Abstract
Although the cholinergic system has long been implicated in the formation of memory, there had been no direct demonstration that activation of this system can actually induce specific behavioral memory. We have evaluated the “cholinergic-memory” hypothesis by pairing a tone with stimulation of the nucleus basalis (NB), which provides acetylcholine to the cerebral cortex. We found that such pairing induces behaviorally-validated auditory memory. NB-induced memory has the key features of natural memory: it is associative, highly-specific and rapidly induced. Moreover, the level of NB stimulation controls the amount of detail in memory about the tonal conditioned stimulus. While consistent with the hypothesis that properly-timed release of acetylcholine (ACh) during natural learning is sufficient to induce memory, pharmacological evidence has been lacking. This study asked whether scopolamine, a muscarinic antagonist, impairs or prevents the formation of NB-induced memory. Adult male rats were first tested for responses (disruption of ongoing respiration) to tones (1–15 kHz), constituting a pre-training behavioral frequency generalization gradient (BFGG). Then, they received a single session of 200 trials of a tone (8.00 kHz, 70 dB, 2 s) paired with electrical stimulation of the NB (100 Hz, 0.2 s). Immediately after training, they received either scopolamine (1.0 mg/kg, i.p.) or saline. Twenty-four hours later, they were tested for specific memory by obtaining post-training BFGGs. The saline group developed CS-specific memory, manifested by maximum increase in response specific to the CS frequency band. In contrast, the scopolamine group exhibited no such memory. These findings indicate that NB-induced specific associative behavioral memory requires the action of intrinsic acetylcholine at muscarinic receptors, and supports the hypothesis that natural memory formation engages the nucleus basalis and muscarinic receptors.
Keywords: Association, Auditory cortex, Cholinergic system, Natural memory, Scopolamine
INTRODUCTION
Acetylcholine (ACh) has long been implicated in learning and memory (Deutsch, 1971; Flood, Landry, & Jarvik, 1981) and continues to be the focus of extensive research (Power, Vazdarjanova, & McGaugh, 2003). For example, pharmacological blockade of the cholinergic system impairs many forms of memory in both animals and humans (Anagnostaras, Maren, Sage, Goodrich, & Fanselow, 1999; Chudasama, Dalley, Nathwani, Bouger, & Robbins, 2004; Lozano, Armengaud, & Gauthier, 2001; Múnera, Gruart, Muñoz, & Delgado-García, 2000; Ravel, Elaagouby, & Gervais, 1994; Rudy, 1996; Schön, Atri, Hasselmo, Tricarico, LoPresti, & Stern, 2005). Cholinergic agonists and cholinesterase antagonists can facilitate memory (Introini-Collison & McGaugh, 1988; Stratton & Petrinovich, 1963), promote recovery of memory from brain damage (Russell, Escobar, Booth, & Bermudez-Rattoni, 1994) and achieve rescue from memory deficits in transgenic mice (Fisher, Brandeis, Chapman, Pittel, & Michaelson, 1998). In addition, several non-cholinergic treatments that facilitate memory, such as adrenergic agents and stress hormones, exert their effects via the cholinergic system (Salinas, Introini-Collison, Dalmaz, & McGaugh, 1997).
Such findings suggest that ACh is released during many types of learning and that its effects throughout the brain may promote the formation of memory and/or increase its strength. The nucleus basalis (NB) of the basal forebrain is a likely candidate for learning-related release of ACh to the cerebral cortex because it is the major source of cortical ACh (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Luiten, Gaykema, Traber, & Spencer, 1987; Mesulam, Mufson, Wainer, & Levey, 1983; Rye, Wainer, Mesulam, Mufson, & Saper, 1984). Also, stimulation of the NB releases ACh in the cortex and produces cortical electroencephalographic (EEG) activation, the waking state accompanying most learning (Casamenti, Deffenu, Abbamondi, & Pepeu, 1986; Celesia & Jasper, 1966; Détári, Juhász, & Kukorelli, 1984; Détári, Rasmusson, & Semba, 1999; Jiménez-Capdeville, Dykes, & Myasnikov, 1997; Rasmusson, Clow, & Szerb, 1992, 1994; Rasmusson, Szerb, & Jordan, 1996).
If acetylcholine and the nucleus basalis are components of neural mechanisms that promote memory, then they might be expected to influence sensory processing, as memories are largely comprised of the record of sensory experience. Indeed, ACh and activation of the NB can modify cortical responses to and representations of sensory events in the primary auditory (Ashe & Weinberger, 1991), somatosensory (Dykes, Tremblay, Warren, & Bear, 1991; Verdier & Dykes, 2001) and visual cortices (Gu, 2003). Most extensively studied in the primary auditory cortex (A1), application of ACh and anti-cholinesterase agents produces long-lasting alteration of acoustic frequency receptive fields (RFs) via muscarinic receptors (Ashe, McKenna, & Weinberger, 1989; McKenna, Ashe, & Weinberger, 1989). Stimulation of the NB produces atropine-sensitive, persistent modification of evoked responses in the auditory cortex, including facilitation of field potentials, cellular discharges and EPSPs elicited by medial geniculate stimulation (Metherate & Ashe, 1991, 1993) in vitro, and facilitation of responses to tones in vivo (Edeline, Hars, Maho, & Hennevin, 1994; Edeline, Maho, Hars, & Hennevin, 1994; Hars, Maho, Edeline, & Hennevin, 1993; Hennevin, Edeline, Hars, & Maho, 1992; Hennevin, Maho, Hars, & Edeline, 1993).
Of particular relevance to memory, pairing a tone with NB stimulation produces shifts in frequency tuning that are specific to (i.e., directed toward) the conditioned stimulus (CS) (Bakin & Weinberger, 1996). As in the case of memory and tuning shifts that develop during classical and instrumental conditioning, NB-induced frequency plasticity is associative, highly specific, rapidly established, discriminative and also consolidates (i.e., becomes stronger over time) and endures (Weinberger, 1998, 2003). NB-induced CS-specific RF plasticity requires the engagement of muscarinic receptors in A1 (Miasnikov, McLin, & Weinberger, 2001). Similar cholinergically-based, muscarinic receptor-dependent CS-specific tuning shifts have been found in the bat, demonstrating species generality (Ji, Gao, & Suga, 2001; Ji & Suga, 2003; Ji, Suga, & Gao, 2005; Ma & Suga, 2005). NB-induced associative tuning shifts presumably consist of more cells becoming preferentially responsive to the CS frequency and thus to an increase in the area of CS representation might be expected, as it is in the case of learning-induced plasticity (Recanzone, Schreiner, & Merzenich, 1993; Rutkowski & Weinberger, 2005). Such specific representational expansions have been confirmed (Kilgard & Merzenich, 1998; Kilgard, Pandya, Vazquez, Gehi, Schreiner, & Merzenich, 2001).
If the NB is normally engaged during associative learning to release ACh and promote memory formation, then pairing a tone with NB stimulation, in the absence of any standard unconditioned stimulus (e.g., food or shock) might be sufficient to induce genuine memory, as behaviorally defined. That, in fact, turned out to be the case. Rats trained with one tone paired with NB stimulation were later tested for specific memory by presenting many different tones, i.e., by obtaining behavioral frequency generalization gradients. A control group received tone and NB stimulation randomly. Memory was assessed by detecting changes in tone-elicited heart rate and respiration. Specific memory for frequency would be revealed by a generalization gradient having its peak (maximal response) at the CS frequency (Pavlov, 1927; Mackintosh, 1974; Mostofsky, 1965). The paired group in these studies did in fact exhibit this CS-specific gradient, whereas the unpaired group had no differential response to any tone (McLin, Miasnikov, & Weinberger, 2002a, 2003; Miasnikov, Chen, & Weinberger, 2006). Moreover, the amount of detail in memory about tonal frequency is controlled by the degree of NB activation; low levels of activation induce associative memory that sound is important without preserving the frequency of the CS whereas higher levels of activation also induce memory of CS frequency specificity (Weinberger, Miasnikov, & Chen, 2006). The nucleus basalis appears to be operating “downstream” of motivational systems because NB stimulation that induces memory is neither rewarding nor aversive (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008).
Overall, the evidence strongly supports the hypothesis that appropriately-timed, phasic release of ACh by the cortical terminals of the nucleus basalis is sufficient for the formation of behavioral memory. However, in the absence of relevant pharmacological findings, memory induction might be attributable to some non-cholinergic effects of stimulation of the nucleus basalis. For example, the NB contains GABAergic cells that release their transmitter in the cerebral cortex, where they inhibit cortical inhibitory interneurons, i.e., promote increased cortical excitation by disinhibition (Dykes, 1997; Freund & Meskenaite, 1992; Jiménez-Capdeville et al., 1997). Therefore, we asked whether scopolamine, a muscarinic antagonist, blocks NB-induced memory. We applied scopolamine after training in order to preclude affecting performance factors during training, such as attention and arousal (McGaugh, 1966).
MATERIALS AND METHODS
The materials and methods were generally the same as those previously reported (Miasnikov et al., 2006, 2008; Weinberger et al., 2006), and thus will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
Subjects and Surgery
The subjects were 16 adult male Sprague–Dawley rats (104 ± 17 days of age, 446 ± 68 g, mean ± s.d.), housed individually with ad libitum food and water, on a 12/12h light–dark cycle (lights on at 7:15 AM). Following several days of adaptation to the vivarium, animals were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. Under general anesthesia (sodium pentobarbital, 40 mg/kg i.p., Abbott Laboratories, North Chicago, IL), an 0.8-mm diameter stainless steel recording epidural screw electrode was inserted over the right primary auditory cortex at the locus showing the largest amplitude evoked potential (200–400 μV) to a contralateral noise burst. Two screws over the frontal sinus served as reference electrodes. A concentric bipolar stainless steel stimulating electrode (#SNEX-100×13, David Kopf Instruments, Tujunga, CA) was implanted through the contralateral (left) hemisphere (45° angle in the frontal plane at AP –2.2, L 3.2; Paxinos & Watson, 1997), into the right nucleus basalis. The final locus was determined by obtaining 1–5 s of auditory cortical EEG activation to stimulation (200–500 μA, pairs of 0.2 ms opposite polarity pulses, 100 Hz, 200–300 ms trains; S88 stimulator, PSIU6 isolation units, Grass Instrument Co., Quincy, MA). A dental acrylic pedestal was built with two aluminum hex threaded standoffs embedded therein, and all leads connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
Stimuli, Recording and Data Analyses
Training and testing took place while each subject was in a box (23 × 23 × 31 cm) supplied with fresh bedding and lined inside with acoustic-damping tile, contained in a double-walled acoustic chamber (Industrial Acoustics Company, Bronx, NY). Acoustic stimuli were pure tones (1.0–15.0 kHz, 2 s duration, cosine 10 ms rise/fall time [10% to 90%], 70 dB SPL) produced by TDT System 3 components (Tucker–Davis Technologies [TDT], Alachua, FL) and delivered to two loudspeakers calibrated for low (electrodynamic, #40-1421, RadioShack, Fort Worth, TX) and high (electrostatic, #ES-1, TDT) frequencies positioned 35 cm above the floor of the box. NBstm current used during training was several times weaker than that used during surgery because the absence of anesthesia greatly reduces the threshold for EEG activation, that was determined as described below.
To assess the induction of memory, we measured changes in the disruption of the ongoing respiration pattern to various tones before and following training (below). Respiration was detected as breathing-related thermal fluctuations with a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly positioned in front of a naris. The details of this device, as well as those for the amplification of respiration signal were previously described (Miasnikov et al., 2006).
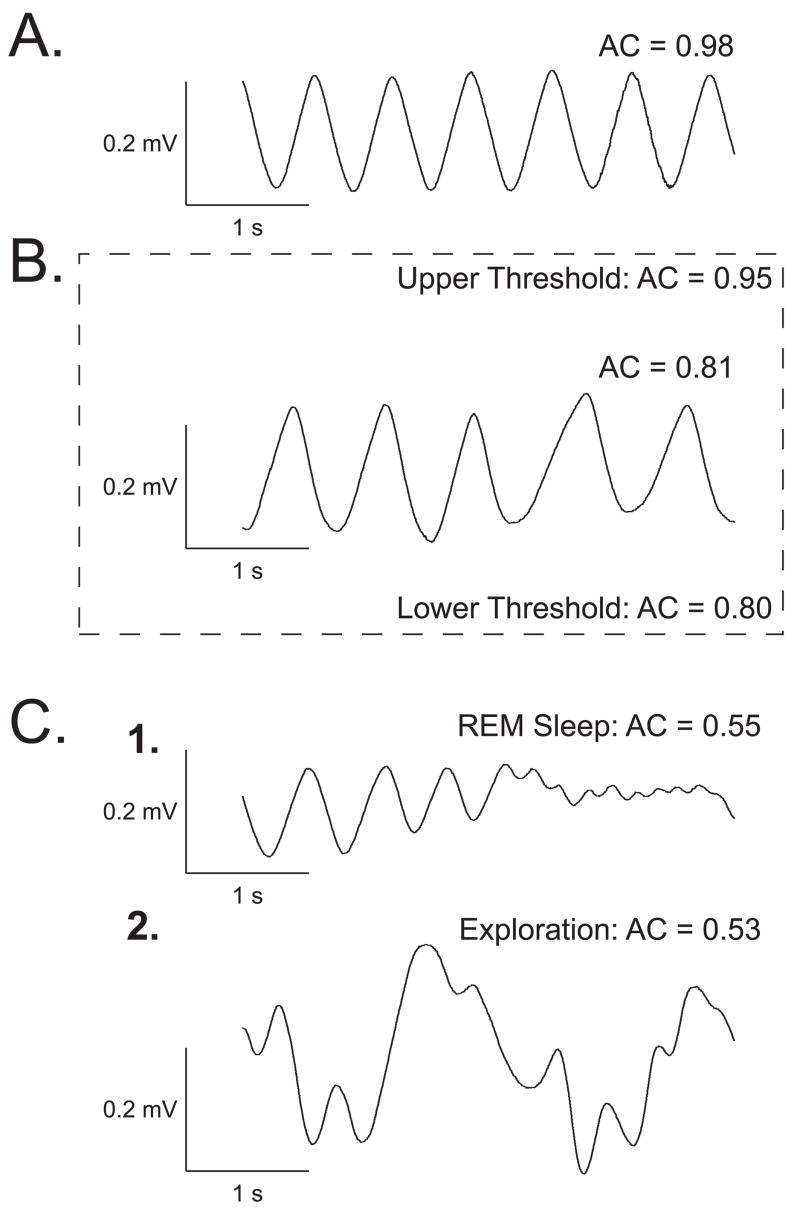
The output signal was fed to a differential band-pass amplifier (1–100 Hz), digitized by two A–D modules, one for on-line calculation of the autocorrelation function (ACF) (100 samples/s), the other for off-line analysis (2,000 samples/s). The highest possible ACF value is 1.0, i.e., a perfect sinusoid. The ACF was used to present tones only when the subject was in a state of quiet waking. This corresponded to ACF values of 0.80–0.95 over a four second pre-tone period. Respiration patterns are characteristic of different behavioral states (Figure 1). An ACF > 0.95 occurs during slow-wave sleep. ACFs ~ 0.75 or lower occur during REM and during active exploration, both states characterized by irregular respiration. We chose the lower criterion of ACF = 0.80 as a conservative means of avoiding any contamination from more activated states, including grooming or the initiation of searching behavior while subjects were still recumbent. These states could have ACF values ~ 0.40–0.75.
Figure 1.
Behavioral state control. Examples of measures of respiration corresponding to four major behavioral states: exploration, quiet waking, slow-wave sleep, paradoxical sleep. The pattern of respiration is characteristic for each state (Weinberger et al., 2006). (A) When respiration is maximally regular (Autocorrelation [AC] function calculated for the 4-s epoch of respiration record exceeds 0.95), the animal responds poorly to tones and its EEG is typical of slow-wave sleep. (B) When respiration is somewhat less regular (AC = 0.80–0.95), the animal generally sits calmly with its eyes opened, is quite responsive to tones, and its EEG is somewhat less synchronized. (C1) When respiration is much less regular (AC < 0.60), with many high-frequency shallow breathing movements, subjects are often non-responsive to tones and the EEG is low-voltage fast, typical of REM sleep. (C2) When respiration is chaotic, with many high-amplitude transients, the EEG is low-voltage fast and the animal is exploring or grooming. The respiration AC range was set at 0.80–0.95 to maximize the probability of presenting training trials when subjects were in the quiet waking state. The relative regularity of respiration and sufficient reactivity to tones during this quiescent state provides an optimal baseline for the detection of tone-elicited disruptions.
Trials meeting the criterion of baseline ACF = 0.80–0.95 for over 4 s were presented if the scheduled inter-trial interval period had passed (30–180 s). This state control was employed to avoid giving stimuli when very high levels of ACh were being released in the cortex, as during exploration or REM sleep (Giovannini, Rakovska, Benton, Pazzagli, Bianchi, & Pepeu, 2001; Jasper & Tessier, 1971; Kametani & Kawamura, 1990; Marrosu, Portas, Mascia, Casu, Fà, Giagheddu, Imperato, Gessa, 1995) to prevent a ceiling effect, thus promoting a physiologically-effective release of ACh by NBstm.
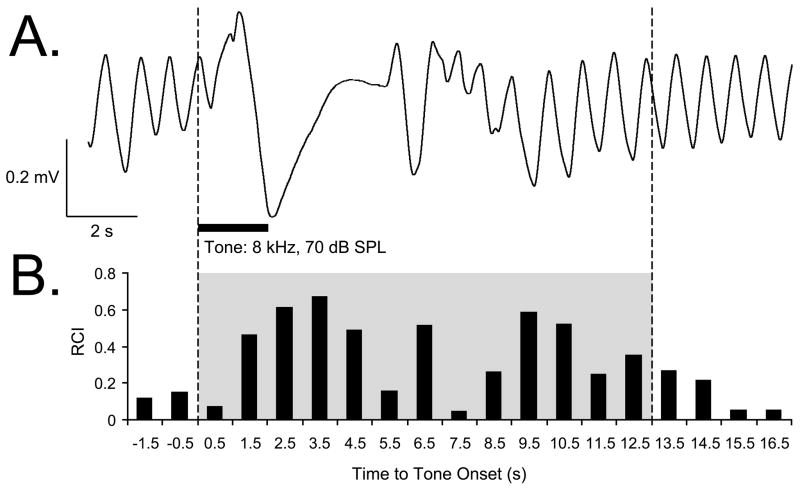
Offline analysis consisted of the calculation of Fast Fourier Transform (FFT) functions for a period of 4 s preceding a trial (Pre) and 24 s following tone onset (Post). Major changes in respiration occurred within 0.5–12.5 s after tone onset. During the quiescent state, the respiration signal is almost completely contained within the bandwidth of 0.975–2.925 Hz. The FFT data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude. RCIs were calculated as: RCIi = (|Posti – Pre|)/(Posti + Pre). A value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. An example of a tone-elicited disruption of respiration is provided in Figure 2. Statistical analyses used SPSS v.15 software (SPSS, Chicago, IL).
Figure 2.
Respiration signal and its quantification. (A) An example of a regular sinusoidal baseline respiration record disrupted by tone presentation. (B) Quantification of the respiration record shown in (A). The “Respiration Change Index” (RCI, see Methods) is sensitive to both increases and decreases in signal amplitude and frequency. The example shows a typical response of a Saline animal to the CS tone, recorded while obtaining the behavioral frequency generalization gradient 24 hours following completion of the pairing session. The shaded area indicates the first 13-s portion of the respiratory record containing the majority of tone-evoked response. The RCI values found within this epoch were used in the behavior data analysis.
Experimental Design
The subjects were assigned to two groups, Saline (n = 8) and Scopolamine (n = 8). After recovery from surgery, NBstm thresholds were determined while subjects were in a quiet waking state. NBstm was delivered every few minutes at increasing levels starting at ~ 50 μA (100 Hz bipolar, 200 ms train) until stimulation reliably elicited 3–8 s epoch of cortical activation (decrease in low frequency activity often accompanied by increase in gamma activity). The current levels used in subsequent training with NB stimulation did not elicit body movements.
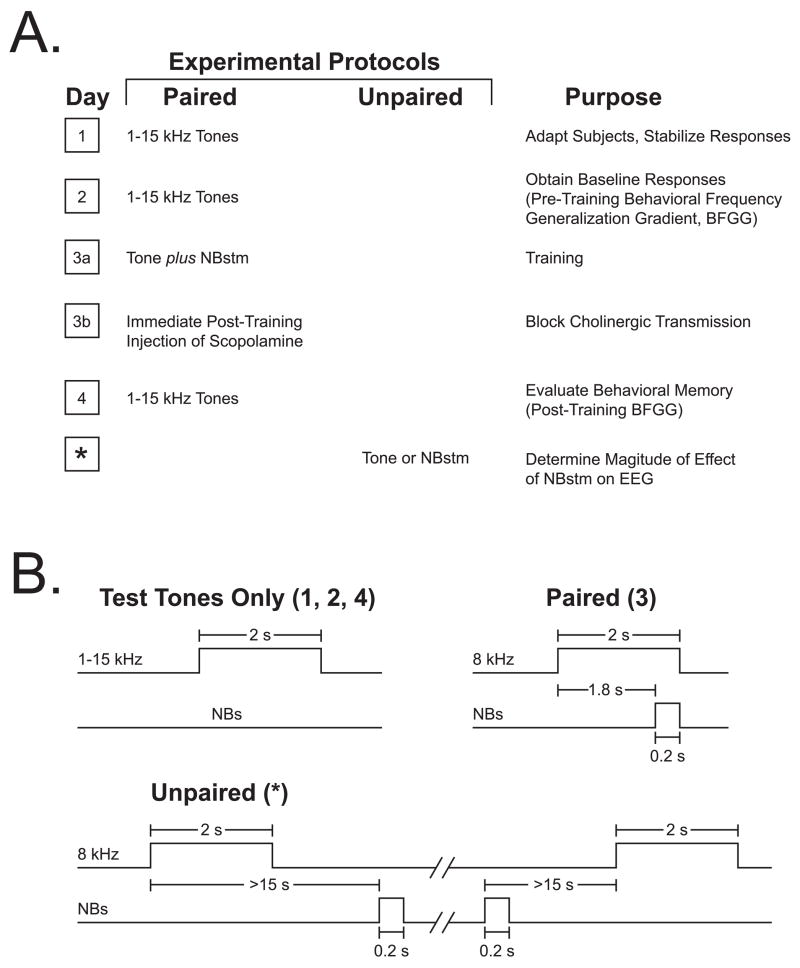
To induce and subsequently evaluate stimulus-specific memory, we used the approach of acquiring behavioral baseline responses to many frequencies, then training with one frequency and testing the training effects with many frequencies. The protocol required four consecutive days (Figure 3A). Days 1–2, obtaining the pre-training baseline response to test tones; the Day 1 session was used to acclimatize subjects to the testing environment and thus data from this session were not analyzed. Day 3 consisted of a single session of training, in which a CS tone was paired with NBstm (below); immediately after training, subjects received an injection of either 1.0 mg/kg (i.p.) scopolamine hydrobromide trihydrate (1 mg/ml with saline as a vehicle, (“scopolamine”) or an saline alone (1 ml/kg). They were then returned to their home cages. On Day 4, post-training responses to tones were obtained. Potential transfer between training and frequency testing sessions was reduced by using different contexts for the two types of session. Thus, animals were delivered to the lab via different circuitous routes and they were trained and injected in the dark (red light) but tested (pre- and post-training) in the light.
Figure 3.
Experimental design. (A) The four main stages of the experiment used to obtain pre-training and post-training behavioral frequency generalization gradients (BFGG) for the Scopolamine group. (The control [“Saline”] group received identical treatment except for the injection of saline.) A fifth stage was used to quantify the effect on the EEG of NBstm alone; such quantification could not be obtained during the pairing session because NBstm was necessarily preceded by the CS tone which itself elicited conditioned EEG changes (McLin et al., 2003). The asterisk indicates that the effects of NBstm were determined within a few days after Day 4, when the last BFGG’s were obtained. (B) Detailed temporal relationships of stimuli for the various phases of the experiment: delivery of test tones (Days 1, 2 and 4), tone–NBstm pairing (Day 3) and unpaired NBstm (*).
The Saline and Scopolamine groups received identical training (Figure 3B). They received 200 trials of a tone (8.00 kHz, 2 s, 70 dB SPL) followed by NBstm (same level as determined post-operatively) that overlapped CS presentation and co-terminated with CS offset (i.e., the CS–US interval was 1.8 s). Inter-trial intervals averaged 80 s (range ~ 25–150 s). On frequency test days, subjects received random presentation of tones of nine different frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25, and 15.00 kHz, 70 dB SPL, constrained only by presenting not more than two stimuli of the same frequency in a row) for 200 trials total. Intervals between tone presentations averaged 94 s. Statistical analyses of respiration responses were based on averaging the data for triplets of frequencies: 1.00–4.50 kHz (lower band), 6.25–9.75 kHz (middle band) and 11.50–15.00 kHz (upper band). The middle frequency band (6.25, 8.00, 9.75 kHz) is referred to as the “CS band” (Weinberger et al., 2006).
Effectiveness of NB Stimulation on the EEG of the Auditory Cortex
To insure that any differences of NB-induced memory were not due to differential effectiveness of NBstm, all subjects received tone and NBstm unpaired within a few days after completion of the post-training assessment (Day 4) of memory induction. This was necessary as it is not possible to determine the effects of NBstm itself during training because it is inevitably preceded by the CS tone which itself acquires the ability to alter the ongoing EEG (McLin et al., 2003). Tones (8.00 kHz) were included to simulate as closely as possible the training conditions without the confound of tone preceding NBstm. Presentation of tones may formally have constituted an extinction session, the subject of which is beyond the scope of the present report.
We quantified changes induced by NBstm by calculating the power spectra of electroencephalographic (EEG) recordings obtained from the auditory cortical electrode. The epidural A1 signal was amplified and filtered (1,000×, band-pass 1–1,000 Hz), digitized at 500 samples/s and processed off-line. The FFT Power was calculated at a frequency resolution of 0.975 Hz for frequencies up to 59.965 Hz. The FFT data were used to calculate an EEG “Power Change Index” (EEG PCI), on a second-by-second basis for each EEG frequency band separately as follows: delta, 0.98–2.92; theta, 2.93–8.78; alpha, 8.79–14.62; beta1, 14.63–20.47; beta2, 20.48–33.15; gamma, 33.16–59.97. EEG PCIs were calculated on each trial as follows: EEG PCIi = (Posti – Pre)/(Posti + Pre), where “Pre” was the first 2 s out of four immediately preceding a trial. The index was sensitive to both increases and decreases in EEG Power. A negative value indicated a decline and a positive value indicated a rise in power within a specified frequency band relative to its baseline (McLin, Miasnikov, & Weinberger, 2002b). In addition, as the major EEG changes were a decrease in alpha power and an increase in gamma power, we calculated an “EEG Activation Index” (EAI) to directly compare the EEG effectiveness of the groups: EAI = (Gamma PCI) + [(Alpha PCI) × (−1.0)]. This yielded generally positive values that gave equal weighting to changes in both alpha and gamma power.
Location of Electrodes
Following the completion of the experiment, an electrolytic lesion (4 ms pulses at 100 Hz, 500 μA for 20-60 s) was made with bipolar current through the stimulating electrode while the animal was under sodium pentobarbital anesthesia. The animal was then given an overdose of sodium pentobarbital and perfused through the heart with saline followed with 3.7% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The brain was removed and the Anterior-to-Posterior (AP) and Medial-to-Lateral (ML) coordinates of the recording electrode relative to Bregma and midline, respectively, were precisely measured on the skull from the interior of the calvaria with a caliper at 0.1 mm resolution. The auditory cortex recording site, which had been determined during surgery by noise burst-induced local field potentials, was estimated by projecting the location of the epidural recording electrode onto the underlying cortical surface. The recording site location was subsequently verified by plotting the obtained coordinates of the mentioned cortical projection onto a stereotaxic map of the auditory and surrounding areas of cortex derived from the Paxinos and Watson (1997) atlas. The AP and ML data collected from individual subjects were combined with respect to experimental protocol, and the groups (Saline vs. Scopolamine) were compared using t-test to determine whether the location of the recording sites differed.
Following several days of post-fixation in paraformaldehyde solution with 0.8 M sucrose added for subsequent tissue cryoprotection, the brain was blocked and sectioned at 50 μm with a freezing microtome. The sections were mounted onto gelatin-coated slides, dried and stained for Nissl substance to recover the electrolytic lesion sites and thus to determine the actual locus of intracranial stimulation. The location of the stimulation site was then projected onto the closest outline of frontal (coronal) sections taken from the Paxinos and Watson (1997) atlas. Based on subcortical structure outlines, the AP, ML, and Dorsal-to-Ventral (DV) coordinates of the tip of the stimulating electrode were determined and converted into standardized atlas dimensions. The AP, ML, and DV data collected from individual subjects were combined with respect to experimental protocol, and the groups (Saline vs. Scopolamine) were compared using a t-test to determine whether the location of the sites of stimulation differed.
RESULTS
Effect of Post-training Scopolamine on Specific Memory Induced by NB Stimulation
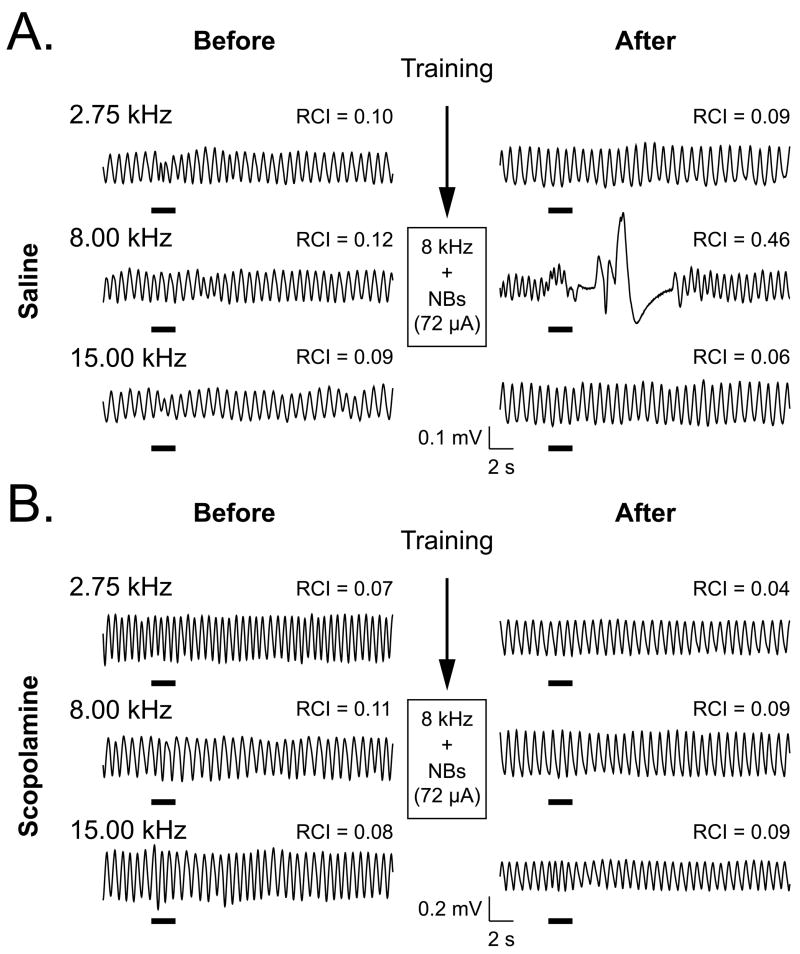
Behavioral frequency generalization gradients (BFGG) were obtained before and after pairing the CS and NBstm. The Scopolamine and Saline groups exhibited the same respiratory responses to tones of 1–15 kHz before training. In contrast, 24 h after training, they exhibited differential responses despite the fact that they had undergone identical pairing of an 8.00 kHz tone with NBstm. Figure 4 provides examples of respiratory records for a single presentation of three tones for a Saline animal and a Scopolamine animal. In this example, both subjects exhibited little response to the CS tone (8.00 kHz), a lower (2.75 kHz) or higher (15.00 kHz) tone prior to training. However, after training, the Saline animal displayed a large response to the CS frequency, but still no responses to the other tones. This pattern of response change is typical of NB-induced behavioral memory (McLin et al., 2002a; Miasnikov et al., 2006; Weinberger et al., 2006). In contrast, the Scopolamine subject displayed neither post-training response to the CS frequency, nor to the other frequencies.
Figure 4.
Effects of paired tone–NBstm training on behavioral memory without and with the block of cholinergic transmission immediately following training. (A) Examples of respiratory waveforms obtained from a Saline subject. Shown are baseline responses to the CS (8.00 kHz) and a lower (2.75 kHz) and higher (15.00 kHz) frequency during Day 2 (“Before”) and Day 4, 24 h post-training (“After”). RCI values indicate the quantified effect of tone on respiration. Before training, responses to all three frequencies were minimal. However, after training, the CS frequency produced a large disruption of respiration (RCI = 0.46). The specificity of this index of NB-induced behavioral memory is indicated by the absence of responses to the lower and higher frequencies. (B) Examples of respiratory waveforms from a subject in the Scopolamine group. Similar to the Saline group, responses before training were minimal. In contrast to the Saline group, there was also a minimal response to the CS frequency (as well as the lower and higher frequencies) after training (CS RCI = 0.09). Note that both subjects had the same level of NBstm (72 μA) during pairing. The thick horizontal bars indicate tone presentation.
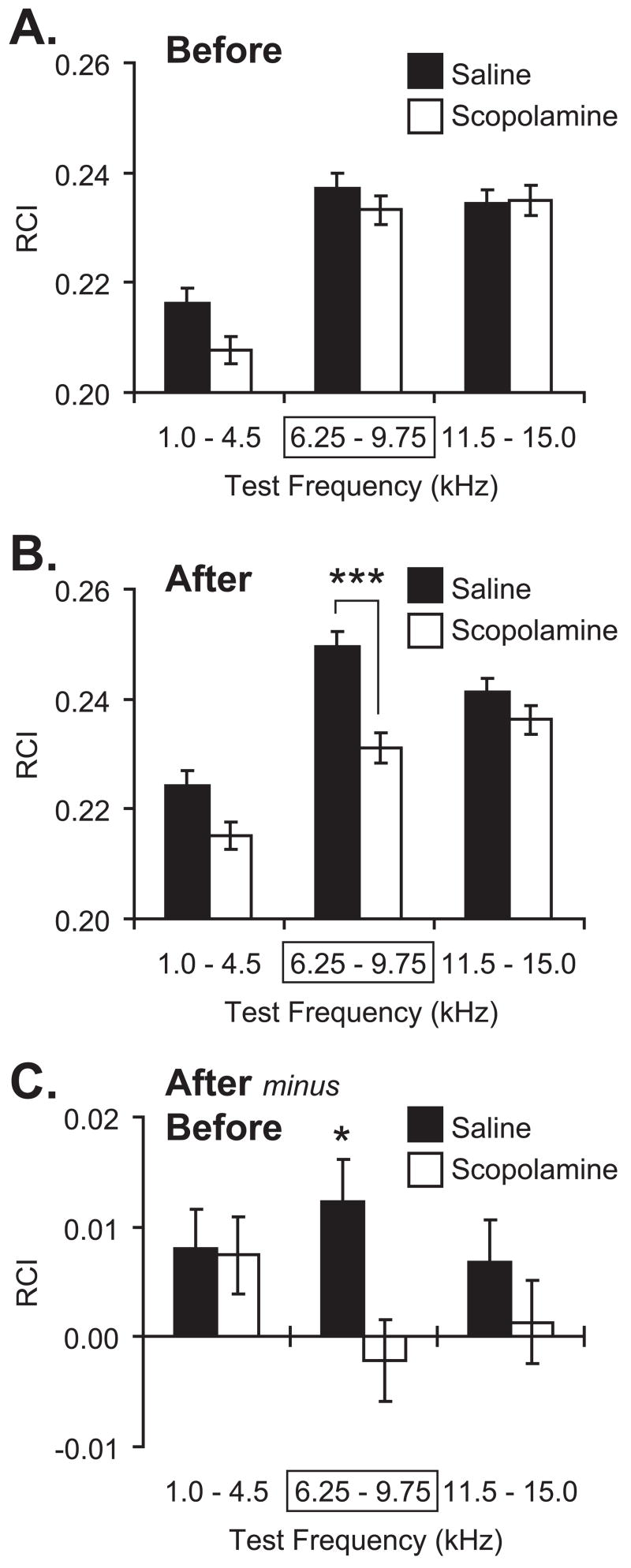
Figure 5 summarizes the group data. Over the 200 presentations of the test tones before pairing, there was substantial response, particularly for the CS frequency band (6.25–9.75 kHz) and the high frequency band (11.5–15.0 kHz). This profile is generally consistent with the audiogram of the rat (Heffner, Heffner, Contos, & Ott, 1994). Although, as mentioned, animals were differentially sensitive to different frequencies (2-way ANOVA, frequency factor: F(2, 41613) = 50.21, p < 0.0001), the difference between the Scopolamine and Saline groups was not significant (F(1, 41613) = 3.44, p > 0.06), and the interaction was not significant (F(2, 41613) = 1.54, p > 0.20) (Figure 5A). Conversely, the groups did differ after pairing (2-way ANOVA, group: F(1, 41600) = 25.37, p < 0.0001); both frequency factor (F(2, 41600) = 37.85, p < 0.0001) and the group × frequency interaction (F(2, 41600) = 3.44, p < 0.05) differed significantly, as well. Post-hoc tests revealed that this difference was limited to the CS frequency band, at which the Saline group exhibited a larger response than the Scopolamine group (Tukey’s test: p < 0.00002). The groups did not differ in response to either the low (p > 0.10) or high (p > 0.75) frequency bands (Figure 5B). These findings indicate that the Saline group had acquired a CS-specific memory following CS–NBstm pairing that could be expressed 24 h after training, whereas the Scopolamine group did not. To better understand the nature of the differences between the groups, within-group analyses were also conducted. They revealed that the Saline group had developed an absolute increase in responses to all three frequency bands, although only the increase at the CS frequency band was statistically significant (Tukey: CS band, p < 0.02; low band, p > 0.25; high band, p > 0.45). The Scopolamine animals also exhibited some changes in responses to test frequencies, but none were significant (Tukey: CS band, p > 0.95; low band, p > 0.30; high band, p > 0.95).
Figure 5.
Effect of post-training scopolamine on NB-induced memory. Graph bars show Mean ± SE of Respiration Change Index, RCI (Y-axis). (A) Pre-training (“Before”) frequency generalization gradients to tones in three frequency bands: “Low” (1.0–4.5 kHz), “CS” (6.25–9.75 kHz [framed with rectangle]; the CS was 8.00 kHz); “High” (11.5–15.0 kHz). There were no significant differences between Saline (black bars) and Scopolamine (white bars) groups before training. (B) Post-training/post-injection (“After”) generalization gradients for the Saline and Scopolamine groups. Note the significant difference in response in the CS band between the Saline and Scopolamine groups. There was no significant differences at the Low and High frequency bands. (C) Comparison of within-group changes (“After minus Before”: post-training minus pre-training responses to test tones) in behavioral generalization gradients. Note that the Saline group had developed a statistically significant increase exclusively within the CS-frequency band. In contrast, the Scopolamine group had not developed any statistically significant changes following pairing/injection. *p < 0.05; ***p < 0.005, two-tailed t-tests.
Location of Electrodes
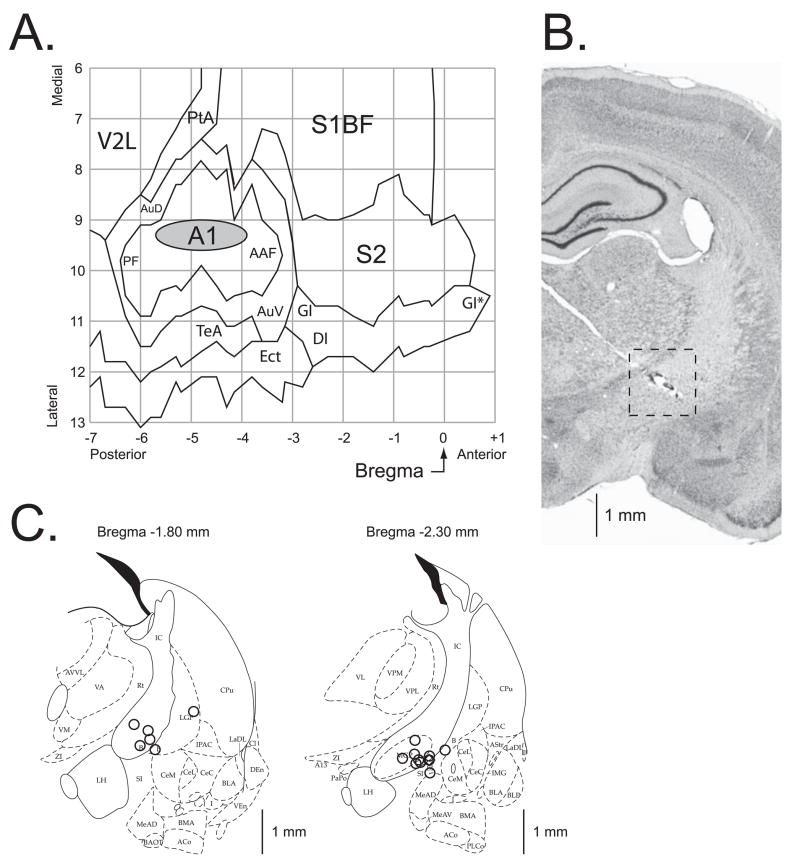
A summary of the location of the electrodes is presented in Figure 6. The cortical sites of recording are summarized in Figure 6A. All of the electrodes were located above the primary auditory cortex. The recording sites of the Saline and Scopolamine groups were intermingled and did not differ either in the AP (t(14) = 0.59, p > 0.55, two-tailed t-test) or the ML (t(14) = 1.38, p > 0.15) dimensions. The center of the location area (grey oval) corresponds to the X-Y coordinates of the mean for the group on the flattened standardized cortical surface, and the horizontal and vertical edges of the oval correspond to the ranges from Mean – SD to Mean + SD for the AP (−4.81 ± 0.91 mm, Mean ± SD) and the ML (9.26 ± 0.30 mm) coordinates, respectively.
Figure 6.
Location of recording and stimulation sites. (A) The auditory cortex EEG recording location. The oval indicates the location of epidural recordings based on their stereotaxic coordinates using a cortical map derived from Paxinos and Watson (1997). The location of the sites for each individual subject in the Saline and Scopolamine groups were precisely measured; they all were found to be over the primary auditory cortex. The sites of recording in Saline and Scopolamine groups overlapped, did not differ statistically (see Results) and thus are shown as a single group. (B) A section stained for Nissl showing the electrode track and the place of lesion (the site of stimulation) in the nucleus basalis. The electrode was implanted by a contralateral approach, to avoid damage to ipsilateral structures. (C) Diagrams of the two coronal sections (AP = −1.8 mm and AP = −2.3 mm) showing the NB stimulation sites; the sites were projected onto outlines of frontal section at closest relevant sections Anterior-to-Posterior (AP) distance relative to Bregma in millimeters (Paxinos & Watson, 1997). The stimulation sites in the Saline and the Scopolamine groups were intermingled and did not differ statistically (see Results) and thus are shown as a single group. In all animals, stimulation was within the caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynart) which projects preferentially to the auditory cortex. Small open circles represent individual stimulation sites. The scale bars in (B) and (C) are roughly equal thus providing a better view on where within the right intracranial region the stimulation sites were located. Abbreviations: B, basal nucleus of Meynert; CeM, amygdala central nucleus medial; CeL, amygdala central nucleus lateral; CPu, caudate–putamen; IC, internal capsule; IPAC, interstitial nucleus of posterior limb of anterior commissure; LGP, lateral globus pallidus; LH, lateral hypothalamus; SI, substantia innominata; SIB, substantia innominata, basal; SIV, substantia innominata, ventral; Au, primary auditory cortex; AAF, anterior auditory Weld; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; PF, posterior auditory Weld; S1BF, primary somatosensory cortex, barrel Weld; S2, secondary somatosensory cortex; TeA, temporal association cortex; V2L, secondary visual cortex, lateral area.
Figure 6B shows the implantation path of the stimulating electrode within the right hemisphere (the 45° angled light electrode trace from the upper left towards the lower right). The stimulation region is delineated with a dashed square around the lesion site found within the ventral portion of internal capsule. The NB stimulation sites from all subjects are shown in Figure 6C. The stimulation sites of the Saline and Scopolamine groups were intermingled and did not differ either in the AP (t(14) = 0.23, p > 0.80, two-tailed t-test), the ML (t(14) = 1.26, p > 0.20), or the DV (t(14) = 1.13, p > 0.25) dimensions. In general, the coordinates of the area of stimulation, as referenced to the coronal plan, were as follows: AP, −2.14 ± 0.25 mm (Mean ± SD); the ML, 3.33 ± 0.40 mm; and the DV, 7.63 ± 0.22 mm. All the located stimulation sites were in the basal forebrain within structures containing corticopetal cholinergic cells, including those that project to the auditory cortex (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Luiten, Gaykema, Traber, & Spenser, 1987; Mesulam, Mufson, Wainer, & Levey, 1983; Rye, Wainer, Mesulam, Mufson, & Saper, 1984).
Verification of Same Effectiveness of NB Stimulation
It might be thought that the failure of the Scopolamine group to display NB-induced memory was due to the use of a less effective NBstm. We compared levels of current used during pairing and found no significant difference: Saline = 68 ± 9 μA; Scopolamine = 67 ± 8 μA (mean ± SD); t(14) = 0.349, p > 0.70, two-tailed. Also, the groups differed neither in weight (t(14) = 1.710, p > 0.10), nor age (t(14) = 1.264, p > 0.20). However, equal stimulating currents do not guarantee equal physiological effectiveness. Therefore, we quantified the amount of change in the EEG induced by NBstm.
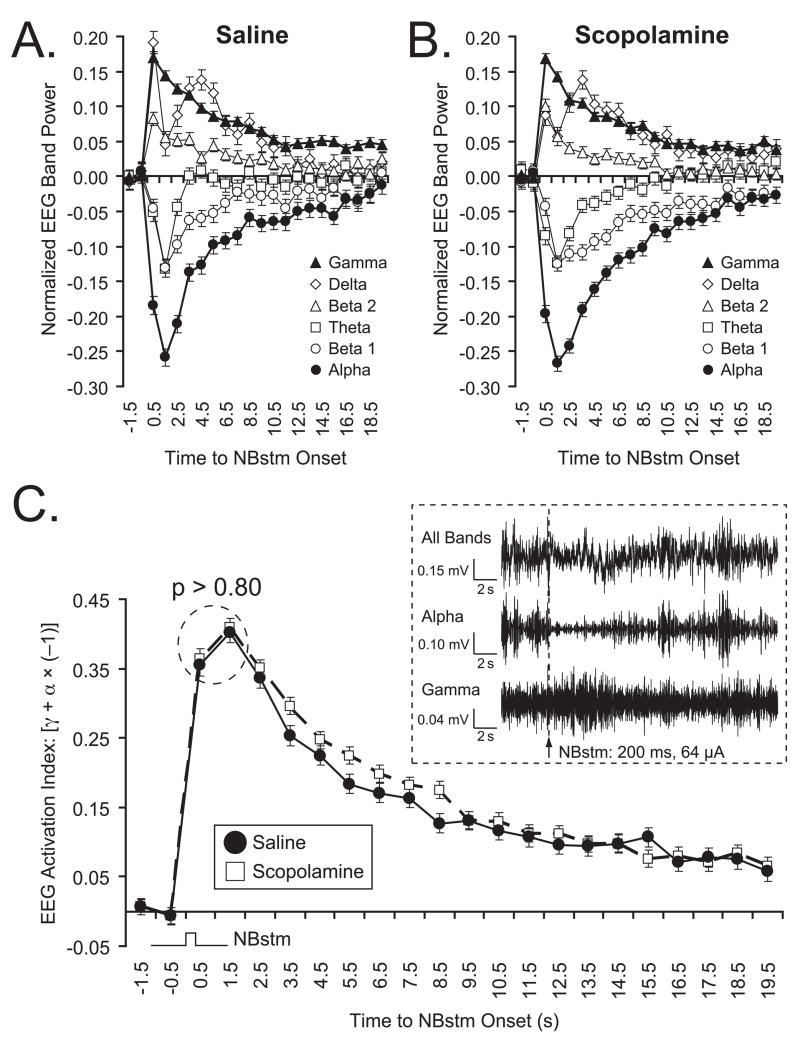
Figures 7A and 7B show the quantified changes in power for all EEG frequency bands: (delta, theta, alpha, beta1, beta2 and gamma) for the Saline and Scopolamine groups, respectively. Band-wise statistical comparison at points of maximal change from baseline (EEG PCI levels immediately preceding NBstm) showed that the effectiveness of NBstm in the Saline and Scopolamine groups did not differ. The EEG power, expressed as EEG PCI, generally declined at alpha (Saline, −0.301 ± 0.013 vs. Scopolamine, −0.308 ± 0.011; mean ± SE, measured at 1.8 s; between-group difference [BGD]: t(3039) = 0.385, p > 0.65); beta1 (BGD 1.8 s; p > 0.70), and theta (BGD 1.8 s; p > 0.95) frequency bands. The power generally increased at beta2 (BGD 0.8 s; p > 0.75), and gamma bands (BGD 0.8 s; p > 0.90). The changes within the delta band were most consistent at a longer latency (3.8 s) but the difference between groups also was not significant (BGD p > 0.65).
Figure 7.
Effectiveness of NBstm (200 presentations) on the auditory cortex (levels of EEG activation) after completion of the training/test protocol in the Saline and Scopolamine groups. (A, B) Group mean EEG spectral changes. For each frequency band, changes were computed as the EEG Power Change Index: EEG PCIi = (Posti – Pre)/(Posti + Pre) where the “Pre” period was the mean of the first 2 s out of four immediately preceding tone onset and post measures were calculated for consecutive periods of 1 s. Note major effects are an increase in gamma (closed triangles) and a decrease in alpha (closed circles) power. The EEG changes are comparable in the two groups. (C) Direct comparison of the levels of EEG activation produced by NBstm in Saline (black circles) and Scopolamine (open squares) groups (Mean ± SE). The graph shows the mean “EEG Activation Index” (EAI) for NBstm alone (Methods). Note that the responses to NBstm were robust in both groups, and there was no significant difference between the peak magnitudes of the EAI values (p > 0.80 for designated data points). Insert: Example of auditory cortical EEG activation by NBstm (200 ms, 100 Hz, 64 μA bipolar stimulation) observed for “All Bands”, and the two frequency bands that exhibited the largest changes in power (“Alpha” and “Gamma”) in an animal from Scopolamine group. “All Bands”: original records obtained with band-pass filters set at 1–1,000 Hz. “Alpha” and “Gamma”: corresponding records band-passed with digital filters set at 8.8–14.6 Hz to emphasize alpha and 33–59 Hz to emphasize gamma bands, respectively. An arrow shows the moment of NB electrical stimulation. Note the EEG activation, including a distinct decrease in higher voltage, slower waves (“Alpha”) and increase in lower voltage faster waves (“Gamma”).
With subjects in a state of quiet waking, the major elicited EEG changes are a decrease in the power of alpha activity and a concomitant increase in the power of gamma activity (McLin et al., 2002b; Miasnikov et al., 2008). Taken together, the two bands form an envelope that represents cortical activation. To determine whether this measure of activation was equal in both groups, we used an EEG Activation Index that combined data from both bands (Methods). These data are shown in Figure 7C, which also gives an example of EEG response to NBstm (“all bands”, “alpha” and “gamma”). The EAI exhibited essentially identical dynamics in the Saline and Scopolamine groups. The peaks of the functions were subjected to a statistical comparison and were found not to differ statistically (t(2) = 0.271, p > 0.80). Therefore, we conclude that the failure of the Scopolamine group to exhibit memory is not attributable to its NBstm effect being functionally weaker than in the Saline group.
DISCUSSION
Summary and Validity of the Findings
The induction of specific behavioral memory by direct intervention in the brain is unusual, if not unique. The present experiment is a logical extension of research on the putative release of acetylcholine from the nucleus basalis during natural learning. It is a consequence of a model of learning-induced associative plasticity in the auditory cortex (Weinberger, 1995, 1998; Weinberger, Ashe, Metherate, McKenna, Diamond, & Bakin, 1990). The model stipulates that as auditory thalamic neurons develop associative increases in discharge to conditioned stimuli, they activate the NB via the amygdala on training trials. This appropriately-timed activation of the NB is hypothesized to release ACh and promote the long-term storage of specific associative memory traces in the primary auditory cortex. We refer to this part of the model as the “NB/ACh Memory Induction Hypothesis”. Our experimental strategy has been to mimic natural associative learning by stimulation of the NB following onset of a tone. Prior findings have revealed the fact of the induction of specific, associative memory for acoustic frequency (McLin et al., 2002a, 2003; Miasnikov et al., 2006). Moreover, they have shown that the level of NB activation can control the degree of auditory frequency detail that is incorporated into memory: low levels of activation induce the memory that “sound is important”, without preserving the information on the frequency of the CS, whereas higher levels of activation also allow formation of the memory for the importance of the CS frequency (Weinberger et al., 2006).
Despite the fact that the NB is the major source of cortical acetylcholine, only indirect evidence has supported the hypothesis that the release and actions of ACh have a causal role in NB-induced memory. Support has been based on established relationships between the NB, ACh and the EEG. First, cholinolytic atropine blocks EEG activation and reduces cortical levels of ACh (Phillis & York, 1968; Szerb, 1964). Second, stimulation of the basal forebrain causes both the release of acetylcholine in the cortex and cortical EEG activation (shift from higher voltage slower waves to lower voltage faster waves) (e.g., Cape & Jones, 1998; Cape, Manns, Alonso, Beaudet, & Jones, 2000; Casamenti et al., 1986; Celesia & Jasper, 1966; Détári, Juhász, & Kukorelli, 1983; Rasmusson et al., 1992, 1994, 1996) and corresponding neural activation (Jiménez-Capdeville et al., 1997). Third, specific neurotoxic lesions of NB cholinergic corticopetal neurons deplete the cortex of ACh and impair EEG activation, i.e., increase slow wave activity and decrease fast (e.g., gamma) waves (Berntson, Shafi, & Sarter, 2002; Wenk, Stoehr, Quintana, Mobley, & Wiley, 1994). Fourth, cholinergic corticopetal neurons in the NB exhibit a strong correlation with the EEG; increased rates of discharge occur with increased activation and vice versa (Chernyshev & Weinberger, 1998; Détári et al., 1984, 1999; Duque, Balatoni, Détári, & Zaborszky, 2000; Manns, Alonso, & Jones, 2000a, 2000b; Szymusiak & McGinty, 1986). Together, these findings support the “NB/ACh Memory Induction” hypothesis and are also consistent with the view that release of cortical ACh is at least part of the mechanism underlying the activation state of the EEG (Détári et al., 1999; Metherate & Ashe, 1992).
The present study has now provided direct evidence to support this hypothesis. The formation of specific associative memory by tone paired with stimulation of the nucleus basalis is impaired or blocked by scopolamine (Figures 4, 5). The most parsimonious interpretation of the findings is that NB-induced memory formation requires the release of acetylcholine in the brain and is not mediated by putative non-cholinergic effects of NB stimulation. However, there are alternative interpretations of the findings.
First, it might be argued that NB stimulation in this study did not induce genuine associative memory because the design did not include a non-associative control group. Such a control group was not employed because previous research has consistently shown that subjects receiving unpaired or random tone and NB stimulation do not form CS-specific memory. Instead they exhibit flat behavioral (respiratory and cardiac) generalization gradients and may even exhibit decreased responses to the “CS” attributable to tone repetition lacking consequences (McLin et al., 2002a, 2003; Miasnikov et al., 2006; Weinberger et al., 2006).
Second, memory induction could have failed because the Scopolamine group received less effective NB stimulation. However, there was no significant difference in the level of NB stimulation and NB stimulation produced the same type and magnitude of EEG activation in both groups. Quantitative analyses revealed significant differences neither in comparisons of individual EEG bands nor in the integral EEG Activation Index that combined data on dominant representative EEG bands, i.e., alpha and gamma (Figure 7). Thus, differences in the expression of memory 24 h after training were not the result of a lesser effectiveness of NBstm in the Scopolamine group.
Third, it might be thought that scopolamine could have interfered with non-mnemonic processes. However, any scopolamine-induced effects on putative performance factors, such as attention and arousal changes during tone–NBstm pairing, can be ruled out because scopolamine was administered post-training. Also, testing for memory was delayed for twenty-four hours, so that the drug was not present at the time of behavioral assessment of memory.
Fourth, given that scopolamine was administered systemically, it was possible that the failure of this group to exhibit specific memory was caused by peripheral effects of the drug. However, an extensive literature, covering numerous tasks and several species including the rat, has established that systemically-administered scopolamine hydrobromide trihydrate affects learning and memory via the brain. These findings include tone and context fear conditioning (rat) (Anagnostaras et al., 1999), avoidance conditioning (mouse) (Flexner, Flexner, & Church, 1991), inhibitory avoidance (rat) (Goto, Kuzuya, Endo, Tajima, & Ikari, 1990), negative patterning (rat) (Moran, 1992), auditory and visual discrimination (rat) (van Haaren & van Hest, 1989), odor discrimination (rat) (De Rosa & Hasselmo, 2000), conditional discrimination (rat) (Wan, Pang, & Olton, 1997) conditioned taste aversion (rat) (Ramírez-Lugo, Miranda, Escobar, Espinosa, & Bermúdez-Rattoni, 2003), 5-choice serial reaction time (monkey) (Spinelli, Ballard, Feldon, Higgins, & Pryce, 2006), operant specific number (rat) (van Haaren & van Hest, 1989), spatial discrimination (rat) (Steckler & Holsboer, 2001), spatial memory (rat) (Pitsikas, 2007), working memory (rat) (Shannon & Yang, 2007), reference memory (rat) (Miyagawa, Honma, & Sato, 1995), recognition memory (rat) (Woolley, Marsden, Sleight, & Fone, 2003), paired associates (human) (Atri, Sherman, Norman, Kirchhoff, Nicolas, Greicius, Cramer, Breiter, Hasselmo, & Stern, 2004) and free recall (human) (Danion, Zimmermann, Willard-Schroeder, Grangé, Welsch, Imbs, & Singer, 1990). Thus, it seems highly likely that the impairing effects of scopolamine on NB-induced specific memory were caused by the block of muscarinic receptors in the brain. The present findings are agnostic regarding any possible critical loci of muscarinic action within the brain. To address these issues, future studies will need to employ locus-specific and receptor-specific manipulation of cholinergic neurotransmission.
Characteristics of Nucleus Basalis-Induced Memory
Specific memory induction by appropriately timed, paired electrical stimulation of the cholinergic nucleus basalis is noteworthy for several reasons. First, NB stimulation sufficient to induce memory is neither rewarding nor punishing (Miasnikov et al., 2008). This contrasts with memory induction that might be induced by brain stimulation that is motivationally significant, such as rewarding stimulation of the ventral tegmentum (Schultz, 2001) and the nucleus accumbens (Day & Carelli, 2007), or aversive stimulation of the periaqueductal gray (Behbehani, 1995), intralaminar thalamic nuclei or anterior cingulate cortex (Sewards & Sewards, 2002). The effects of substitution of a brain reward or punishment for an environmental reward or punishment, while interesting, would hardly be surprising. Thus, it seems likely that the memory-inducing function of the NB is “downstream” of motivational detection and the assignment of positive or negative valence. This location in the information processing stream should enable the NB to promote memory across a variety of situations and types of motivation.
Second, the present findings provide, in part, an anatomical–physiological basis for the actions of cholinergic agents on memory. Certainly, other groups of cholinergic cells are involved in learning and memory. Recent studies of the magnitude and release of ACh have found locus-dependent competition (McIntyre, Pal, Marriott, & Gold, 2002) or cooperation between different structures (McIntyre, Marriott, & Gold, 2003). The roles of various cholinergic cells in the numerous processes underlying learning and memory might be clarified by using the strategy of natural learning mimicry, i.e., by determining the extent to which their activation might induce specific memory. Similar considerations apply to other neuromodulatory systems, such as noradrenergic and serotonergic. Thus, pairing a sensory stimulus with activation of the locus coeruleus and raphe, respectively, could be undertaken given the present state of knowledge of the nucleus basalis and memory induction.
Third, the cholinergic induction of specific behavioral memory raises the issue of what sort of memory has been induced. In the absence of an external biologically-significant reinforcer, what is the nature of an association with the tonal conditioned stimulus? We suggest that the subjects learned that “8.00 kHz is important”, nothing more and nothing less. In short, they would now associate the CS frequency with behavioral relevance, although having no information on why the CS tone has become relevant. Such a memory would increase the salience of 8.00 kHz compared to other tones, but by itself would not predict any sensory or motivationally-significant reinforcer. This hypothesis can be tested by determining if this “information” can be transferred to a new situation. For example, increased salience to the CS frequency might interfere with an ongoing task or even retard new learning. Even more interesting, subjects might be able to use this information to facilitate the solution of a novel problem.
Beyond such transfer studies, NB-induced specific memory accomplishes the task of a “dissection” of memory. Workers have long recognized that associative memories have several components, including sensory content, associations between discrete stimuli, between a stimulus and a context, hedonic content, and even the outcome of greater understanding of the “causal fabric” of the environment. NB-induced memory may provide for an elemental memory in which the sensory content of the signal stimulus is encoded and stored without many of the usual associations or memory components. Such artificially-induced memory should be subject to a simpler and more elemental analysis than natural memories. Already, there is some characterization of the neural underpinning of NB-induced memory. It is accompanied by a retuning of the primary auditory cortex to favor processing of signal frequencies (reviewed in Weinberger, 2007). Such an increase in cells responding preferentially to signal stimuli of increased salience might constitute a neurobiological substrate of a “memory code” for the increased behavioral importance of environmental events (Rutkowski & Weinberger, 2005). Given the feasibility of inducing specific, behaviorally-validated memories and the findings to date, this approach to the neurobiology of learning and memory holds considerable promise.
Acknowledgments
We thank Nataliya Gross, Gabriel K. Hui, Julia Martinson and Jacquie Weinberger for assistance. This study was funded by the NIDCD/NIH, DC-02938.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: Dose-effect analysis. Neuropsychopharmacology. 1999;21(6):731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4(1):44–54. doi: 10.1002/syn.890040106. [DOI] [PubMed] [Google Scholar]
- Ashe JH, Weinberger NM. Acetylcholine modulation of cellular excitability via muscarinic receptors: Functional plasticity in auditory cortex. In: Richardson RT, editor. Activation to acquisition: Functional aspects of the basal forebrain cholinergic system. Boston: Birkhäuser; 1991. pp. 189–246. [Google Scholar]
- Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behavioral Neuroscience. 2004;118(1):223–236. doi: 10.1037/0735-7044.118.1.223. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Progress in Neurobiology. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. European Journal of Neuroscience. 2002;16(12):2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. Journal of Neuroscience. 1998;18(7):2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. Journal of Neuroscience. 2000;20(22):8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamenti F, Deffenu G, Abbamondi AL, Pepeu G. Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Research Bulletin. 1986;16(5):689–695. doi: 10.1016/0361-9230(86)90140-1. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Chernyshev BV, Weinberger NM. Acoustic frequency tuning of neurons in the basal forebrain of the waking guinea pig. Brain Research. 1998;793(1–2):79–94. doi: 10.1016/s0006-8993(98)00163-2. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW. Cholinergic modulation of visual attention and working memory: Dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learning and Memory. 2004;11(1):78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danion JM, Zimmermann MA, Willard-Schroeder D, Grangé D, Welsch M, Imbs JL, Singer L. Effects of scopolamine, trimipramine and diazepam on explicit memory and repetition priming in healthy volunteers. Psychopharmacology. 1990;102(3):422–424. doi: 10.1007/BF02244116. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13(2):148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behavioral Neuroscience. 2000;114(1):32–41. [PubMed] [Google Scholar]
- Détári L, Juhász G, Kukorelli T. Effect of stimulation of vagal and radial nerves on neuronal activity in the basal forebrain area of anaesthetized cats. Acta Physiologica Hungarica. 1983;61(3):147–154. [PubMed] [Google Scholar]
- Détári L, Juhász G, Kukorelli T. Firing properties of cat basal forebrain neurones during sleep–wakefulness cycle. Electroencephalography and Clinical Neurophysiology. 1984;58(4):362–368. doi: 10.1016/0013-4694(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(11):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Détári L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of Neurophysiology. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Mechanisms controlling neuronal plasticity in somatosensory cortex. Canadian Journal of Physiology and Pharmacology. 1997;75(5):535–545. [PubMed] [Google Scholar]
- Dykes RW, Tremblay N, Warren RA, Bear MF. Cholinergic modulation of synaptic plasticity in sensory neocortex. In: Richardson RT, editor. Activation to acquisition: Functional aspects of the basal forebrain cholinergic system. Boston: Birkhäuser; 1991. pp. 325–345. [Google Scholar]
- Edeline JM, Hars B, Maho C, Hennevin E. Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Experimental Brain Research. 1994;97(3):373–386. doi: 10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Maho C, Hars B, Hennevin E. Non-awaking basal forebrain stimulation enhances auditory cortex responsiveness during slow-wave sleep. Brain Research. 1994;636(2):333–337. doi: 10.1016/0006-8993(94)91033-2. [DOI] [PubMed] [Google Scholar]
- Fisher A, Brandeis R, Chapman S, Pittel Z, Michaelson DM. M1 muscarinic agonist treatment reverses cognitive and cholinergic impairments of apolipoprotein E-deficient mice. Journal of Neurochemistry. 1998;70(5):1991–1997. doi: 10.1046/j.1471-4159.1998.70051991.x. [DOI] [PubMed] [Google Scholar]
- Flexner LB, Flexner JB, Church AC. Long-term suppression in mice of the development of complementary memory storage sites: Effect of a muscarinic antagonist. Pharmacology, Biochemistry, and Behavior. 1991;39(3):689–694. doi: 10.1016/0091-3057(91)90148-u. [DOI] [PubMed] [Google Scholar]
- Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Research. 1981;215(1–2):177–185. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. γ-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(2):738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Goto T, Kuzuya F, Endo H, Tajima T, Ikari H. Some effects of CNS cholinergic neurons on memory. Journal of Neural Transmission Supplementum. 1990;30:1–11. doi: 10.1007/978-3-7091-3345-3_1. [DOI] [PubMed] [Google Scholar]
- Gu Q. Contribution of acetylcholine to visual cortex plasticity. Neurobiology of Learning and Memory. 2003;80(3):291–301. doi: 10.1016/s1074-7427(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56(1):61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73(2):244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Edeline J-M, Hars B, Maho C. Fifth conference on the neurobiology of learning and memory. Brain and memory: Modulation and mediation of neuroplasticity. Irvine, CA: Center for the Neurobiology of Learning and Memory, University of California; 1992. Basal forebrain (BF) stimulation enhances tone-evoked responses in the auditory cortex of awake rats. Abs. No. 40. [Google Scholar]
- Hennevin E, Maho C, Hars B, Edeline JM. Basal forebrain (BF) stimulation facilitates tone-evoked responses during slow-wave sleep in the rat auditory cortex. Abs. No. 69.16. Society for Neuroscience Abstracts. 1993;19(1):164. [Google Scholar]
- Introini-Collison IB, McGaugh JL. Modulation of memory by post-training epinephrine: Involvement of cholinergic mechanisms. Psychopharmacology. 1988;94(3):379–385. doi: 10.1007/BF00174693. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Ji W, Gao E, Suga N. Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. Journal of Neurophysiology. 2001;86(1):211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: Effect of atropine. Journal of Neurophysiology. 2003;90(3):1904–1909. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N, Gao E. Effects of agonists and antagonists of NMDA and ACh receptors on plasticity of bat auditory system elicited by fear conditioning. Journal of Neurophysiology. 2005;94(2):1199–1211. doi: 10.1152/jn.00112.2005. [DOI] [PubMed] [Google Scholar]
- Jiménez-Capdeville ME, Dykes RW, Myasnikov AA. Differential control of cortical activity by the basal forebrain in rats: A role for both cholinergic and inhibitory influences. Journal of Comparative Neurology. 1997;381(1):53–67. [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep–wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279(5357):1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. Journal of Neurophysiology. 2001;86(1):326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology. 2001;187(4):249–254. doi: 10.1007/s003590100196. [DOI] [PubMed] [Google Scholar]
- Luiten PGM, Gaykema RPA, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Research. 1987;413(2):229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9335–9340. doi: 10.1073/pnas.0503851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. Journal of Neuroscience. 2000a;20(4):1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. Journal of Neuroscience. 2000b;20(24):9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fà M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep–wake cycle in freely moving cats. Brain Research. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153(742):1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Cooperation between memory systems: Acetylcholine release in the amygdala correlates positively with performance on a hippocampus-dependent task. Behavioral Neuroscience. 2003;117(2):320–326. doi: 10.1037/0735-7044.117.2.320. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Pal SN, Marriott LK, Gold PE. Competition between memory systems: Acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. Journal of Neuroscience. 2002;22(3):1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna TM, Ashe JH, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse. 1989;4(1):30–43. doi: 10.1002/syn.890040105. [DOI] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. The effects of electrical stimulation of the nucleus basalis on the electroencephalogram, heart rate, and respiration. Behavioral Neuroscience. 2002b;116(5):795–806. [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Research. 1991;559(1):163–167. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation elicits neocortical activation and facilitates thalamocortical synaptic transmission: Intracellular and extracellular recordings in rat auditory cortex. Abs. No. 411.8. Society for Neuroscience Abstracts. 1992;18(2):975. [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14(2):132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008 doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, III, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. NeuroReport. 2001;12(7):1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Miyagawa M, Honma T, Sato M. Effects of subchronic exposure to toluene on working and reference memory in rats. Neurotoxicology and Teratology. 1995;17(6):657–664. doi: 10.1016/0892-0362(95)02008-x. [DOI] [PubMed] [Google Scholar]
- Moran PM. Scopolamine deficits in negative patterning discrimination: Evidence for a role of the central cholinergic system in retention but not acquisition of non-spatial configural association learning. Behavioural Brain Research. 1992;48(2):187–197. doi: 10.1016/s0166-4328(05)80156-1. [DOI] [PubMed] [Google Scholar]
- Mostofsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. [Google Scholar]
- Múnera A, Gruart A, Muñoz MD, Delgado-García JM. Scopolamine impairs information processing in the hippocampus and performance of a learned eyeblink response in alert cats. Neuroscience Letters. 2000;292(1):33–36. doi: 10.1016/s0304-3940(00)01430-0. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Anrep GV, translator. New York: Dover Publications; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Phillis JW, York DH. Pharmacological studies on a cholinergic inhibition in the cerebral cortex. Brain Research. 1968;10(3):297–306. doi: 10.1016/0006-8993(68)90201-1. [DOI] [PubMed] [Google Scholar]
- Pitsikas N. Effects of scopolamine and L-NAME on rats’ performance in the object location test. Behavioural Brain Research. 2007;179(2):294–298. doi: 10.1016/j.bbr.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of Learning and Memory. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Ramírez-Lugo L, Miranda MI, Escobar ML, Espinosa E, Bermúdez-Rattoni F. The role of cortical cholinergic pre- and post-synaptic receptors in taste memory formation. Neurobiology of Learning and Memory. 2003;79(2):184–193. doi: 10.1016/s1074-7427(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Research. 1992;594(1):150–154. doi: 10.1016/0006-8993(92)91041-c. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Clow K, Szerb JC. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience. 1994;60(3):665–677. doi: 10.1016/0306-4522(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Szerb JC, Jordan JL. Differential effects of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and N-methyl-D-aspartate receptor antagonists applied to the basal forebrain on cortical acetylcholine release and electroencephalogram desynchronization. Neuroscience. 1996;72(2):419–427. doi: 10.1016/0306-4522(95)00523-4. [DOI] [PubMed] [Google Scholar]
- Ravel N, Elaagouby A, Gervais R. Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behavioral Neuroscience. 1994;108(2):317–324. doi: 10.1037//0735-7044.108.2.317. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. Journal of Neuroscience. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiology of Learning and Memory. 1996;65(1):73–81. doi: 10.1006/nlme.1996.0008. [DOI] [PubMed] [Google Scholar]
- Russell RW, Escobar ML, Booth RA, Bermudez-Rattoni F. Accelerating behavioral recovery after cortical lesions. II. In vivo evidence for cholinergic involvement. Behavioral and Neural Biology. 1994;61(1):81–92. doi: 10.1016/s0163-1047(05)80047-0. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Introini-Collison IB, Dalmaz C, McGaugh JL. Posttraining intraamygdala infusions of oxotremorine and propranolol modulate storage of memory for reductions in reward magnitude. Neurobiology of Learning and Memory. 1997;68(1):51–59. doi: 10.1006/nlme.1997.3776. [DOI] [PubMed] [Google Scholar]
- Schön K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. Journal of Neuroscience. 2005;25(40):9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7(4):293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. The medial pain system: Neural representations of the motivational aspect of pain. Brain Research Bulletin. 2002;59(3):163–180. doi: 10.1016/s0361-9230(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Yang L. A non-spatial, stimulus-comparison working memory task in rats and disruption by scopolamine. Neuroscience. 2007;145(3):955–962. doi: 10.1016/j.neuroscience.2006.12.055. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR. Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology. 2006;51(2):238–250. doi: 10.1016/j.neuropharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Interaction between the cholinergic system and CRH in the modulation of spatial discrimination learning in mice. Brain Research. 2001;906(1–2):46–59. doi: 10.1016/s0006-8993(01)02555-0. [DOI] [PubMed] [Google Scholar]
- Stratton LO, Petrinovich L. Post-trial injections of an anti-cholinesterase drug and maze learning in two strains of rats. Psychopharmacologia. 1963;5(1):47–54. doi: 10.1007/BF00405574. [DOI] [PubMed] [Google Scholar]
- Szerb JC. The effect of tertiary and quaternary atropine on cortical acetylcholine output and on the electroencephalogram in cats. Canadian Journal of Physiology and Pharmacology. 1964;42(3):303–314. doi: 10.1139/y64-036. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Research. 1986;370(1):82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A. The effects of scopolamine and methylscopolamine on visual and auditory discriminations in male and female Wistar rats. Pharmacology, Biochemistry, and Behavior. 1989;32(3):707–710. doi: 10.1016/0091-3057(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Verdier D, Dykes RW. Long-term cholinergic enhancement of evoked potentials in rat hindlimb somatosensory cortex displays characteristics of long-term potentiation. Experimental Brain Research. 2001;137(1):71–82. doi: 10.1007/s002210000646. [DOI] [PubMed] [Google Scholar]
- Wan RQ, Pang K, Olton DS. Nonhippocampal muscarinic receptors are required for nonspatial working memory. Pharmacology, Biochemistry, and Behavior. 1997;58(2):361–367. doi: 10.1016/s0091-3057(97)00146-9. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annual Review of Neuroscience. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiology of Learning and Memory. 2003;80(3):268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J. Retuning auditory cortex by learning: A preliminary model of receptive field plasticity. Concepts in Neuroscience. 1990;1(1):91–132. [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. Journal of Neuroscience. 1994;14(10):5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Sleight AJ, Fone KC. Reversal of a cholinergic-induced deficit in a rodent model of recognition memory by the selective 5-HT6 receptor antagonist, Ro 04-6790. Psychopharmacology. 2003;170(4):358–367. doi: 10.1007/s00213-003-1552-5. [DOI] [PubMed] [Google Scholar]