
Strain 3D7 parasites stably transformed with reporter constructs containing the indicated 5’-FR were harvested through sexual development [11,20] and assayed for epifluorescence, (A) Pfs16 (5’-FR bp −783 to 1), (C) Calmodulin (5’-FR 993 bp [14]) (D) α-tubulin (5’-FR bp −1211 to 1) (E) α-tubulin II (5’-FR bp −1134 to 1) (F) PfsMR5 (5’-FR bp −1011 to −79) (G) Pfs230 (5’-FR bp −1595 to −25) (H) Pfs28 (5’-FR bp −1263 to 1). (B & I) Immunofluoresence assay of wt 3D7 parasites using (B) mouse anti-recombinant Pfs16 antiserum(1:1,000) [11] or (I) mouse anti-recombinant Pfs28 (1:500), Malaria Research & Reference Reagent Resource Center, MRA-18 [11]. The developmental stages of the parasites are indicated: Asexual ring and trophozoite stages (asex), Schizont (S), Gametocyte stages I–V (I–V), Gametes (Gm), Exflagellating males (Exf), and Released microgamete (micro).
GFP expression continues through all 5 stages of gametocytogenesis in 5’16.GFP parasites (Fig 1A). A similar time course of expression is observed for native Pfs16 in wt 3D7 gametocytes by IFA [8–10,12]. Pfs16 protein levels increase through stage II and remain high through the production of mature stage V gametocytes. Following emergence of the gamete from the RBC, native Pfs16 is shed with the parasitophorous vacuole membrane (PVM). In contrast, 5’16.GFP gametes continue to fluorescence for 1 hour after RBC emergence. This difference is consistent with the distinct subcellular locations of GFP and native Pfs16. In 5’16.GFP cells GFP is expressed as a soluble cytoplasmic protein [13], while native Pfs16 has a predicted transmembrane domain and has been reported to be associated with the PVM [8–10,12]. Consequently, native Pfs16 is not associated with the gamete after the PVM is released during RBC emergence.
As a control to demonstrate asexual expression of GFP and DsRed, 993 bp of the 5’-FR of calmodulin [14] and base pairs −1211 to +9 of alpha-tubulin were inserted upstream of GFP in pDH.TgA.22 (pDH.TgA.22.5’CAM.GFP) or DsRed in pCBM.BSD (pCBM.BSD.5’αtub.RED), respectively. Parasites transformed with pDH.TgA.22.5’CAM.GFP (5’CAM.GFP) expressed GFP in the majority of asexual and sexual stage parasites (stages I–V and gametes), while those transformed with pCBM.BSD.5’αtub.RED (5’αtub.RED) expressed DsRed in the majority of schizonts and both male and female gametocytes (Fig. 1C & D). This pattern is consistent with the transcription pattern reported for native calmodulin and native alpha-tubulin. Moreover, it demonstrates that with the 5’-FR GFP or DsRed can be expressed in both asexual and sexual stage parasites and that the level of reporter gene expression is high enough to visualize individual exflagellating microgametocytes. Unfortunately in Fig. 1C the individual fluorescent microgametocytes are difficult to see in the photograph due to the limited focal plane of the 100x objective, their rapid flagellar movement, and their small size in relation to the residual body of the microgametocyte.
To evaluate the 5’-FR of α-tubulin II, wt 3D7 parasites were transformed with pCBM.BSD.5’αtubII.Red (5’αtubII.Red), which contained −1134 bp 5’ to α-tubulin II start codon inserted upstream from DsRed. In contrast to the pCBM.BSD.5’αtub.Red parasites, 5’αtubII.Red parasites did not express DsRed until stage II of gametocytogenesis and then only a subpopulation of gametocytes were positive (Fig. 1E). DsRed expression continued as the 5’αtubII.Red gametocytes matured to stage V and were able to exflagellate. The remaining macrogametes were not fluorescent (Fig. 1E). This DsRed expression pattern is consistent with male-specific expression, as has been reported for native α-tubulin II using anti-α-tubulin II antibodies [6].
Like α-tubulin II, PfsMR5 has also been shown by IFA to be expressed only in male gametocytes, but it is not detected until male gametocytes mature to stage IV–V [7]. Base pairs −1011 to −79 upstream of the PfsMR5 start codon were inserted 5’ to GFP in pDH.TgA.22 (pDH.TgA.22.5’MR5.GFP) and the resulting plasmid was transformed into wt 3D7 parasites. Transformed parasites (5’MR5.GFP) did not begin to express GFP until they developed into stage IV–V gametocytes and then only a subpopulation of stage V parasites were fluorescent (Fig. 1F). As with 5’αtubII.Red parasites the fluorescent population could be stimulated to exflagellate, consistent with male-specific PfsMR5 protein expression. Native PfsMR5 transcript levels are similar to the DsRed and native protein expression pattern, not peaking until stage IV gametocytes. The data for PfsMR5 indicate that the 1011 bp upstream of the start codon regulate both sex- and stage- specificity.
Expression of GFP under the control of −1595 to −25 bp upstream of the Pfs230 start codon in pDH.TgA.22 (pDH.TgA.22.5’230.GFP) was first observed in transformed parasites (5’230.GFP) at stage II of gametocytogenesis and continued in both male and female parasites even following gametogenesis (Fig. 1G). The time course of GFP expression in 5’230.GFP cells is similar to the protein expression profile observed by IFA and northern blot analysis.
GFP expression in parasites transformed with plasmid pDH.TgA.22 containing −1263 bp 5’ to the Pfs28 start codon inserted upstream of GFP was first visualized in stage IV gametocytes and continued through gametogenesis (plasmid, pDH.TgA.22.5’28.GFP and transformed parasite line, 5’Pfs28.GFP) (Fig. 1H). The native Pfs28 transcript level is consistent with the GFP expression pattern. This suggests that during gametogenesis in P. falciparum the 5’-FR alone is sufficient to induce stage-specific expression that corresponds to transcription. Studies of the P. berghei ortholog of Pfs28, P28, suggest that following gametogenesis protein expression levels increase significantly as the parasite continues to develop into an ookinete [5]. In P. berghei this later increase in protein translation is regulated by a U-rich region located in the 3’ FR, which was not included in the pDH.TgA.22.5’28.GFP construct used in this study [15]. It is possible that Pfs28 protein expression also increases during the gamete-to-ookinete transition. However, in contrast to P. berghei, P. falciparum ookinetes are not formed in vitro. Therefore, further investigation of the later stages in P. falciparum requires in vivo analysis in the mosquito midgut which is beyond the scope of this study. By IFA, anti-Pfs28 antiserum reacts weakly with structures inside stage IV and V gametocytes and the surface of gametes 1 hour after emergence, which is consistent with the presence of Pfs28 transcript and GFP expression in the 5’Pfs28.GFP cell line (Fig. 1I).
The data presented clearly indicate that the presence of the 5’-FR of the genes tested was sufficient to drive reporter gene expression that mirrored transcript levels of the native gene (Table 1). The 5’-FR was also all that appeared to be required to reproduce the appropriate sex- and stage-specific induction of protein expression during intraerythrocytic sexual development within the human host. As expected, GFP and DsRed were expressed as cytoplasmic proteins and consequently had a different subcellular localization pattern than the membrane-associated proteins evaluated in this study. The set of reporter constructs described here can be used to initiate protein expression at each stage of gametocytogenesis. The transformed-parasite lines generated allow gametocyte development to be monitored during the intraerythrocytic cycle and serve as a base to further dissect the mechanisms regulating stage- and sex-specific expression.
Table 1.
Summary of the Reporter Gene Expression Results
| Common Name | PlasmoDB ID | 5’ Flanking Region | Initiation of Reporter Gene Expression |
|---|---|---|---|
| Pfs16 | PFD0310w | −783 to 1 | Subpopulation of schizonts & early rings |
| α-tubulin II | PFD1050w | −1134 to 1 | Stage II, male gametocytes |
| Pfs230 | PFB0405w | −1595 to −25 | Stage II gametocytes |
| PfsMR5 | PFB0400w | −1011 to −79 | Stage IV, male gametocytes |
| Pfs28 | PF10_0302 | −1263 to 1 | Stage IV gametocytes |
| α-tubulin | PFI0180w | −1211 to 1 | Schizonts |
The common names and www.PlasmoDB.org ID numbers of the selected genes are indicated in the first two columns. The third column lists the base pairs 5’ to the selected gene that were inserted upstream of the reporter gene in the transformation vector. The first stage at which fluorescent parasites were observed following stable transformation is listed in the fourth column. In each case the GFP expression pattern corresponds with the sex and stage-specific IFA data and the transcript pattern determined by microarray and northern analysis. Wild type 3D7 parasite lines were transformed with reporter constructs containing the coding region of green fluorescence protein (GFP) [17] or red fluorescent protein from Discosoma sp. reef coral (DsRed) (Clontech, Mountain View, CA) between the XbaI and BamHI sites in P. falciparum transformation plasmids, pDH.TgA.22 [18] or pCBM.BSD [19] as indicated. The 5’-FR of the selected genes were amplified using polymerase chain reaction and then inserted 5’ to the coding region of the reporter gene between the SacII and XbaI sites. Pyrimethamine (15 ng ml−1)- or blasticidin (5 µM)-resistant parasites were assayed directly by fluorescence microscopy (Axiovert 200/Axiovision 4.3, Zeiss) to evaluate GFP or DsRed expression.
The unique expression pattern driven by each of the 5’-FR of the gametocyte-specific genes tested here indicates that each region contains distinct regulatory elements. One hundred and fifty six genes with homology to transcription associated proteins (TAPs) were identified in the P. falciparum genome, which results in a TAP/genome ratio that is only a third of that found in most other eukaryotes [16]. Thirteen of the predicted TAPs are specific to gametocytes and 39 are expressed in gametocytes as well as other stages of the life cycle. None of these has yet been linked to the expression of a specific gametocyte gene or stage. Bioinformatic approaches to identifying regulatory regions have been hampered by the A/T richness of the P. falciparum untranslated regions and the diversity of possible gametocyte expression patterns. The set of transformed parasites generated in this work provide an important step in experimentally defining the regulatory sequences involved in stage- and sex-specific gene expression during gametocytogenesis.
Acknowledgements
This investigation received financial support from Public Health Service grants AI069314 and AI48826 from the National Institute of Allergy and Infectious Disease. We thank B. Czesny for technical support, I. Rupp and Dr. J. Heller for critical reading of the manuscript, and Malaria Research & Reference Reagent Resource Center for generously providing reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: DsRed, Discosoma sp. reef coral red fluorescent protein; FR, flanking region; GFP, green fluorescent protein; IFA, Indirect immunofluorescent assay; PfsMR5, Pfs230 paralog; PVM, parasitophorous vacuole membrane; RBCs, red blood cells; TAPs, transcription associated proteins
References
- 1.Lobo CA, Kumar K. Sexual differentiation and development in the malaria parasite. Parasitol Today. 1998;14:146–150. doi: 10.1016/s0169-4758(97)01210-6. [DOI] [PubMed] [Google Scholar]
- 2.Young JA, Fivelman QL, Blair PL, et al. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Silvestrini F, Bozdech Z, Lanfrancotti A, et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:100–110. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Lasonder E, Ishihama Y, Andersen JS, et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature. 2002;419:537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 5.Mair GR, Braks JA, Garver LS, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawlings DJ, Fujioka H, Fried M, Keister DB, Aikawa M, Kaslow DC. Alpha-tubulin II is a male-specific protein in Plasmodium falciparum. Mol Biochem Parasitol. 1992;56:239–250. doi: 10.1016/0166-6851(92)90173-h. [DOI] [PubMed] [Google Scholar]
- 7.Eksi S, Williamson KC. Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PFB0400w. Mol Biochem Parasitol. 2002;122:127–130. doi: 10.1016/s0166-6851(02)00091-9. [DOI] [PubMed] [Google Scholar]
- 8.Bruce MC, Carter RN, Nakamura K, Aikawa M, Carter R. Cellular location and temporal expression of the Plasmodium falciparum sexual stage antigen Pfs16. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 9.Dechering KJ, Thompson J, Dodemont HJ, Eling W, Konings RN. Developmentally regulated expression of Pfs16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 1997;89:235–244. doi: 10.1016/s0166-6851(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 10.Lobo CA, Konings RN, Kumar N. Expression of early gametocyte-stage antigens Pfg27 and Pfs16 in synchronized gametocytes and non-gametocyte producing clones of Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:151–154. doi: 10.1016/0166-6851(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 11.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143:90–99. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Lanfrancotti A, Bertuccini L, Silvestrini F, Alano P. Plasmodium falciparum: mRNA co-expression and protein co-localisation of two gene products upregulated in early gametocytes. Exp Parasitol. 2007;80(1):15–26. doi: 10.1016/j.exppara.2007.01.021. 116: 497–503. [DOI] [PubMed] [Google Scholar]
- 13.Kadekoppala M, Kline K, Akompong T, Haldar K. Stable expression of a new chimeric fluorescent reporter in the human malaria parasite Plasmodium falciparum. Infect Immun. 2000;63(2):467–471. doi: 10.1128/iai.68.4.2328-2332.2000. 68: 2328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabb BS, Triglia T, Waterkeyn JG, Cowman AF. Stable transgene expression in Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:131–144. doi: 10.1016/s0166-6851(97)00143-6. [DOI] [PubMed] [Google Scholar]
- 15.Braks JA, Mair GR, Franke-Fayard B, Janse CJ, Waters AP. A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 2008;36:1176–1186. doi: 10.1093/nar/gkm1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulson RM, Hall N, Ouzounis CA. Comparative genomics of transcriptional control in the human malaria parasite Plasmodium falciparum. Genome Res. 2004;14:1548–1554. doi: 10.1101/gr.2218604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanWye JD, Haldar K. Expression of green fluorescent protein in Plasmodium falciparum. Mol Biochem Parasitol. 1997;87:225–229. doi: 10.1016/s0166-6851(97)00059-5. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Kirkman LA, Wellems TE. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci U S A. 1996;93:1130–1134. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Mamoun C, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ifediba T, Vanderberg JP. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]