Summary
Interference occurs when a substance or process falsely alters an assay result.
Interferences are classified as endogenous or exogenous. Endogenous interference originates from substances present in the patient’s own specimen. Exogenous interferences are substances introduced into the patient’s specimen.
To perform interference studies, proper planning is required.
Interference from haemolysis, icterus and lipaemia are most frequently studied. Haemolysis affects more analytes than does any other type of interference.
Protein interferences are most often associated with paraproteins and predominantly with IgM or IgG and rarely with IgA.
Drug interference may be due to the parent drug, metabolite(s) or additives in the drug preparation.
Collection tube components can affect determination of analytes.
Carryover interference typically occurs when analyte from a high concentration sample (or reagent) is incompletely removed by the analytical system’s washing process, whether probe, mixer or cuvette washing.
Immunoassay interferences are most commonly due to antibodies (generally polyclonal). They may be autoantibodies (e.g. in thyroid disease) or heterophile antibodies that predominantly interfere in two-site immunometric (sandwich) assays, forming a bridge between capture and detection antibodies.
Determining if interference is significant requires deviation limits from the original result.
Once interferences are identified during method evaluation or in general use, there is a need to establish procedures for handling affected results as part of the quality system.
Introduction
Interference occurs when a substance or process falsely alters an assay result. This may lead to inappropriate further tests, incorrect diagnoses, and treatments with potentially unfavourable outcomes for the patient. The most frequently performed interference studies are for the serum indices, haemolysis, icterus and lipaemia.
Classifying Interferences
Interferences are classified as endogenous or exogenous.
Endogenous interference originates from substances found naturally in the patient sample. They may be natural substances or health-related factors: haemolysis (haemoglobin and other substances), bilirubin, lipids, proteins, antibodies (autoantibodies, heterophile antibodies), excessive analyte concentration, and cross-reacting substances, e.g. bicarbonate on chloride ion selective electrode (ISE),1 ketones on creatinine by Jaffé technique.
Exogenous interference results from substances not naturally found in the patient’s specimen, including drugs (parent drug, metabolites, and additives), poisons, herbal products, IV fluids, substances used as therapy (e.g. antibodies, digi-bind). It may also arise from collection tube components, test sample additives such as preservatives added to quality control (QC) and calibration materials, processes affecting the sample (e.g. transport, storage, centrifugation), clots (post-refrigeration in heparin plasma, slow-clotting serum) and carryover contamination.
Where to Start
It is most important to understand that interferences may be method or analyser dependent. From a practical view, the starting point for interference testing should always include an assessment of the manufacturer’s method specifications. Nowadays kit inserts usually include statements on interference studies conducted by the manufacturer.
What Next
It is then necessary to plan an interference testing procedure by referring to the literature,2–4 obtaining the required materials, and establishing testing methods and procedures. Preferably, interference studies should mimic actual processes, testing increasing concentrations of the interferent with the analyte of interest at least at two levels, the first at a decision point and the second at an increased analyte concentration.
Haemolysis
There are three basic methods of preparation of haemolysates for interference assessment. These differ in the physical and mechanical techniques employed for red and white cell lysis.
Methods for preparation of haemolysate
Osmotic shock (Meites’ method)5: White cells and platelets are first removed to minimise their potential contribution to the analyte concentration.
Freezing/thawing of whole blood followed by the osmotic shock protocol.
Shearing (multiple needle aspirations) where cells are lysed progressively to provide a range of haemolysis.6
Methods 2 and 3 will include a contribution from white cell and platelet lysis. The preferred method will depend on the analyte of interest. The shearing method more closely mimics the actual pathological processes of haemolysis.7 However, it requires practice to obtain a wide haemolysis range and may not produce graded increases in haemoglobin concentration.
Mechanisms of interference from haemolysis
Additive: released intracellular substances, e.g. K, LD, AST are co-measured with the analyte in serum or plasma.
Spectral: most notably at wavelengths of 415, 540 and 570 nm where haemoglobin shows strong absorbance peaks; e.g. ALP, GGT may be affected.
Chemical: where there may be cross-reaction by free haemoglobin or other cellular constituents with the analyte of interest, e.g. red cell adenylate kinase interference in CK assays.
Dilutional: intracellular fluid contamination in serum or plasma, seen in severe haemolysis e.g. with Na, Cl.
When to Reject Haemolysed Samples
Having established for each analyte haemolysis cut-off values above which the assay is considered compromised, samples can be rejected as unsuitable for analysis. With some analytical platforms, an upper limit on haemolysis detection may dictate the cut-off (e.g. 5 g/L on Beckman DxC800 and DxC600 systems), while for other systems it is up to the laboratory to determine (e.g. a haemoglobin concentration of 6 g/L on the Roche Modular/Integra systems).6
Icterus (Jaundice)
High serum or plasma bilirubin concentrations can cause spectral interference with assays near the bilirubin absorbance peak of ~ 456 nm. Chemical interference e.g. with peroxidase-catalysed reactions may also occur. The most widely published studies are on the effects of bilirubin interference in Jaffé methods for creatinine measurement. Interference testing is performed with commercial bilirubin standards or with patient samples. The highest bilirubin concentration tested should be at least 500 μmol/L. Results are compared with those obtained by methods known to be interference-free, for example, by chromatography or tandem mass spectrometry.8,9
Lipaemia
Interference studies are less easily performed. Unlike haemoglobin or bilirubin, it is difficult to obtain suitable material to mimic lipid interference. Although patient samples are theoretically desirable, these are rarely suitable for interference studies. Intralipid, a fat emulsion containing soybean oil, egg yolk phospholipids and glycerin is commonly used for both interference studies and for setting lipaemia indices based on absorbance (LI). However, lipids in patient specimens are far more complex than in Intralipid, hence there is a poor relationship between triglyceride concentration and LI in patient samples. The interference mechanism is due either to (1) light scatter causing measurement errors in photometric methods e.g. with enzymes, or (2) volume displacement by lipid causing a decreased aqueous phase e.g. with indirect ISE methods.
Methods to Remove Lipid Interference
To assess the extent of lipid interference, a mechanism to remove or minimise the lipid concentration is required. Preferably the final triglyceride concentration in the cleared sample should be <15 mmol/L. Methods for clarification include the following:
Ultracentrifugation (e.g. Airfuge): ~15 min, ~20 psi air pressure, and 90,000 rpm.
High speed centrifugation: ~13,000 rpm or higher for 15 min; infranatant (clearer lower fraction) transferred to a new tube and re-centrifuged.
Lipid clearing agent e.g. Lipoclear used at 1 part per 9 parts sample.
For indirect ISEs, use of direct ISEs for comparison.10
Ultracentrifugation if available is certainly superior to high speed centrifugation, especially for grossly lipaemic samples. For example, a sample with a triglyceride of 59.2 mmol/ L became 17.3 mmol/L after two high speed spins but 8.1 mmol/L after ultracentrifugation (method 1).
Proteins
Most protein interferences are associated with paraproteins (monoclonal immunoglobulins), predominantly with IgM and IgG. Paraproteins can interfere with all types of automated assays (spectrophotometric, immunonephelometric, immunoturbidimetric) including total bilirubin, phosphate, HDL-cholesterol, GGT, CRP, and glucose.11,12
Mechanisms of interference
Unique physicochemical interactions between the paraprotein and method reagent result in precipitation of the paraprotein. Generally, precipitation is initiated by the first reagent, which is usually a buffer. Testing for interference may require change of sample type, assay with a different method, or precipitation studies at a number of sample dilutions.9,10
Protein affects indirect ISE methods due to the volume displacement effect, with the most noticeable errors being in sodium estimation (pseudohyponatraemia in hyperproteinaemia and pseudohypernatraemia in hypoproteinaemia).13 In general, samples with total protein concentration >100 or <40 g/L require sodium estimation to be performed on direct ISEs.
Drugs
Drug interferences often go unrecognised in the laboratory due to lack of relevant patient medication information. Interference may be due to the parent drug, its metabolite or additives. The best source for identifying drug interferences is Young’s Effects Online database3 or Young’s textbook.4 If the drug is not listed in these references, try contacting the reagent supplier or drug manufacturer.
Testing protocols in the laboratory for drug interference may include analysis of:
Saline or pooled serum spiked with the drug of interest.
Samples collected in different tubes e.g. gel-free or alternate supplier.
Samples by a known interference-free method e.g. chromatography, mass spectrometry.
Samples collected from volunteers who have taken the drug in the same concentrations suspected to be causing the interference.
Drug interference may be (1) chemical where the parent drug, metabolites or additives cross-react, (2) drugs or additives may act as accelerators or inhibitors of the assay, or (3) photometric where the parent drug, metabolites or additives may have similar absorption peaks to that of the measured chromogen.
Tube Components
The interference mechanism may be
Additive: where the interfering substance is present in the tube.14
Chemical: inhibition or activation in the assay by tube components.
Non-specific adsorption or non-specific binding in immunoassays.15
Testing protocols for tube component interference may include analysis of:
Samples collected in plain tubes (e.g. without gel) or tubes obtained from an alternate supplier.
Samples assessed by an alternate or reference method.
QC material with known analyte concentrations is added to the suspect collection tube, in an attempt to replicate the interference.16
Carryover
Typically interference is additive from high analyte concentrations in preceding samples (or reagent). The analyte is incompletely removed during washing processes,17,18 particularly in automated assays where the expected concentration range varies widely e.g. hCG, tumour markers. Carryover can be very tedious to confirm.
Interference testing can be basically divided into probe or cuvette wash testing.
Probe Wash Testing
A sample with high concentration analyte is analysed several times followed by several aliquots of sample with low concentration. If the washing is incomplete, the first of the low concentration samples is high (carryover) and decreases in the subsequent samples.
Cuvette Wash Testing
Cuvette re-use sequences may make cuvette carryover difficult to identify. Many aliquots of a low concentration sample may need to be analysed to ensure re-use of the cuvette previously containing a high concentration of analyte.
The probe or cuvette wash testing processes must be repeated several times to confirm the presence of carryover.
Immunoassay
Immunoassay interferences are more complex and usually difficult to resolve.19
Endogenous interferences
Serum Indices: There is no documented evidence of interference by icterus. Lipaemia interference is confined to immunonephelometric and immunoturbidimetric assays. Ideally, grossly lipaemic samples should be cleared for all assays to minimise volume displacement errors (see lipaemia removal). Haemolysis interference is rare. However, TnT is a commonly performed immunoassay that is affected by haemolysis.20
Antibody (generally polyclonal antibodies) interferences are the most commonly encountered e.g. autoantibodies in autoimmune diseases (thyroid disease), heterophile antibodies especially in two-site immunometric (sandwich) assays forming a bridge between capture and detection antibodies. Generally, heterophile antibodies do not interfere in competitive binding, immunonephelometric or immunoturbidimetric assays. In this type of interference human anti-animal antibodies, especially human anti-mouse antibodies (HAMA), are the cause. The heterophile immunoglobulins develop as a result of treatment of patients with drugs attached to mouse monoclonal antibodies or in individuals exposed to mouse proteins e.g. farmers, university laboratory and pet shop workers, or even contact with household pets.
Rheumatoid factors can behave like heterophile antibodies.
Hook Effect (antigen excess): In immunoassays with very wide measurement ranges and high analyte concentration, antigen excess results in false low values e.g. myoglobin, prolactin, hCG, serum free light chains, urinary albumin.
Other binding proteins (e.g. complement, paraproteins) are capable of binding to assay antibodies causing interference.
Exogenous interferences
Exogenous substances that have been reported to cause interferences include:
Tube components resulting in binding of antibodies or analytes
Fibrin clots
Monoclonal antibodies in cancer therapy
Digi-bind in digoxin toxicity
Fludrocortisone derivatives with cortisol
Testing Protocols
Heterophile antibodies can be tested for as follows.
Measurement by an alternate method that uses antibodies raised in other animal species but otherwise has close agreement with the usual test method.
Pre-treatment of specimen by (A) prior extraction using gel-filtration chromatography, (B) precipitation with PEG 6000 (25–40%), (C) heating to 70–90º C for heat-stable analytes such as drugs, or (D) using heterophile blocking tubes to remove interference.
Addition of blocking reagent (e.g. non-immune serum from the same species as reagent antibodies, non-immune mouse monoclonals, species-specific polyclonal IgG). A suggested ratio to start is 9 parts sample to 1 part blocking reagent.
Serial dilutions are recommended as a last resort. If the results are non-linear it may mean that heterophile antibodies are present. However, to obtain an accurate result the sample will have to be tested by an alternative method.
Hook Effect
This requires performing serial dilutions (1/20, 1/50, 1/100) and checking the linearity. It is also helpful to check other clinically-related results e.g. for myoglobin check CK results.
Decision Limits (Allowable Deviation Limits)
The next step in determining if interference is present is to develop cut-off limits for acceptable deviation from the original results. Setting cut-off limits must include clinical as well as statistical correlations to ensure the appropriate balance between bias (where results may be rejected even with small interference contribution) and utility (not withholding potentially useful information for clinical need).
Suggested allowable deviation limits in literature are:
Generally a change of 3 to 10% from original results confirms interference.21,22
Specifically it is suggested for enzymes and ISE methods a change of >5%, and for other analytes a change of >10%.23
Sample difference assessed by paired t-test, and if p<0.05 it is considered statistically significant, and interference is occurring.24
Use of intra-individual biological variation with desirable specification for total error.25
Heterophile antibodies: a difference between initial and treated results of 3 to 5 SD suggests possible heterophile interference;>5 SD indicates definite heterophile interference.26
Interference and Quality Systems
Developing a quality system for handling potential interferences in the routine laboratory is likely to involve education of staff (laboratory, collection, clinical), development or modification of standard operating procedures, establishing detection process alerts (e.g. delta checks, analyser flags, critical limits, reports review, awareness of discrepant clinical result and the necessity for clinical consultation). Once a significant new interference has been identified, it is essential to contact the manufacturer to discuss possible assay modifications.
Worked Example
AST Haemolysis study – Modified Osmotic Shock Method
Obtain whole blood sample (Li Heparin, ~ 10 mL in gel free tubes) with normal profile (normal lipids and bilirubin), and centrifuge.
Remove plasma (do not remove buffy coat) and replace with equal volume of normal saline (9 g/L).
Re-suspend cells and centrifuge.
Repeat saline wash 3–5 times.
In final wash, replace saline with distilled water and store at −20º C overnight
Thaw, mix, and centrifuge to remove stroma.
Transfer supernatant (haemolysate) to a clean tube and measure [Hb].
Prepare stock solutions of haemolysate in saline to give [Hb] range 0–100 g/L in increments of 5 or 10 g Hb/L.
Prepare two pooled plasma samples free of any visible haemolysis with AST ~30 U/L and ~100 U/L respectively.
To 900 μL of each of the pooled plasma samples add 100 μL of saline as baseline.
Measure AST and [Hb] or haemolysis index (HI) on these samples five times (baseline values).
To 900 μL of pooled plasma sample add 100 μL of each of the Hb stock solutions.
Measure AST and [Hb] or HI on spiked samples singularly or in duplicate.
Analyse data; the [Hb] or HI value where the AST concentration is >5% of the baseline value will be the HI cut-off limit (50 mg/dL). Refer to the Table and Figure.
Table.
Results of AST haemolysis study performed on a Hitachi Modular D analyser: A, AST - normal baseline activity; B, AST - elevated baseline activity.
| Part A | Part B | ||||
|---|---|---|---|---|---|
|
| |||||
| HI (mg/dL) | AST (U/L) | % Change from baseline value | HI (mg/dL) | AST (U/L) | % Change from baseline value |
| 5 | 40 | 14 | 112 | ||
| 44 | 42 | 5 | 55 | 118 | 5 |
| 103 | 45 | 13 | 104 | 121 | 8 |
| 160 | 50 | 25 | 208 | 133 | 19 |
| 321 | 59 | 48 | 295 | 142 | 27 |
| 425 | 66 | 65 | 401 | 154 | 38 |
| 556 | 76 | 90 | 595 | 178 | 59 |
| 701 | 85 | 113 | 715 | 191 | 71 |
Figure.
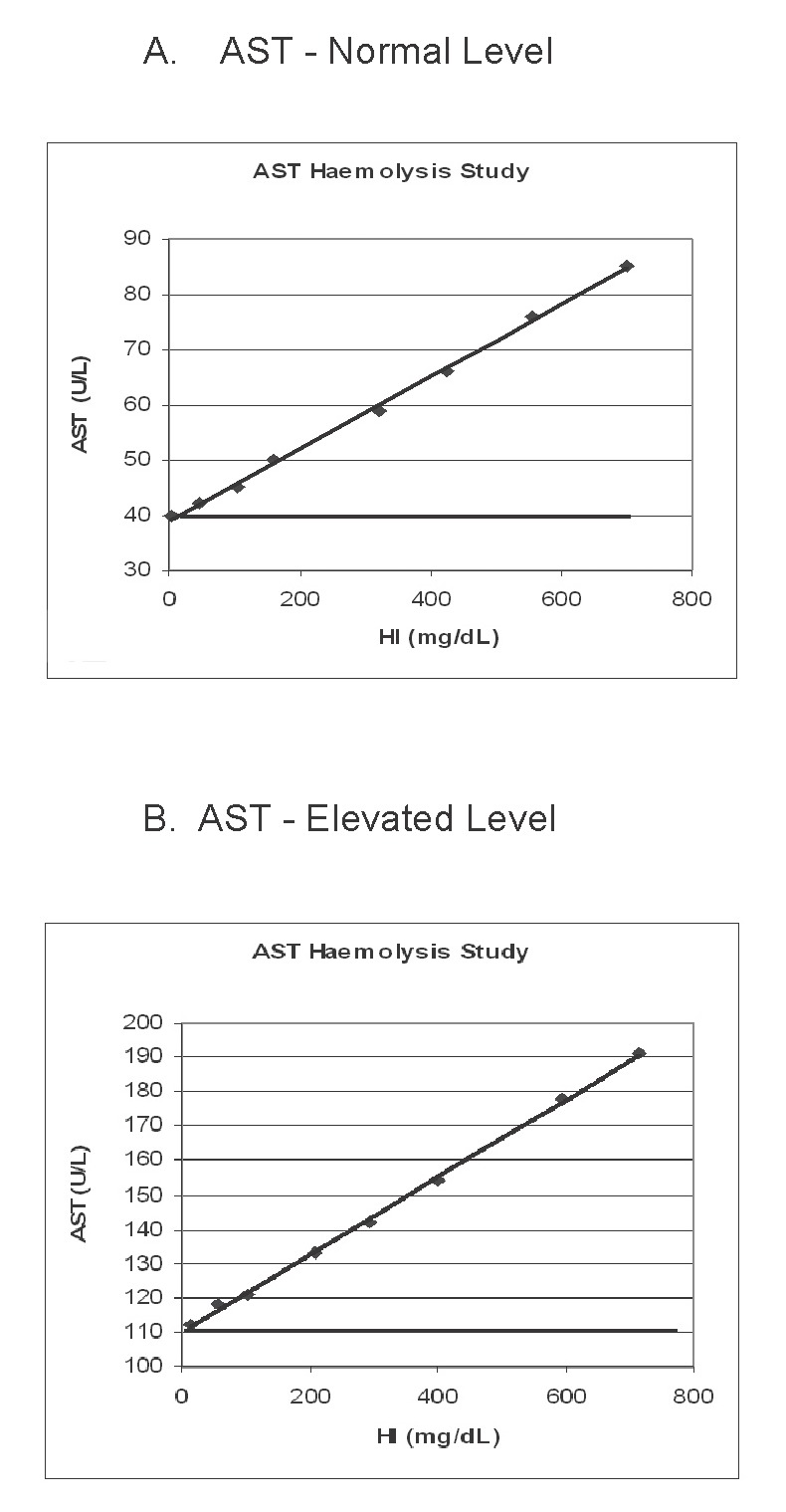
Effect of haemolysis on AST activity: A, AST - normal baseline activity; B, AST - elevated baseline activity.
Footnotes
Competing Interests: None declared.
References
- 1.Dimeski G, Clague AE. Bicarbonate interference with chloride ion selective electrodes. Clin Chem. 2004;50:1106–7. doi: 10.1373/clinchem.2004.033589. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. Interference Testing in Clinical Chemistry; approved guideline. Wayne, PA, USA: CLSI; 2005. CLSI document EP7-P. [Google Scholar]
- 3.Young’s Effects Online. [(Accessed 21 May 2008)]. https://www.fxol.org/aaccweb/
- 4.Young DS. Effects of drugs on clinical laboratory tests. 5. Vol. 2. Washington DC, USA: AACC Press; 2000. [Google Scholar]
- 5.Meites S. Reproducibly simulating hemolysis, for evaluating its interference with chemical methods. Clin Chem. 1973;19:1319. [PubMed] [Google Scholar]
- 6.Dimeski G, Clague AE, Hickman PE. Correction and reporting of potassium results in haemolysed samples. Ann Clin Biochem. 2005;42:119–23. doi: 10.1258/0004563053492739. [DOI] [PubMed] [Google Scholar]
- 7.Dimeski G. Effects of hemolysis on the Roche ammonia method for Hitachi analyzers. Clin Chem. 2004;50:976–7. doi: 10.1373/clinchem.2003.028993. [DOI] [PubMed] [Google Scholar]
- 8.Owen LJ, Keevil BG. Does bilirubin cause interference in Roche creatinine methods? Clin Chem. 2007;53:370–1. doi: 10.1373/clinchem.2006.075846. [DOI] [PubMed] [Google Scholar]
- 9.Dimeski G, McWhinney B, Jones B, Mason R, Carter A. Extent of bilirubin interference with Beckman creatinine methods. Ann Clin Biochem. 2008;45:91–2. doi: 10.1258/acb.2007.007079. [DOI] [PubMed] [Google Scholar]
- 10.Dimeski G, Mollee P, Carter A. Effects of hyperlipidemia on plasma sodium, potassium and chloride measurements by an indirect ion-selective electrode measuring system. Clin Chem. 2006;52:155–6. doi: 10.1373/clinchem.2005.054981. [DOI] [PubMed] [Google Scholar]
- 11.Pantanowitz L, Horowitz GL, Upalakalin JN, Beckwith BA. Artifactual hyperbilirubinemia due to paraprotein interference. Arch Pathol Lab Med. 2003;127:55–9. doi: 10.5858/2003-127-55-AHDTP. [DOI] [PubMed] [Google Scholar]
- 12.Dimeski G, Carter A. Rare IgM interference with Roche/Hitachi Modular glucose and gamma-glutamyltransferase methods in heparin samples. Clin Chem. 2005;51:2202–4. doi: 10.1373/clinchem.2005.053561. [DOI] [PubMed] [Google Scholar]
- 13.Dimeski G, Barnett RJ. Effects of total plasma protein concentration on plasma sodium, potassium and chloride measurements by an indirect ion selective electrode measuring system. Crit Care Resusc. 2005;7:12–5. [PubMed] [Google Scholar]
- 14.Dimeski G, Carter A. Magnesium contamination from Terumo blood collection tubes. Clin Chem. 2006;52:1612–4. doi: 10.1373/clinchem.2005.064683. [DOI] [PubMed] [Google Scholar]
- 15.Bowen RA, Chan Y, Ruddel ME, Hortin GL, Csako G, Demosky SJ, et al. Immunoassay interference by a commonly used blood collection tube additive, the organosilicone surfactant silwet L-720. Clin Chem. 2005;51:1874–82. doi: 10.1373/clinchem.2005.055400. [DOI] [PubMed] [Google Scholar]
- 16.Kricka LJ, Park JY, Senior MB, Fontanilla R. Processing controls in blood collection tubes reveals interference. Clin Chem. 2005;51:2422–3. doi: 10.1373/clinchem.2005.060392. [DOI] [PubMed] [Google Scholar]
- 17.Dimeski G, Cheung K, Ormiston B. Interference from bicarbonate reagent in magnesium measurements on the BM/Hitachi 747. Clin Chem. 1994;40:851–2. [PubMed] [Google Scholar]
- 18.Redondo FL, Bermudez P, Cocco C, Colella F, Graziani MS, Fiehn W, et al. Evaluation of Cobas Integra 800 under simulated routine conditions in six laboratories. Clin Chem Lab Med. 2003;41:365–81. doi: 10.1515/CCLM.2003.058. [DOI] [PubMed] [Google Scholar]
- 19.Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25:105–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Hermsen D, Apple F, Garcia-Beltran L, Jaffe A, Karon B, Lewandrowski E, et al. Results from a multicenter evaluation of the 4th generation Elecsys Troponin T assay. Clin Lab. 2007;53:1–9. [PubMed] [Google Scholar]
- 21.Ryder KW, Glick MR. Erroneous laboratory results from hemolyzed, icteric, and lipemic specimens. Clin Chem. 1993;39:175–6. [PubMed] [Google Scholar]
- 22.Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem. 1986;32:470–5. [PubMed] [Google Scholar]
- 23.Vermeer HJ, Thomassen E, de Jonge N. Automated processing of serum indices used for interference detection by the laboratory information system. Clin Chem. 2005;51:244–7. doi: 10.1373/clinchem.2004.036301. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–6. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- 25.Westgard QC. Desirable specifications for total error, imprecision, and bias, derived from biological variation. [(Accessed 21 May 2008)]. http://www.westgard.com/biodatabase1.htm.
- 26.Preissner CM, Dodge LA, O’Kane DJ, Singh RJ, Grebe SK. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin Chem. 2005;51:208–10. doi: 10.1373/clinchem.2004.040501. [DOI] [PubMed] [Google Scholar]


