Summary
The neurotransmitter GABA exerts a strong negative influence on the production of adult-born olfactory bulb interneurons via tightly regulated, non-synaptic GABAergic signaling. After discussing some findings on GABAergic signaling in the neurogenic subventricular zone (SVZ), we provide data suggesting ambient GABA clearance via two GABA transporter subtypes and further support for a non-vesicular mechanism of GABA release from neuroblasts. While GABA works in cooperation with the neurotransmitter glutamate during embryonic cortical development, the role of glutamate in adult forebrain neurogenesis remains obscure. Only one of the eight metabotropic glutamate receptors (mGluRs), mGluR5, has been reported to tonically increase the number of proliferative SVZ cells in vivo, suggesting a local source of glutamate in the SVZ. We show here that glutamate antibodies strongly label subventricular zone (SVZ) astrocytes, some of which are stem cells. We also show that some SVZ neuroblasts express one of the ionotropic glutamate receptors, AMPA/kainate receptors, earlier than previously thought. Collectively, these findings suggest that neuroblast-to-astrocyte GABAergic signaling may cooperate with astrocyte-to-neuroblast glutamatergic signaling to provide strong homeostatic control on the production of adult-born olfactory bulb interneurons.
Introduction
The production of adult born neurons persists in two brain regions, the subventricular zone (SVZ, also called subependymal layer (Boulder Committee, 1970)) and the dentate gyrus subgranular zone (SGZ) in the hippocampus. The SVZ contains the largest pool of dividing neural stem cells (NSCs) in the adult mammalian brain, including in humans (Sanai et al., 2004; Curtis et al., 2007). Division of NSCs generate transit amplifying cells, which in turn divide to give rise to neuroblasts (Doetsch et al., 1999a). These latter cells migrate along the rostral migratory stream (RMS) to the olfactory bulb where they differentiate into interneurons (Bryans, 1959; Altman, 1969; Luskin, 1993; Swarzenski et al., 1996). Adult-born neuron production is an ongoing process. Estimates suggest that 10,000 to 30,000 neurons migrate to the olfactory bulb every day (Lledo et al., 2006). This high turnover rate necessitates profound homeostatic control mechanisms at the level of NSCs, and their proliferative and migrating neuroblasts. Homeostatic mechanisms provide a balance between proliferation, migration and survival of stem cells and neuroblasts in the neurogenic microenvironment (also called niche) to achieve adequate adult born neuron production. Improving our understanding of these control mechanisms will move us one step closer to promoting self-repair in the adult-injured brain.
The adult neurogenic niche contains many factors that control neural stem cell (NSCs) and neuroblast proliferation and migration (Hagg, 2005). One of these factors, the neurotransmitter GABA, plays critical roles on the different steps of adult neurogenesis. GABA decreases the proliferation of NSCs and neuroblasts (Nguyen et al., 2003; Liu et al., 2005) while also reducing neuroblast migration (Bolteus and Bordey, 2004). GABA thus exerts a negative influence on neuroblast production. During embryonic neurogenesis another neurotransmitter glutamate works in cooperation with GABA to control cortical development (for review see Owens and Kriegstein, 2002; Schlett, 2006). However, the role of glutamate in adult forebrain neurogenesis remains obscure. It is also unknown whether glutamate could cooperate with GABA to provide a homeostatic control on adult-born neuron production.
Here we review the recent findings that GABAergic signaling in the SVZ and RMS plays an important role during adult neurogenesis. We also highlight questions that remain to be addressed. We then discuss published data suggesting the existence of glutamatergic signaling in the SVZ and present new data on the presence of AMPA/kainate-type glutamate receptors in neuroblasts. Finally, we discuss possible interactions between GABA and glutamate signaling that provides a homeostatic control of adult neurogenesis.
Materials and Methods
Immunohistochemistry
50-100 μm-thick slices were obtained from CD1 or C57Bl6 mice that were 24-30 days old. Free-floating sections were blocked (PBS + 0.3% Triton X100 + 2% BSA +/− 0.3% Tween-20) and incubated in the following primary antibodies for 2 hrs at RT or overnight at 4°C: goat anti-doublecortin (1:100, Santa Cruz, USA), guinea pig anti-glutamate-aspartate transporter (GLAST, 1:500, Chemicon, USA), mouse anti-glutamate (1:2000, Sigma, USA) (Platel et al., 2005), rabbit anti-GABA (1:500, Sigma, USA), rabbit anti-GAT1 (1:100, Chemicon, USA), and rabbit anti-VGAT (1:1000, Synaptic Systems, Germany). After several washes, slices were incubated with the appropriate secondary antibody (Alexa Fluor series at 1:1000, Invitrogen, USA; or Cyanine series at 1:500, Jackson Labs) for 1 hr at room temperature. Images were acquired on an Olympus FluoView 300 confocal microscope.
Calcium imaging
Acute slices were loaded (45 min at 37°C) with the Ca2+-sensitive dyes fluo-4 AM or Oregon Green BAPTA 1-AM (4-5 μM in DMSO with 20% Pluronic acid F-127), then washed for at least 30 min before imaging. Acute brain slices containing the SVZ or RMS were prepared as previously described (Wang et al., 2003). Ca2+ imaging experiments were performed on an Olympus Fluoview 300 confocal microscope equipped with a 60x objective. The frequency of cytosolic Ca2+ increases was calculated using Calsignal written by JCP (Platel et al., 2006). Images were acquired every 1.16 to 6 s. F0 (i.e. baseline) is the mean fluorescence intensity measured throughout all of the regions of interest, and F is the mean fluorescence intensity in a single cell. A change in fluorescence was considered to be a Ca2+ increase if it was superior to 15% F/F0 increase. Kainate was applied by pressure (Picospritzer II, General Valves) while DNQX was bath applied. Data are expressed as mean ± standard error. Statistical analysis used a two-tailed t-test except where noted.
Cell organization and identity in the neurogenic forebrain
The SVZ-ependymal region contains at least four different cell types defined by their morphology, ultrastructure, and molecular markers (Smart, 1961; Altman, 1963; 1969; Blakemore, 1969; Privat and Leblond, 1972; Kishi, 1987; Sucher and Deitcher, 1995; Lois et al., 1996; Jankovski and Sotelo, 1996; Doetsch et al., 1997; Peretto et al., 1997; Mercier et al., 2002). Neuroblasts (also called type A cells (Lois et al., 1996)) migrate in chains to the olfactory bulb along the rostral migratory stream (RMS). Cells that express glial fibrillary acidic protein (GFAP, also called type B cells (Lois et al., 1996) or SVZ astrocytes (Doetsch et al., 1999a)) ensheath the chains of migrating neuroblasts and display several features of mature astrocytes (Liu et al., 2006b). Scattered, highly proliferative progenitors (transit amplifying cells or type C cells) form clusters next to the neuroblasts. The SVZ is separated from the ventricular cavity by a layer of ependymal cells. Immunohistochemical studies have revealed that a cluster of 3-5 neuroblasts is tightly encapsulated by one or two astrocytes in a coronal plane (Lois et al., 1996; Peretto et al., 1997; Bolteus and Bordey, 2004) (Fig. 1A-C). Based on electron microscopy data, the extracellular space between neuroblasts or between neuroblasts and astrocytes is ∼20-50 nm (Doetsch et al., 1997; Peretto et al., 1999). Although this distance is on the same order as that of the synaptic cleft, synapses are absent between SVZ cells (Doetsch et al., 1997; Liu et al., 2005). This arrangement and cell:cell proximity lend itself to a nonsynaptic signaling between neuroblast:neuroblast and neuroblast:astrocyte.
Figure 1. Organizations of the adult subventricular zone.
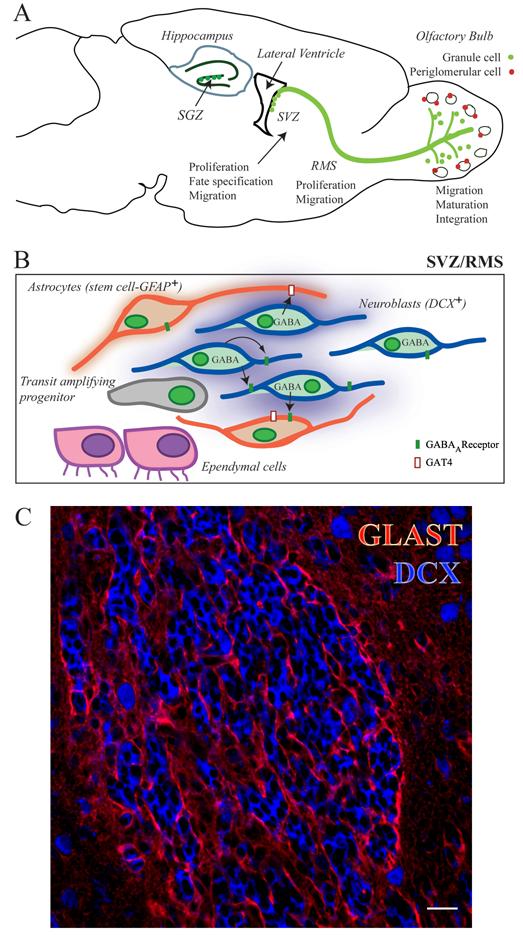
(A) Diagram of a sagittal rodent brain section illustrating the two neurogenic regions, the SVZ/olfactory system and the hippocampal subgranular zone (SGZ). In the SVZ, neuroblasts are continually generated and migrate as chains (not illustrated) throughout the SVZ, join the RMS and differentiate into interneurons in the olfactory bulb (OB), including granule cells (green) and periglomerular cells (red). The different stages of neurogenesis are marked along the migratory path of the neuroblasts. The SGZ is located at the base of the granule cell layer in the hilus of the dentate gyrus (DG). (B) Diagram illustrating the cellular organization in the SVZ/RMS. SVZ/RMS neuroblasts (blue) that express doublecortin (DCX) and migrate to the olfactory bulb are ensheathed by astrocytes (red). Ependymal cells (purple) line the wall of the lateral ventricle in the SVZ. Scattered transit amplifying progenitors (grey) are present along the SVZ and RMS. Astrocytes express GFAP and constitute a pool of neural stem cells. GABA released from neuroblasts diffuses and activates GABAA receptors (green rectangles) in neuroblasts as well as astrocytes. GABA is also taken up by GABA transporters (mouse GAT4) in astrocytes. (C) Confocal microscopy photograph illustrating the cellular organization in the RMS: astrocytes labeled by GLAST antibodies (red) ensheath clusters of neuroblasts labeled by DCX antibodies (blue) in a coronal section. Scale bar: 20 μm.
SVZ astrocytes have stem cell attributes, including self-renewal and multipotency (Chiasson et al., 1999; Doetsch et al., 1999a; Laywell et al., 2000; Capela and Temple, 2002; Doetsch et al., 2002; Garcia et al., 2004). It remains unclear whether every SVZ astrocyte can behave as a stem cell. Nevertheless, here we will refer to SVZ astrocytes as NSCs acknowledging that only a subpopulation of SVZ or RMS astrocytes may have stem cell features.
GABA signaling molecules in the SVZ and RMS
The SVZ represents a local GABAergic network where GABA, synthesized and released by neuroblasts, provides paracrine (i.e. nonsynaptic) signaling both between neuroblasts (Stewart et al., 2002; Wang et al., 2003; Nguyen et al., 2003; Bolteus and Bordey, 2004) and from neuroblasts to NSCs (Liu et al., 2005)(see diagram in Fig. 1B and GABA immunostaining in Fig. 2A). Briefly, neuroblasts release GABA via an unknown Ca2+-dependent mechanism (Liu et al., 2005). Liu et al. showed that GABA released from neuroblasts was independent of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, which is the minimum machinery required for exocytosis. GABAA receptors are expressed on both NSCs (Liu et al., 2005) and neuroblasts (Wang et al., 2003). GABA released either spontaneously or following electrical activation of SVZ cells activates GABA receptors in surrounding neuroblasts and NSCs (Bolteus and Bordey, 2004; Liu et al., 2005). Once released GABA is taken up into astrocytes via high affinity GABA transporters, GAT4 (Bolteus and Bordey, 2004). Thus, GABA signaling molecules are expressed in the SVZ and RMS, suggesting the presence of a tightly regulated GABAergic signaling in the SVZ and RMS. However, key pieces of the puzzle are missing, including GABA release mechanisms, the presence of signaling molecules in transit amplifying cells, and the presence of GABAB receptors in SVZ cells.
Figure 2. Expression of GABA signaling molecules in the SVZ.
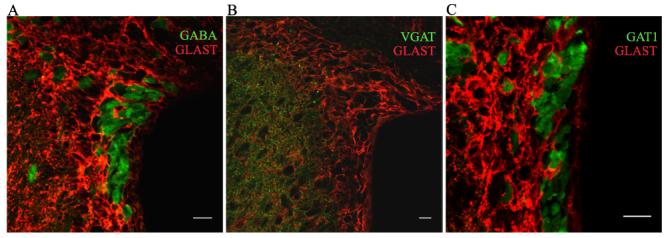
(A) GABA immunostaining (green) in the SVZ shows the presence of GABA in some SVZ cells, but not in GLAST-positive cells (red, i.e. astrocytes). (B) Immunolabeling for vesicular GABA transporters, VGATs (green), is sparse or nonexistent in SVZ cells. Prominent punctate labeling, by contrast, is observed in the striatum in the left of the panel. (C) Positive labeling for the GABA transporter GAT1 (green) in the SVZ is observed mainly in cells that do not stain positive for GLAST (red). Scale bars: 10 μm.
Our new data suggest that vesicular GABA transporters, VGATs, are absent from RMS and SVZ cells (Fig. 2B). This finding is consistent with the lack of the SNARE complex and synapsin 1 in neuroblasts (Liu et al., 2005). Here we show using immunostaining that another high affinity GABA transporter, GAT1, is also expressed in the SVZ and RMS in GLAST-negative cells, which are likely neuroblasts (Fig. 2C). It is, however, unknown whether these transporters are functional and work in reverse, thus contributing to GABA release as previously reported for other cell types (Cammack and Schwartz, 1993; Cammack et al., 1994). One way to address this question is to acutely isolate neuroblasts and use a “sniffer” patch, which consists of an outside-out patch from a GABAA receptor-expressing cell, to examine whether spontaneous GABA release can be detected from neuroblasts. Liu et al (2005) used a variation of this method. First, they lifted a neuroblast from an acute slice after obtaining a cell-attached recording. They then moved a “sniffer” patch obtained from either a nearby astrocyte or a striatal neuron to detect GABA release from the neuroblast. If spontaneous release was not detected, they electrically depolarized the cell-attached neuroblast to induce GABA release. Although this method is efficient at monitoring GABA release, it is technically more challenging than acutely dissociating neuroblasts. The presence of GABAB receptors in SVZ cells also remains unclear and need to be examined using immunostaining and functional assays such as perforated patch clamp recordings or calcium imaging.
Is GABA release from neuroblasts dynamic and regulated by other microenvionmental signals? GABA release is Ca2+-dependent and may thus be regulated by factors increasing intracellular Ca2+ in migrating neuroblasts. Migrating neuroblasts display high frequency of spontaneous Ca2+ transients (Fig. 3). In acute slices loaded with the calcium indicator Oregon Green BAPTA 1-AM, 61% of the cells in the RMS displayed spontaneous Ca2+ transients at a mean frequency of 15.5 peaks per hour. It is, however, unknown whether these Ca2+ transients are intrinsic, regulated or induced by microenvironmental factors. The neurotransmitter glutamate is a good candidate for regulating Ca2+ activity (see below). It is also possible that the cerebrospinal fluid gradient of Slit (Sawamoto et al., 2006) contributes to regulation of Ca2+ dynamics in migrating SVZ neuroblasts, as shown in migrating cerebellar granule cell precursor (Xu et al., 2004). Intracellular Ca2+ activity determines the state of excitability of migrating neuroblasts that are not able to generate action potentials while migrating in the SVZ and RMS. The properties of the Ca2+ activity in migrating neuroblasts have not yet been reported in the neurogenic forebrain, however, we are presently exploring this issue.
Figure 3. Neuroblasts display spontaneous Ca2+ transients.
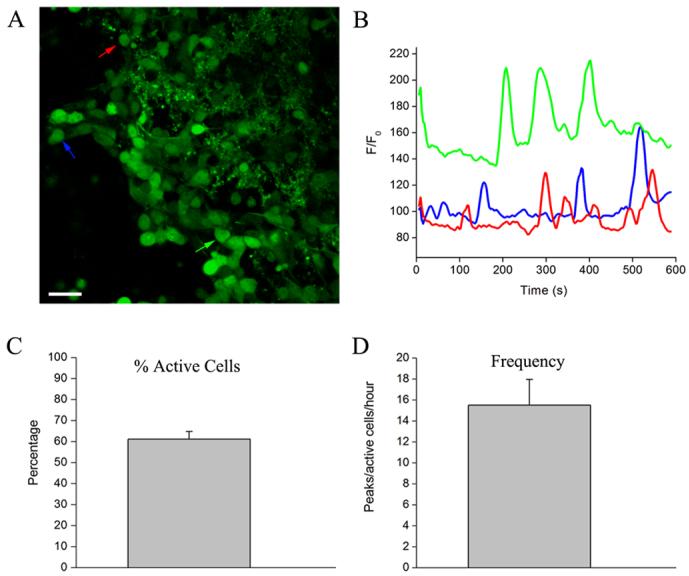
(A) Photograph of a confocal section displaying Oregon Green loading in an acute coronal slice containing the RMS. Cells denoted by arrows (red, blue, and green) exhibit spontaneous Ca2+ activity as shown in (B). (B) Ca2+ activity graphs of cells shown in (A). (C-D) Bar graphs of the percentage of cells displaying spontaneous Ca2+ transients (C) and of the frequency of these transients (D) in 3 slices. 31 to 65 cells were analyzed per slice (mean of 51.3, n=3).
GABA: a negative regulator of neuroblast production in the SVZ
Functionally, GABAA receptor activation in neuroblasts results in a reduction of their speed of migration in the proximal RMS (Bolteus and Bordey, 2004) and also the number of proliferative SVZ neuroblasts (Nguyen et al., 2003). It was at first surprising to find that ambient GABA decreased the speed of neuroblast migration in acute sagittal slices. However, two recent studies provide some additional support for this finding. Heck et al (2007) found that application of the GABAA receptor antagonist, bicuculline, induced an increase in the migration speed of cortical neurons in organotypic neocortical slices from embryonic day 18–19. This increase in speed resulted in aberrant accumulation of neurons in the upper cortical layers. In the second study, Gascon et al (2006) showed that GABA regulates dendritic growth in cultured neuroblasts from the SVZ or in acute slices. In particular, bicuculline promoted the rapid stabilization of new dendritic segments. The inhibitory effect of GABA on the speed of neuroblast migration may thus be due to a stabilization of their processes resulting in a loss of motility. GABA does not only alter the behavior of neuroblasts, but it was also shown to reduce the number of proliferative NSCs via tonic GABAA receptor activation (Liu et al., 2005). GABA's effect on NSC proliferation is reminiscent of the studies showing that tonic GABAA receptor activation limits the proliferation of cortical radial glia (LoTurco et al., 1995; Haydar et al., 2000). In addition, the functions of GABA on adult neurogenesis integrate well with the tight correlation between the behavior of NSCs and neuroblasts; local GABA signaling provides information to NSCs about the size of the neuroblast pool. As more neuroblasts are generated, more GABA is expected to be released into the extracellular space, resulting in increased GABAA receptor activation in NSCs. Since astrocytes generate neuroblasts (Doetsch et al., 1999a), an increase in the number of neuroblasts seems to serve as a negative feedback control of astrocyte proliferation and therefore neuroblast production. This negative feedback reconciles well with the constant migration of neuroblasts to the olfactory bulb, which would limit ambient GABA accumulation in the SVZ, and with increased proliferation of astrocytes following elimination of neuroblasts (Doetsch et al., 1999b).
Local glutamatergic signaling in the neurogenic forebrain
One candidate molecule to counteract the inhibitory effect of GABA on progenitor proliferation and neuroblast migration is the excitatory neurotransmitter glutamate. Based on published data and new data presented here we propose that glutamatergic and GABAergic signaling maintains homeostasis of the production of adult born neurons. We will address this issue by discussing the two following questions. First, does glutamatergic signaling exist in the SVZ and RMS? Second, what are the functions of the different glutamate receptors on adult neurogenesis?
The existence of glutamatergic signaling requires identification of source cells that release glutamate, target cells that express glutamate receptors, and removal mechanisms to limit extracellular glutamate accumulation and receptor desensitization.
Sources of glutamate
Among the possible sources of glutamate, we favor NSCs. NSCs display astrocytic properties (Liu et al., 2006b) and mature astrocytes release glutamate via several mechanisms (Evanko et al., 2004). Nevertheless, it is possible that ependymal cells, which in many ways resemble astrocytes (Liu et al., 2006b), release glutamate as well. It is unlikely that neuroblasts, which are GABAergic (Bolteus and Bordey, 2004; Liu et al., 2005), release glutamate although immature cells may transiently release both glutamate and GABA (Gutierrez, 2005). We thus performed immunostaining for glutamate as previously described (Platel et al., 2005). Figure 4A shows that glutamate antibodies strongly labeled SVZ astrocytes while weakly labeled neuroblasts. Astrocytes and neuroblasts were identified by GLAST and doublecortin (DCX) immunostaining, respectively (Braun et al., 2003a; Liu et al., 2006a). SVZ astrocytes may thus be a source of glutamate.
Figure 4. Astrocytes contain glutamate while neuroblasts express AMPA-type glutamate receptors.
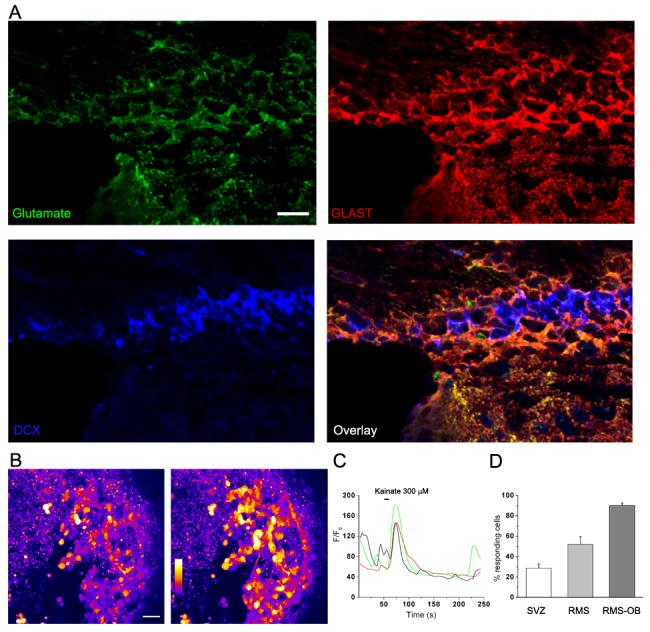
(A) Photograph of a confocal section displaying co-immunostaining for glutamate (green), GLAST (red), and DCX (blue) in the SVZ. Scale bar: 10 μm. (B) Photographs of an acute slice containing the RMS-OB and loaded with fluo 4-AM. Image before (left panel) and after (right panel) application of kainate (300 μM, 10 s). In this slice, 85% of the cell responded to kainate suggesting the functional expression of AMPA receptors in RMS cells. The slice was made from a postnatal day 25 mouse. (C) Ca2 activity traces of three representative cells showing some spontaneous activity and Ca2+ increases in response to kainate application. (D) Bar graphs of the percentage (%) of cells responding to kainate application as a function of their location along the rostro-caudal axis, i.e. SVZ, RMS, and RMS of the OB (RMS-OB). An increasing percentage of neuroblasts acquire functional AMPA receptors during migration to the OB.
Glutamate receptor expression
Very little is known about glutamate receptor expression in the SVZ or RMS. Glutamatergic signals are conveyed by different glutamate (Glu) receptor subtypes, namely ionotropic NMDA and non-NMDA or AMPA/kainate subtypes (Sommer and Seeburg, 1992; Hollmann and Heinemann, 1994) and metabotropic (mGlu; group-I–III subtypes (Pin and Duvoisin, 1995; Conn and Pin, 1997; Cartmell and Schoepp, 2000)). Regarding NSCs, our recent study suggests that they do not express functional AMPA or NMDA receptors as shown using patch clamp recordings of SVZ astrocytes (Liu et al., 2006b). It remains unclear whether NSCs express mGluRs. Regarding neuroblasts, it is thought that they do not express AMPA and NMDA receptors until they enter the granule cell layer of the olfactory bulb (Carleton et al., 2003). However, our recent data suggest that some neuroblasts begin expressing functional AMPA/kainate receptors in the SVZ (Fig. 4B-D). To optimize our detection of AMPA receptors, kainate was applied at 300 μM. At this concentration, kainate produces incompletely desensitizing activation of AMPA receptors (Huettner, 1990; Lerma et al., 1993) but would lead to desensitization of kainate receptors. Kainate was pressure applied for 10 sec onto SVZ and RMS cells in acute brain slices and intracellular Ca2+ concentration was monitored with fluo 4 fluorescence. Kainate significantly increased intracellular Ca2+ in 29 ± 4% of the SVZ cells (Fig. 4C, n=6 slices). About 90% of the kainate-induced Ca2+ increases in the SVZ was blocked by the competitive AMPA/kainate receptor antagonist DNQX (60 μM). The large percentage of cells responding to kainate and their morphology suggest that some of the responding cells are neuroblasts. We observed a progressive increase in the number of cells responding to kainate application along the rostral-caudal axis. In the RMS, 52 ± 10% of the cells (n=4) responded to kainate while nearly all of the cells responded to kainate (90 ± 2%, n=3) in the RMS of the olfactory bulb. A study also reported that 40 to 60% of progenitors examined 16 h after plating neurospheres from neonatal rat SVZ expressed functional AMPA, kainate and NMDA receptors (Brazel et al., 2005). It is, however, difficult to extrapolate data obtained from plated neurospheres because the culture medium contains growth factors, which may affect the differentiation of neuroblasts. Using immunostaining, it was reported that mGluR5 (group I subtype coupled to phospholipase C and IP3-regulated intracellular stores) are expressed in the postnatal SVZ (Di Giorgi Gerevini et al., 2004). However, it is not known which cell types express these receptors. In addition, the mGluR3 agonist, N-acetylaspartylglutamate, induced Ca2+ increases in 45% of cultured cells from SVZ neurospheres (Brazel et al., 2005). Finally, glutamate receptor expression in transit amplifying cells has not been explored.
Glutamate uptake
Once released, glutamate is not degraded in the extracellular space but needs to be taken up into cells. Extracellular glutamate levels are regulated by high affinity transporters named EAAT1 (GLAST), EAAT2 (GLT-1), EAAT3-5 in human (rodent homologs, Palacin et al., 1998). NSCs in the SVZ express GLAST and GLT-1 (Braun et al., 2003b; Bolteus and Bordey, 2004). It remains unknown whether neuroblasts and transit amplifying cells express some of the other glutamate transporters.
Glutamate: a positive regulator of adult neurogenesis
Only one study thus far has examined the effect of glutamate receptor inhibition on the number of dividing cells in the SVZ in vivo. Di Giorgi-Gerevini et al. (2005) reported that adult mice lacking mGluR5 or treated with mGluR5 antagonists showed a dramatic reduction in the number of proliferating cells in the SVZ (Giorgi-Gerevini et al., 2005). This in vivo study shows that mGluR5 are tonically activated by ambient glutamate suggesting a local source of glutamate in the SVZ consistent with our strong immunoreactivity in SVZ astrocytes (see Fig. 4). The authors also used neurospheres from the forebrain of embryonic day 20 mice making comparison with in vivo data difficult. Using cultured SVZ cells plated from perinatal SVZ neurospheres, Brazel et al. (2005) showed that group II mGluR stimulation for 24 hours reduced basal level of apoptosis and increased the proliferation of presumably transit amplifying cells, but did not increase the NSC pool. They also showed that kainate and glutamate increased cell proliferation and reduced the level of apoptosis, but only when NMDA receptors were blocked. From these findings, glutamate may exert a positive influence on adult forebrain neurogenesis by increasing the number of SVZ cells via mGluR activation. The function of AMPA receptors in neuroblasts remains unclear. AMPA receptors reduce DNA synthesis of presumably radial glia in acute embryonic neocortical slices (LoTurco et al., 1995). In other systems, AMPA receptor activation was reported to exert a positive proliferative effect (for review see Schlett, 2006). Collectively, it can be speculated that glutamate acting at both ionotropic and metabotropic receptors positively affects the production of adult-born neurons.
Concluding remarks
Data discussed and presented here support the emerging idea that a homeostatic balance between GABA and glutamate signaling may be present even before synaptic transmission is established (Fig. 5). It may underlie fundamental processes of adult neurogenesis, such as proliferation and survival of progenitor cells. Interactions between GABA and glutamate signaling likely occur at different levels leading to a homeostatic control of adult neurogenesis. For example, glutamate released form SVZ astrocytes may lead to Ca2+-dependent GABA release from neuroblasts via AMPA receptor activation as previously reported in the embryonic intermediate zone (Poluch and Konig, 2002). GABA is then expected to act as a negative feedback regulator of SVZ astrocyte proliferation. The diversity of glutamate receptors, as opposed to GABA receptors, allow for a fine control of the different steps of adult neurogenesis. Controls exerted on multiple stages of neuron production may limit an acute alteration of cell production (by e.g. gene mutations or damages) to significantly impair neurogenesis. Alternatively, a chronic disruption of the homeostatic balance may profoundly affect neurogenesis and lead to NSC exhaustion or aberrant proliferation. Clinically, novel drugs affecting GABA and glutamate signaling molecules may prove to be relevant for therapeutic applications aimed at promoting cell replacement by endogenous progenitors during the course of neurodegenerative diseases.
Figure 5. Hypothetical model illustrating a homeostatic control of neuroblast production by GABA and glutamate.
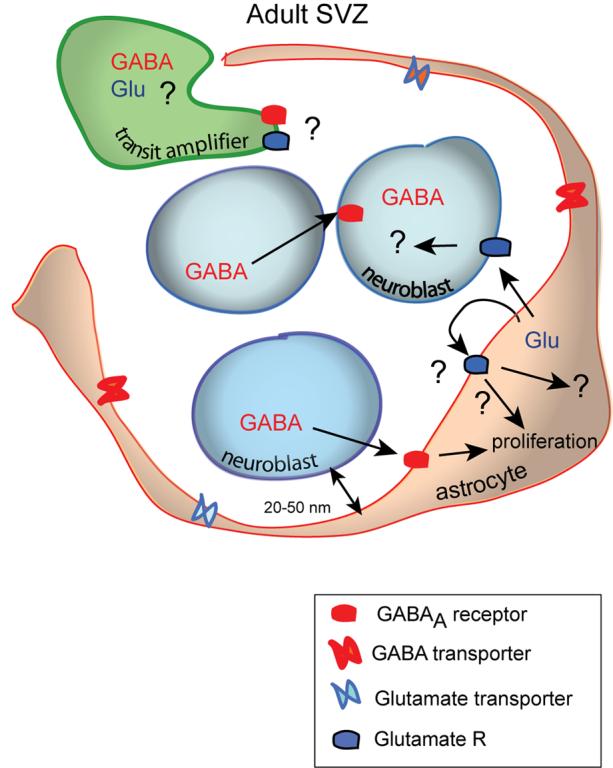
Neuroblasts express GABAA receptors and AMPA receptors in the SVZ and RMS. Neuroblasts release GABA that activates GABAA receptors on themselves and surrounding astrocytes. In turn, astrocytes surrounding neuroblasts release glutamate which may activate AMPA receptors on migrating neuroblasts. mGluRs are expressed in the SVZ but the identity of the cell bearing these receptors is unknown. GABAA receptor activation by ambient GABA leads to a decrease in the number of proliferative astrocytes and neuroblasts while mGluR activation by ambient glutamate induces an increase in the number of proliferative cells in the SVZ. The function of AMPA receptors in neuroblasts remains unknown.
Acknowledgment
This work was supported by a grant from the National Institute of Health R01 NS048256 and DC007681 (A.B.).
REFERENCES
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. The ultrastructure of the subependymal plate in the rat. J Anat. 1969;104:423–433. [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulder Committee Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra SK, et al. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003a;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra SK, et al. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003b;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Nunez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131:55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Bryans WA. Mitotic activity in the brain of the adult rat. Anatomical Record. 1959;133:65–73. [Google Scholar]
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J Physiol (Lond) 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. 81-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der KD. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Di Giorgi Gerevini V, Caruso A, Cappuccio I, et al. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Evanko DS, Zhang Q, Zorec R, Haydon PG. Defining pathways of loss and secretion of chemical messengers from astrocytes. Glia. 2004;47:233–240. doi: 10.1002/glia.20050. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gascon E, Dayer AG, Sauvain MO, et al. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi-Gerevini V, Melchiorri D, Battaglia G, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck N, Kilb W, Reiprich P, et al. GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb Cortex. 2007;17:138–148. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006b;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu X, Bolteus AJ, Balkin DM, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006a;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, et al. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Malgrange B, Breuskin I, et al. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull. 1997;42:9–21. doi: 10.1016/s0361-9230(96)00116-5. [DOI] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res Bull. 1999;49:221–243. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Platel JC, Boisseau S, Dupuis A, et al. Na+ channel-mediated Ca2+ entry leads to glutamate secretion in mouse neocortical preplate. Proc Natl Acad Sci U S A. 2005;102:19174–19179. doi: 10.1073/pnas.0504540102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Dupuis A, Boisseau S, et al. Synchrony of spontaneous calcium activity in mouse neocortex before synaptogenesis. Eur J Neurosci. 2006 doi: 10.1111/j.1460-9568.2007.05367.x. In press. [DOI] [PubMed] [Google Scholar]
- Poluch S, Konig N. AMPA receptor activation induces GABA release from neurons migrating tangentially in the intermediate zone of embryonic rat neocortex. Eur J Neurosci. 2002;16:350–354. doi: 10.1046/j.1460-9568.2002.02068.x. [DOI] [PubMed] [Google Scholar]
- Privat A, Leblond CP. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol. 1972;146:277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- Smart I. The subependymal layer of the mouse brain and its cellular production as shown by radioautography after thymidine-H3 injection. J Comp Neurol. 1961;116:325–349. [Google Scholar]
- Sommer B, Seeburg PH. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992;13:291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Deitcher DL. PCR and patch-clamp analysis of single neurons. Neuron. 1995;14:1095–1100. doi: 10.1016/0896-6273(95)90257-0. [DOI] [PubMed] [Google Scholar]
- Swarzenski BC, O'Malley KL, Todd RD. PTX-sensitive regulation of neurite outgrowth by the dopamine D3 receptor. Neuroreport. 1996;7:573–576. doi: 10.1097/00001756-199601310-00047. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol (Lond) 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HT, Yuan XB, Guan CB, et al. Calcium signaling in chemorepellant Slit2-dependent regulation of neuronal migration. Proc Natl Acad Sci U S A. 2004;101:4296–4301. doi: 10.1073/pnas.0303893101. [DOI] [PMC free article] [PubMed] [Google Scholar]


