Abstract
The heme oxygenase (HO) enzymes catalyze the rate-limiting step of heme breakdown, and may accelerate oxidative injury to neurons exposed to heme or hemoglobin. HO-1 and HO-2 are activated in vitro by the phosphatidylinositol 3-kinase (PI3K)/Akt and protein kinase C (PKC)/CK2 pathways, respectively. The present study tested the hypotheses that CK2, PKC, and PI3K inhibitors would reduce both HO activity and neuronal vulnerability to hemoglobin in murine cortical cultures. Oxidative cell injury was quantified by LDH release and malondialdehyde assays. HO activity was assessed by carbon monoxide assay. Consistent with prior observations, treating primary cortical cultures with hemoglobin for 16h resulted in release of approximately half of neuronal LDH and a seven-fold increase in malondialdehyde. Both endpoints were significantly reduced by the CK2 inhibitors 4,5,6,7-tetrabromobenzotriazole (TBB) and 2-dimethyl-amino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT), and by the PKC inhibitor GF109203X; the PI3K inhibitors LY294002 and wortmannin had no effect. None of these inhibitors altered basal HO activity. The 1.9-fold activity increase observed after hemoglobin treatment was largely prevented by LY294002 and LY303511, a structural analog of LY294002 that does not inhibit PI3K activity. It was not reduced by wortmannin, TBB or GF109203X. These results suggest that the protective effect of CK2 and PKC inhibitors in this model is not dependent on reduction in HO activity. In this culture system that expresses both HO-1 and HO-2, HO activity does not appear to be primarily regulated by the PKC/CK2 or PI3K pathways.
Keywords: cell culture, free radical, hemoglobin toxicity, intracerebral hemorrhage, mouse, oxidative stress
Introduction
The heme oxygenase (HO) enzymes catalyze the breakdown of heme to iron, carbon monoxide (CO), and biliverdin. Under physiologic conditions, this reaction contributes to the tight cellular regulation of heme that maintains its concentration within the nontoxic range (Taketani, 2005). Under most pathologic conditions, HO also appears to be beneficial (Otterbein et al., 2003), particularly against oxidative injury (Doré, 2002). Proposed mechanisms underlying this phenomenon include the cytoprotective effect of low concentrations of biliverdin and CO (Parfenova and Leffler, 2008), conversion of lipophilic heme-iron to a more soluble form that is then sequestered by ferritin (Balla et al., 2007), and activation of undefined signaling pathways that are unrelated to heme breakdown (Lin et al., 2007). Conversely, in some in vitro and in vivo models, HO increases or accelerates oxidative injury, due at least in part to iron release that exceeds sequestration capacity (Lamb et al., 1999; Dennery et al., 2003; Song et al., 2007).
Manifestation of the pro-oxidant effect of HO may be more likely in neurons surrounding an intracranial hematoma for three reasons. First, the preferred HO substrate, hemin, is present in gross excess in the days after hemorrhage (Letarte et al., 1993). Second, most central neurons constitutively express heme oxygenase-2 (Ewing and Maines, 1997), resulting in relatively high baseline HO activity (Doré, 2002). Third, central neurons appear to have very little ability to upregulate ferritin synthesis after hemorrhage (Wu et al., 2003), resulting in rather limited iron-binding capacity. Consistent with a pro-oxidant effect of HO under these circumstances, decreasing HO-2 expression or HO activity is protective in several models of hemoglobin toxicity or intracerebral hemorrhage (Huang et al., 2002; Rogers et al., 2003; Koeppen et al., 2004; Gong et al., 2006; Qu et al., 2007).
Since HO has both pro-oxidant and antioxidant effects, a strategy that aims to attenuate its activity may be preferable to complete inhibition. Both HO-1 and HO-2 are phosphoproteins. Phosphorylation of HO-1 by Akt/PKB on Ser188 increases its activity 1.6-fold in vitro (Salinas et al., 2004), while phosphorylation of HO-2 by CK2 at Ser79 increases activity between two and fourfold (Boehning et al., 2003). In the latter study, CK2 activity was directly regulated by protein kinase C (PKC). We hypothesized that inhibiting these regulatory pathways would reduce HO activity sufficiently to mitigate heme-mediated neuronal injury. This hypothesis was tested in an in vitro model of hemoglobin neurotoxicity, using murine primary cortical cell cultures that constitutively express HO-2 and induce HO-1 after hemoglobin exposure (Rogers et al., 2003).
Materials and Methods
Cortical cell cultures
Mixed cortical cultures, containing approximately 50% neurons and 50% glial cells, were prepared from fetal B6129 mice at 14-16 days gestation, following a protocol that has previously been described in detail (Rogers et al., 2003). Plating medium contained Minimal Essential Medium (MEM, Invitrogen, Carlsbad, CA), 5% equine serum (Hyclone, Logan, UT), 5% fetal bovine serum (Hyclone), and 2 mM glutamine. Cultures were incubated at 37°C in a 5% CO2 atmosphere. During the first ten days in vitro, two-thirds of the culture medium was replaced twice weekly with medium similar to plating medium, except that it lacked fetal bovine serum. After day 11, medium was exchanged daily.
Cytotoxicity experiments
Experiments were conducted at 12-16 days in vitro. Cultures were washed with MEM containing 10 mM glucose (MEM10), without serum. All exposures to hemoglobin alone or with inhibitors were conducted in this medium at 37°C in a 5% CO2 atmosphere. Hemoglobin exposure concentrations were determined from prior studies using this model, which demonstrated that 3–10 μM hemoglobin produced widespread neuronal injury with overnight treatment (Rogers et al., 2003), without significantly injuring astrocytes (Chen-Roetling and Regan, 2006). Inhibitors were dissolved in dimethyl sulfoxide (DMSO), and were added to culture medium from concentrated stock solutions. Direct comparisons were made on cultures containing equal DMSO concentrations (0.1–0.45%), including those subjected to medium exchanges only or exposed only to hemoglobin.
Assessment of injury
At the end of the exposure period, all cultures were inspected under phase-contrast microscopy. Neuronal death was quantified by lactate dehydrogenase (LDH) release assay, as previously described (Regan et al., 2001). This assay correlates well with assessment of neuronal death by cell counts after staining with trypan blue (Koh and Choi, 1988), which stains cells with disrupted membranes. LDH values were scaled to those in sister cultures treated with N-methyl-D aspartate (NMDA, 300 μM) for 24 hours, which causes near-100% neuronal death without injuring astrocytes. The mean basal LDH activity in sister cultures subjected to medium exchange (sham wash) alone was subtracted from all values to quantify the signal that was specific to the cytotoxic exposure, following the protocol of Koh and Choi (1988). Hemoglobin-mediated lipid peroxidation was also quantified via malondialdehyde assay, as previously detailed (Benvenisti-Zarom et al., 2005).
HO activity assay
HO activity was quantified by assaying CO production, following a modification of the procedure of Vreman and Stevenson (1988). Cultures were treated for defined intervals with enzyme inhibitors or phorbol 12-myristate-13-acetate (PMA), alone or with hemoglobin; control cultures were exposed to an equal concentration of the DMSO vehicle only. After the defined exposure interval, cultures were washed, and cells were then collected in Dulbecco's Phosphate Buffered Saline (DPBS) with 3X concentration of inhibitors or DMSO vehicle. Samples of this suspension (40 μl) were then diluted in 2 ml septum-sealed glass vials with equal volumes of freshly prepared 75 μM hemin and 4.5 mM NADPH (final reactant concentrations 25 μM hemin, 1.5 mM NADPH, total volume 120 μl); control vials lacked NADPH. Vials were purged for 4 sec with CO-free air at a flow rate of 250mL/min. Reactions were then run for 15 minutes at 37°C in a water bath under reduced light, and were terminated by quick-freezing vials on dry ice. CO was then quantified in the vial head space by gas chromatography (Peak Laboratories, Mountain View, CA). HO activity was expressed as nanomoles CO produced per hour per milligram protein. Protein concentrations were determined by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL).
Statistical Analysis
Data were analyzed with one-way analysis of variance. Differences between groups were then assessed with the Bonferroni Multiple Comparisons test.
Results
Cultures treated with 3 μM hemoglobin for 16 h released 54.6±3.7% of neuronal LDH. The glial monolayer remained intact, consistent with prior observations that glia were not injured even when treated with 30 μM hemoglobin for 5 days (Chen-Roetling and Regan, 2006). Cell counts of representative cultures (n = 5) demonstrated that 50.6±1.7% of cells were neurons and 49.4±1.7% were glia.
CK2 and PKC inhibitors attenuate hemoglobin neurotoxicity
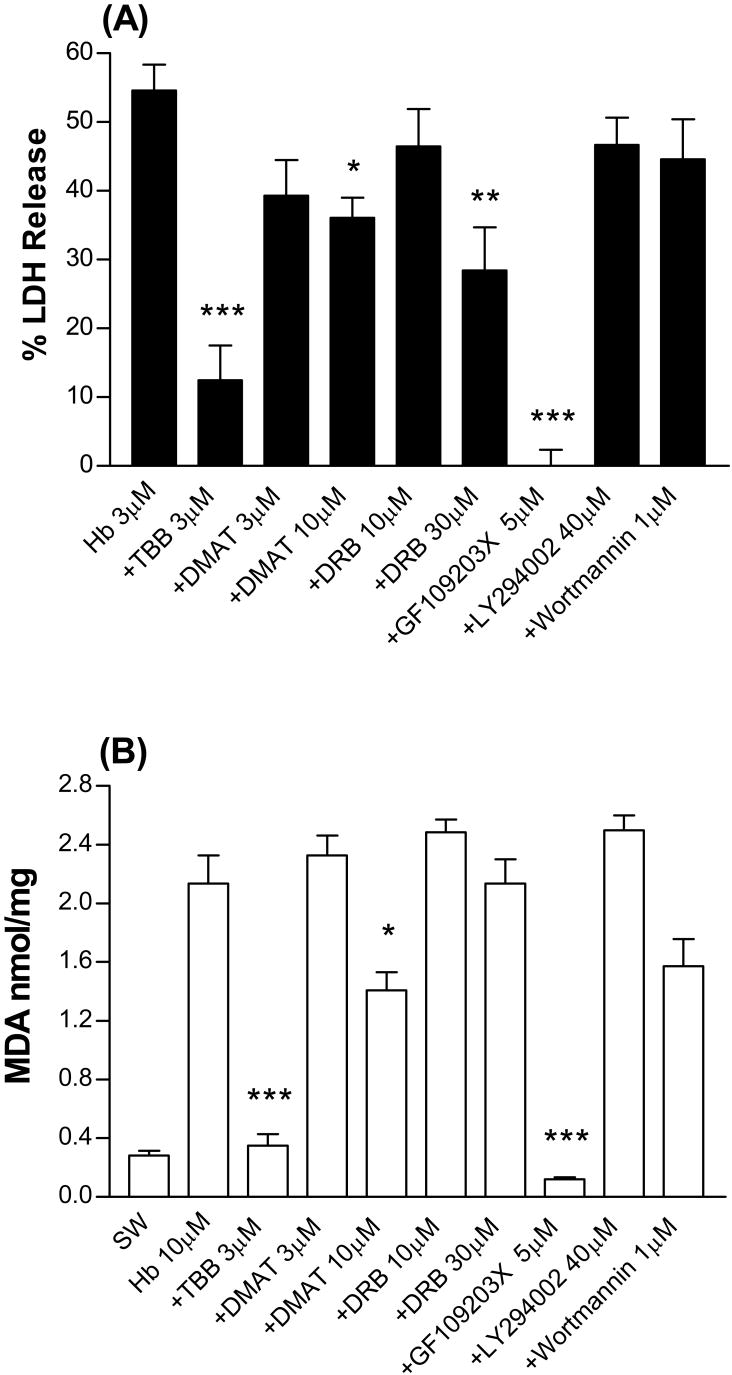
4,5,6,7-tetrabromobenzotriazole (TBB) is a potent (IC50 0.9 μM) and highly selective inhibitor of CK2 that has minimal or no effect on other protein kinases (Sarno et al., 2001). At a concentration of 3 μM, TBB prevented most of the neuronal death produced by hemoglobin treatment (Fig. 1A). Higher concentrations of TBB were tested but were found to be toxic per se with overnight exposure. 2-dimethyl-amino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT) shares the selectivity of TBB but is more potent, inhibiting CK2 with an IC50 of 0.15 μM (Pagano et al., 2004). It was also protective, but surprisingly less so than TBB, reducing injury by about one-third at 10 μM. 5,6-dichloro-1-(b-D-ribofuranosyl)benzimidazole (DRB) is a CK2 inhibitor that is less potent and less selective than either TBB or DMAT (IC50 4-10 μM, Zandomeni et al., 1986). It was ineffective at 10 μM but reduced injury by about half at 30 μM. GF109203X is a PKC inhibitor that prevented HO-2 activation in hippocampal neurons by blocking upstream activation of CK2 (Boehning et al., 2003). At the concentration used in the latter study (5 μM), it prevented all neuronal injury produced by hemoglobin.
Figure 1.
Protective effect of CK2 and protein kinase C inhibitors. (A). Mixed astrocyte-neuron cultures were treated with hemoglobin (Hb) 3 μM alone or with indicated concentrations (μM) of TBB, DMAT, DRB, GF109203X, LY294002, or wortmannin for 16 h. LDH activity in the culture medium is scaled to the mean value in sister cultures treated with 300 μM NMDA for 24 h (= 100), which produced near-100% neuronal death. The mean LDH activity in sister cultures subjected to medium exchange only was subtracted from all values to yield the signal specific to hemoglobin or NMDA neurotoxicity. *P<0.05, **P<0.01, ***P < 0.001 v. mean value in cultures treated with hemoglobin, Bonferroni multiple comparisons test, mean ± SEM, n=8-42/condition. (B) Mean culture malondialdehyde (MDA, nmol/mg protein, ±SEM, n = 5-21/condition) in cultures subjected to medium exchange (sham wash, SW) only or treated for 16 hours with Hb 10 μM alone or with indicated concentrations of inhibitors as defined above. Statistical comparisons are the same as in A.
Malondialdehyde is a sensitive marker of hemoglobin-mediated lipid peroxidation in cell culture (Benvenisti-Zarom et al., 2005; Benvenisti-Zarom et al., 2006) and in vivo (Sadrzadeh et al., 1984; Lim et al., 2000; Qu et al., 2005), and is frequently assayed to quantify hemoglobin-mediated injury. Consistent with prior observations, culture malondialdehyde was increased 7.6-fold by hemoglobin treatment (Fig. 1B), and was significantly reduced by concomitant treatment with TBB, DMAT, and GF109203X, but not by DRB.
Phosphatidylinositol 3-kinase inhibitors have no effect on hemoglobin neurotoxicity
LY294002 and wortmannin are cell-permeable phosphatidylinositol 3-kinase (PI3K) inhibitors, with IC50 values of 1.4 μM (Vlahos et al., 1994) and 3 nM (Yano et al., 1993), respectively. At concentrations sufficient to completely block PI3K activity and reduce HO-1 phosphorylation in cultured cells (Nakamura et al., 1995; Bulleit and Cui, 1998; Salinas et al., 2004), they had no effect on LDH release or malondialdehyde production (Fig. 1 A, B).
CK2, PKC and PI3K inhibitors have no effect on HO activity
We hypothesized that HO activity would be reduced by treating cells with kinase inhibitors. After washout of serum, cultures were treated with neuroprotective concentrations of TBB, DMAT, DRB or GF109203X for 30 minutes, or with an equal volume of DMSO vehicle alone. In vehicle-treated cultures, mean culture HO activity was 1.02 ± 0.03 nmol CO/mg protein/hour (Table 1). This value was not significantly altered by any of the CK2 or PKC inhibitors. Additional cultures were treated with 10 μM TBB, the concentration reported to reduce HO activity in rat hippocampal cultures (Boehning et al., 2003), which is toxic with overnight exposure in these mouse cortical cultures but is tolerated for 30 minutes; again no change was observed. The PI3K inhibitors LY294002 and wortmannin likewise had no effect on HO activity.
Table 1.
Effect of inhibitors on HO activity.
| Condition | HO Activity |
|---|---|
| SW | 1.02 ± 0.03 |
| TBB 3 μM | 1.05 ± 0.07 |
| TBB 10 μM | 1.02 ± 0.05 |
| DMAT 10 μM | 1.01 ± 0.08 |
| DRB 30 μM | 0.94 ± 0.09 |
| GF109203X 5 μM | 0.96 ± 0.03 |
| LY294002 40 μM | 1.11 ± 0.09 |
| Wortmannin 1 μM | 1.00 ± 0.03 |
Mean HO activity (nmol CO/mg protein/h ± SEM, n = 5-31/condition) in cultures subjected to medium exchange (sham-wash, SW) only or treated for 30 minutes with indicated concentrations of TBB, DMAT, DRB, GF109203X, LY294002 and Wortmannin.
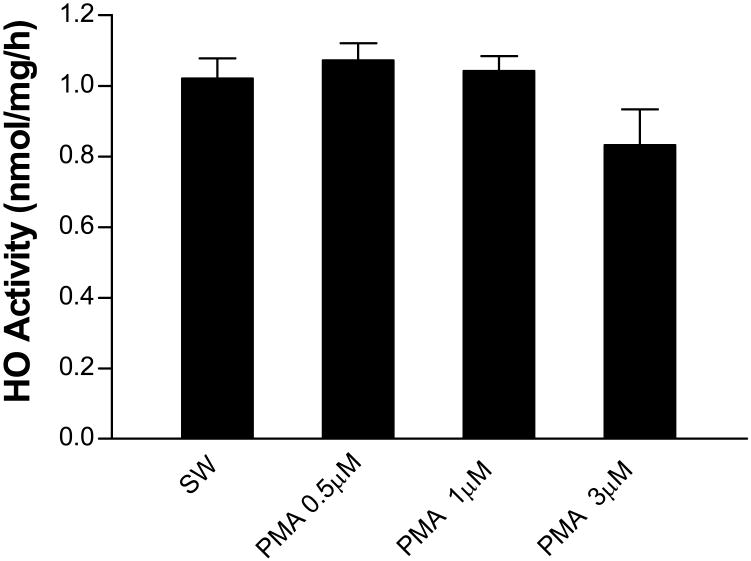
The PKC activator phorbol 12-myristate-13-acetate (PMA) does not increase HO activity
In rat hippocampal cultures, PMA treatment rapidly triples HO activity (Boehning et al., 2003). However, in these murine cortical cultures, the same concentration of PMA had no effect (Fig. 2). A similar result was observed with higher PMA concentrations (1-3 μM).
Figure 2.
Protein kinase C activator phorbol 12-myristate-13-acetate (PMA) has no effect on HO activity. Mean HO activity (nmol CO/mg protein/h ±SEM, n = 6-15/condition) in cultures subjected to medium exchange (sham-wash, SW) only or treated for 30 minutes with indicated concentrations of PMA.
Effect of CK2, PKC, and PI3K inhibitors on HO activity in hemoglobin-treated cultures
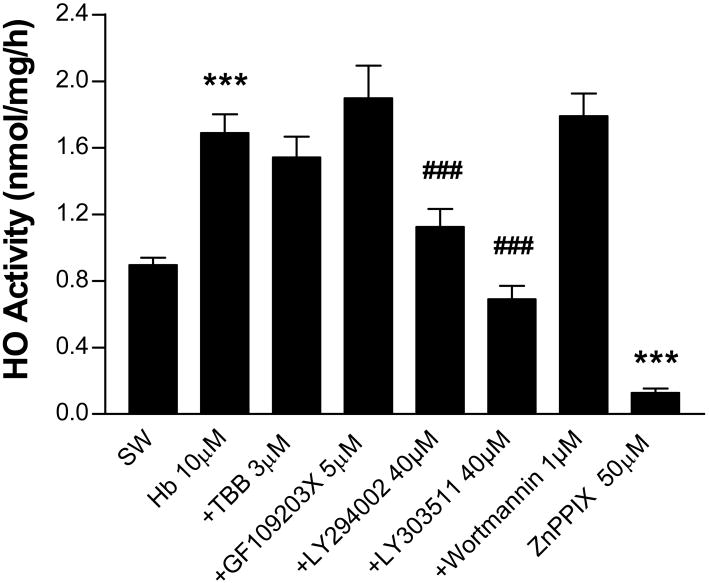
We next hypothesized that the cytoprotective effect of CK2 and PKC inhibitors was due to a reduction in HO activity after hemoglobin treatment. Prior studies have demonstrated that cell death begins in this model about 4 hours after exposure onset and progresses over the following day (Rogers et al., 2003). We therefore treated cultures with hemoglobin plus inhibitors or vehicle for four hours. Cultures were then washed, harvested, and assayed for HO activity. In cultures treated with hemoglobin plus vehicle, activity increased 1.9-fold compared with cultures treated with vehicle and medium exchange (sham wash) only (Fig. 3). This value was not altered by TBB or GF109203X at their neuroprotective concentrations. Most of the increase produced by hemoglobin was prevented by concomitant treatment with LY294002. However, HO activity was also reduced by LY303511, a structural analog of LY294002 that does not inhibit PI3K (El-Kholy et al., 2003). Wortmannin had no effect. In agreement with prior observations (Appleton et al., 1999), CO production was almost completely prevented by the HO inhibitor zinc protoporphyrin IX (50 μM).
Figure 3.
Effect of CK2, protein kinase C, and phosphatidylinositol 3-kinase inhibitors on HO activity in hemoglobin-treated cultures. Mean HO activity (nmol CO/mg protein/h ±SEM, n = 5-15/condition) in cultures subjected to sham-wash (SW) only or treated for 4 hours with hemoglobin (Hb) 10 μM alone or with indicated concentrations of TBB, GF109203X, LY294002, LY303511, or wortmannin. Additional controls were treated with the HO inhibitor zinc protoporphyrin IX (50 μM) in the reaction vials. ***P<0.001 v. sham wash, ###P<0.001 v. Hb alone, Bonferroni multiple comparisons test.
Discussion
These results suggest the following conclusions. First, CK2 and PKC inhibitors are protective in this injury model, but their effect is not dependent on an alteration in HO activity. Second, inhibiting the PI3K pathway has no effect on hemoglobin neurotoxicity. Third, HO activity in this mixed neuron/astrocyte culture system under the reported experimental conditions does not appear to be primarily regulated by either the PKC/CK2 or PI3K pathways.
In prior studies using this murine cortical cell culture system, the effect of HO-1 and HO-2 on hemoglobin toxicity has been extensively characterized. Both HO-1 and HO-2 are expressed at baseline in both neurons and astrocytes (Rogers et al., 2003; Chen-Roetling et al., 2005); HO-1 is robustly induced in astrocytes but not neurons by hemoglobin treatment (Benvenisti-Zarom et al., 2006). Knockout studies have demonstrated that HO-1 expression protects astrocytes from hemoglobin, but has minimal or no effect on neuronal injury (Chen-Roetling and Regan, 2006). In contrast, constitutively-expressed HO-2 accelerates neuronal injury while protecting astrocytes (Rogers et al., 2003; Chen and Regan, 2004). The observation that LY294002 selectively attenuated the increase in HO activity associated with hemoglobin exposure without altering neuronal vulnerability to hemoglobin is consistent with an effect on HO-1. However, astrocytes in these mixed cultures were not injured by treatment with hemoglobin plus LY294002 despite a reduction in HO activity, which is consistent with prior observations that hemoglobin-mediated astrocyte injury requires at least a 3 day exposure even in HO-1 knockout cells (Chen-Roetling and Regan, 2006).
Although LY294002 was initially described as a highly selective PI3K inhibitor (Vlahos et al., 1994), a variety of nonspecific effects have been delineated by concomitant testing of its negative control, LY303511, and wortmannin. These include inhibition of voltage-dependent calcium and potassium channels (El-Kholy et al., 2003; Callaghan et al., 2006), NF-kappaB binding activity (Kim et al., 2005), inducible nitric oxide synthase expression (Kim et al., 2005), and cell proliferation (Kristof et al., 2005). In addition, it sensitizes tumor cells to drug-induced apoptosis by increasing hydrogen peroxide production via a PI3K-independent mechanism (Poh and Pervaiz, 2005). Since HO activity after hemoglobin treatment was reduced by both LY294002 and LY303511, but not by wortmannin, it is unlikely to be due to PI3K inhibition.
The mechanism that mediates the protective effect of CK2 inhibitors in this oxidative injury model remains undefined. CK2 is a multifunctional kinase with over 300 identified substrates (Pagano et al., 2006). It is strongly expressed in the brain (Blanquet, 2000), where it has been reported to regulate long-term potentiation (Kimura and Matsuki, 2008), synaptic plasticity (Reikhardt et al., 2003), development (Jauch et al., 2002), and the circadian clock (Blau, 2003). Its effect on iron-mediated or other oxidative neuronal injury has not been previously investigated. However, in contrast to the present results, three lines of evidence predict that its activity would actually be beneficial. First, CK2 phosphorylates NADPH oxidase (Park et al., 2001), which catalyzes reduction of oxygen to superoxide. Since the CK2 inhibitor DRB enhances superoxide production, phosphorylation by CK2 appears to inactivate the oxidase and reduce cellular oxidative stress. Second, CK2 also phosphorylates Nrf2 at its transcription activation domains, facilitating its nuclear translocation and the induction of a variety of antioxidant genes (Apopa et al., 2008). Third, CK2 has a robust anti-apoptotic effect (Pagano et al., 2006). In neurons, this may be mediated at least in part by phosphorylation and inactivation of BAD, a pro-apoptotic protein (Klumpp et al., 2004). In light of these observations, the cytoprotection provided by CK2 inhibitors against hemoglobin neurotoxicity, without a concomitant reduction in HO activity, was unexpected.
A mechanistic link between CK2 activity and iron-catalyzed protein degradation has recently been reported by Takahashi et al., who observed that α-synuclein is phosphorylated by CK2 in neuroblastoma cells treated with ferrous chloride, leading to formation of oligomers and inclusion bodies (Takahashi et al., 2007). Hyperphosphorylation of α-synuclein by CK2 in cultured rat primary neurons has similarly been reported by Ishii et al., who also observed that CK2 was recovered in the same fraction as phosphorylated α-synuclein in the brains of some patients with Lewy body disease (Ishii et al., 2007). Although α-synuclein aggregation has not been observed in this hemoglobin neurotoxicity model (unpublished observations), iron-mediated protein degradation is a prominent and consistent feature (Chen-Roetling and Regan, 2006). The effect of CK2 phosphorylation on other protein targets of iron-catalyzed oxidation remains to be determined.
The inefficacy of PKC/CK2 inhibitors and PMA on HO activity in these cultures is at variance with observations in rat hippocampal cultures, in which PMA rapidly increased activity threefold, and TBB prevented this increase (Boehning et al., 2003). However, the present findings are concordant with those of Leffler et al., who reported that PMA and TBB had no effect on HO activity in piglet cerebral microvessels (Leffler et al., 2003; Leffler et al., 2005). If phosphorylation of HO-2 produces a physiologically relevant increase in its activity, the dominant kinase pathway may vary with species, cell type, and/or experimental model. It is noteworthy that murine HO-2 contains putative phosphorylation sites for several kinases in addition to CK2, including Itk, Src, Abl, protein kinase A, and DNA PK, and it also has PDK1 Binding and Erk D-Domain motifs (Obenauer et al., 2003). Further investigation of the post-translational regulation of HO-2 activity in other injury models seems warranted.
Acknowledgments
Funding for this study was provided by a grant from the National Institutes of Health (NS050662).
References
- Apopa PL, He X, Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J Biochem Mol Toxicol. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- Appleton SD, Chretien ML, McLaughlin BE, Vreman HJ, Stevenson DK, Brien JF, Nakatsu K, Maurice DH, Marks GS. Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab Disp. 1999;27:1214–1219. [PubMed] [Google Scholar]
- Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- Benvenisti-Zarom L, Chen J, Regan RF. The oxidative neurotoxicity of clioquinol. Neuropharmacology. 2005;49:687–694. doi: 10.1016/j.neuropharm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Benvenisti-Zarom L, Chen-Roetling J, Regan RF. Inhibition of the ERK/MAP kinase pathway attenuates heme oxygenase-1 expression and heme-mediated neuronal injury. Neurosci Lett. 2006;398:230–234. doi: 10.1016/j.neulet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Blanquet PR. Casein kinase 2 as a potentially important enzyme in the nervous system. Prog Neurobiol. 2000;60:211–246. doi: 10.1016/s0301-0082(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Blau J. A new role for an old kinase: CK2 and the circadian clock. Nat Neurosci. 2003;6:208–210. doi: 10.1038/nn0303-208. [DOI] [PubMed] [Google Scholar]
- Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- Bulleit RF, Cui H. Methylmercury antagonizes the survival-promoting activity of insulin-like growth factor on developing cerebellar granule neurons. Toxicol Appl Pharmacol. 1998;153:161–168. doi: 10.1006/taap.1998.8561. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Zhong J, Keef KD. Signaling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gbetagamma subunits. Am J Physiol Heart Circ Physiol. 2006;291:H2541–2546. doi: 10.1152/ajpheart.00420.2006. [DOI] [PubMed] [Google Scholar]
- Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem Biophys Res Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Cultured astrocytes from heme oxygenase-1 knockout mice are more vulnerable to heme-mediated oxidative injury. J Neurosci Res. 2005;82:802–810. doi: 10.1002/jnr.20681. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Regan RF. Effect of heme oxygenase-1 on the vulnerability of astrocytes and neurons to hemoglobin. Biochem Biophys Res Commun. 2006;350:233–237. doi: 10.1016/j.bbrc.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennery PA, Visner G, Weng YH, Nguyen X, Lu F, Zander D, Yang G. Resistance to hyperoxia with heme oxygenase-1 disruption: role of iron. Free Radic Biol Med. 2003;34:124–133. doi: 10.1016/s0891-5849(02)01295-9. [DOI] [PubMed] [Google Scholar]
- Doré S. Decreased activity of the antioxidant heme oxygenase enzyme: implications in ischemia and in Alzheimer's disease. Free Radic Biol Med. 2002;32:1276–1282. doi: 10.1016/s0891-5849(02)00805-5. [DOI] [PubMed] [Google Scholar]
- El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB. The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. Faseb J. 2003;17:720–722. doi: 10.1096/fj.02-0802fje. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Maines MD. Histochemical localization of heme oxygenase-2 protein and mRNA expression in rat brain. Brain Res Brain Res Protoc. 1997;1:165–174. doi: 10.1016/s1385-299x(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Gong Y, Tian H, Xi G, Keep RF, Hoff JT, Hua Y. Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochir Suppl. 2006;96:232–236. doi: 10.1007/3-211-30714-1_50. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Ishii A, Nonaka T, Taniguchi S, Saito T, Arai T, Mann D, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human alpha-synuclein: Implication for alpha-synucleinopathies. FEBS Lett. 2007;581:4711–4717. doi: 10.1016/j.febslet.2007.08.067. [DOI] [PubMed] [Google Scholar]
- Jauch E, Melzig J, Brkulj M, Raabe T. In vivo functional analysis of Drosophila protein kinase casein kinase 2 (CK2) beta-subunit. Gene. 2002;298:29–39. doi: 10.1016/s0378-1119(02)00921-6. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi KH, Park JW, Kwon TK. LY294002 inhibits LPS-induced NO production through a inhibition of NF-kappaB activation: independent mechanism of phosphatidylinositol 3-kinase. Immunol Lett. 2005;99:45–50. doi: 10.1016/j.imlet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kimura R, Matsuki N. Protein kinase CK2 modulates synaptic NMDA receptors and synaptic plasticity in the hippocampus. J Physiol. 2008 doi: 10.1113/jphysiol.2008.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp S, Maurer A, Zhu Y, Aichele D, Pinna LA, Krieglstein J. Protein kinase CK2 phosphorylates BAD at threonine-117. Neurochem Int. 2004;45:747–752. doi: 10.1016/j.neuint.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Dickson AC, Smith J. Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. J Neuropathol Exp Neurol. 2004;63:587–597. doi: 10.1093/jnen/63.6.587. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: Differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988;8:2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristof AS, Pacheco-Rodriguez G, Schremmer B, Moss J. LY303511 (2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one) acts via phosphatidylinositol 3-kinase-independent pathways to inhibit cell proliferation via mammalian target of rapamycin (mTOR)- and non-mTOR-dependent mechanisms. J Pharmacol Exp Ther. 2005;314:1134–1143. doi: 10.1124/jpet.105.083550. [DOI] [PubMed] [Google Scholar]
- Lamb NJ, Quinlan GJ, Mumby S, Evans TW, Gutteridge JMC. Haem oxygenase shows pro-oxidant activity in microsomal and cellular systems: implications for the release of low-molecular-mass iron. Biochem J. 1999;344:153–158. [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol. 2005;289:H1442–1447. doi: 10.1152/ajpheart.00464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Balabanova L, Sullivan CD, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;285:H292–297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]
- Letarte PB, Lieberman K, Nagatani K, Haworth RA, Odell GB, Duff TA. Hemin: levels in experimental subarachnoid hematoma and effects on dissociated vascular smooth muscle cells. J Neurosurg. 1993;79:252–255. doi: 10.3171/jns.1993.79.2.0252. [DOI] [PubMed] [Google Scholar]
- Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM, Baumman H, Halliwell B, Lim SK. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 2000;58:1033–1044. doi: 10.1046/j.1523-1755.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Takahashi N, Sasaki T, Tanaka S, Udagawa N, Murakami H, Kimura K, Kabuyama Y, Kurokawa T, Suda T, et al. Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett. 1995;361:79–84. doi: 10.1016/0014-5793(95)00153-z. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Cesaro L, Meggio F, Pinna LA. Protein kinase CK2: a newcomer in the ‘druggable kinome’. Biochem Soc Trans. 2006;34:1303–1306. doi: 10.1042/BST0341303. [DOI] [PubMed] [Google Scholar]
- Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun. 2004;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des. 2008;14:443–453. doi: 10.2174/138161208783597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lee SM, Lee JH, Kim YS, Bae YS, Park JW. Phosphorylation of the leucocyte NADPH oxidase subunit p47(phox) by casein kinase 2: conformation-dependent phosphorylation and modulation of oxidase activity. Biochem J. 2001;358:783–790. doi: 10.1042/0264-6021:3580783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh TW, Pervaiz S. LY294002 and LY303511 sensitize tumor cells to drug-induced apoptosis via intracellular hydrogen peroxide production independent of the phosphoinositide 3-kinase-Akt pathway. Cancer Res. 2005;65:6264–6274. doi: 10.1158/0008-5472.CAN-05-0152. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg. 2007;106:428–435. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- Regan RF, Wang Y, Ma X, Chong A, Guo Y. Activation of extracellular signal-regulated kinases potentiates hemin toxicity in astrocyte cultures. J Neurochem. 2001;79:545–555. doi: 10.1046/j.1471-4159.2001.00590.x. [DOI] [PubMed] [Google Scholar]
- Reikhardt BA, Kulikova OG, Borisova GY, Aleksandrova IY, Sapronov NS. Status of the “protein kinase CK2-HMG14” system in age-related amnesia in rats. Neurosci Behav Physiol. 2003;33:799–804. doi: 10.1023/a:1025101516128. [DOI] [PubMed] [Google Scholar]
- Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad Biol Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh SMH, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin: A biologic Fenton reagent. J Biol Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- Salinas M, Wang J, Rosa de Sagarra M, Martin D, Rojo AI, Martin-Perez J, Ortiz de Montellano PR, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004;578:90–94. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (“casein kinase-2”) FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- Song L, Song W, Schipper HM. Astroglia overexpressing heme oxygenase-1 predispose co-cultured PC12 cells to oxidative injury. J Neurosci Res. 2007;85:2186–2195. doi: 10.1002/jnr.21367. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ko LW, Kulathingal J, Jiang P, Sevlever D, Yen SH. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur J Neurosci. 2007;26:863–874. doi: 10.1111/j.1460-9568.2007.05736.x. [DOI] [PubMed] [Google Scholar]
- Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vreman HJ, Stevenson DK. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988;168:31–38. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakemura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- Zandomeni R, Zandomeni MC, Shugar D, Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986;261:3414–3419. [PubMed] [Google Scholar]