Abstract
Tissue-type plasminogen activator (tPA) is found in the intravascular space and in the central nervous system. The low-density lipoprotein receptor–related protein (LRP) is expressed in neurons and in perivascular astrocytes. During cerebral ischemia, tPA induces the shedding of LRP's extracellular domain from perivascular astrocytes, and this is followed by the development of cerebral edema. Protein kinase B (Akt) is a serine/threonine kinase that plays a critical role not only in cell survival but also in the regulation of the permeability of the blood-brain barrier. We found that, in the early phases of the ischemic insult, the interaction between tPA and LRP induces Akt phosphorylation (pAkt) in perivascular astrocytes and inhibits pAkt in neurons. Coimmunoprecipitation studies indicate that pAkt and LRP's intracellular domain interact in perivascular astrocytes and that this interaction is dependent on the presence of tPA and results in the development of edema. Together, these results indicate that, in the early stages of cerebral ischemia, the interaction between tPA and LRP in perivascular astrocytes induces the activation of a cell signaling event mediated by pAkt that leads to increase in the permeability of the blood-brain barrier.
Introduction
Tissue-type plasminogen activator (tPA) is a highly specific serine proteinase and one of the 2 main plasminogen activators.1 In the intravascular space, tPA is primarily a thrombolytic enzyme; and based on this property, tPA is the only FDA-approved medication for the treatment of patients with acute ischemic stroke.2 Unfortunately, treatment with tPA has been associated not only with a complete or nearly complete recovery in 30% of patients with acute ischemic stroke, but also with a 10-fold increase in the incidence of hemorrhagic complications.2 A deleterious role for tPA in the ischemic brain has also been indicated by animal studies demonstrating that, after middle cerebral artery occlusion (MCAO), there is an increase in endogenous tPA activity within the ischemic tissue3–5 and that either genetic deficiency of tPA3,6 or its inhibition with neuroserpin4,7 is associated with neuronal survival, decrease in the volume of the ischemic lesion, and preservation of the barrier function of the blood-brain barrier (BBB).8,9
The neurovascular unit (NVU) is assembled by endothelial cells, the basement membrane neurons, and perivascular astrocytes.10 One of the main functions of the NVU is the regulation of the passage of substances from the intravascular space into the brain. During cerebral ischemia, there is a progressive increase in tPA activity in the NVU that has been associated with the development of cerebral edema.5 The low-density lipoprotein (LDL) receptor–related protein (LRP) is a member of the LDL receptor gene family that interacts with multiple ligands, including tPA.11 In the central nervous system (CNS), LRP is found in neurons and in perivascular astrocytes. In neurons, LRP has been implicated in cellular signal transduction pathways.12 In perivascular astrocytes, the interaction between tPA and LRP has been demonstrated to increase the permeability of the BBB.5,8,13
Protein kinase B (PKB), also known as Akt, is a serine/threonine protein kinase with oncogenic and antiapoptotic properties.14 The expression of Akt in the CNS increases in response to cellular stress or injury, suggesting a role for Akt in cell survival.15 During cerebral ischemia, there is a rapid and transient induction of Akt phosphorylation (serine 473) in neurons16,17 that has been considered to be a neuroprotective response.16,18–20 However, it has been recently suggested that Akt phosphorylation also has a direct effect on the permeability of the BBB.21–23
In previous work, we demonstrated that, during cerebral ischemia, the interaction between tPA and LRP in perivascular astrocytes induces shedding of LRP's ectodomain into the basement membrane in those areas of the NVU with early signs of developing edema.5 Here we show that the interaction between tPA and LRP in perivascular astrocytes induces phosphorylation of Akt- and pAkt-dependent increase in the permeability of the BBB. Together, our results indicate that the interaction between tPA and LRP in the NVU under ischemic conditions induces the activation of a cell signaling event mediated by phosphorylation of Akt that leads to increase in the permeability of the BBB. Moreover, our results indicate that LRP phosphorylates Akt in perivascular astrocytes subjected to hypoxic/ischemic conditions. Finally, our data suggest that, during cerebral ischemia, phosphorylation of Akt has a dual function: a deleterious role in perivascular astrocytes and a neuroprotective effect in neurons.
Methods
Animal model of cerebral ischemia and quantification of Evans blue dye extravasation
Transient occlusion of the middle cerebral artery (tMCAO) was induced in wild-type (WT) C57BL/6J, tPA-deficient (tPA−/−), and plasminogen deficient (Plg−/−) mice24 with a 6-0 silk suture advanced from the common carotid artery into the middle cerebral artery as described elsewhere.25 Plg−/− mice lack plasminogen in both the intravascular and extravascular compartments, including the brain parenchyma. After 60 minutes of ischemia, the suture was withdrawn and animals were reperfused. Cerebral perfusion in the distribution of the middle cerebral artery was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed, North Royalton, OH), and only animals with a more than 70% decrease in cerebral perfusion after occlusion and complete recovery after suture withdrawal were included in this study. The rectal and masseter muscle temperatures were controlled at 37°C with a homoeothermic blanket. Immediately after tMCAO, a subgroup of tPA−/− mice was placed on a stereotactic frame and intracortically injected at bregma: −1 mm, mediolateral 3 mm, and dorsoventral 3 mm, with 2 μL of either phosphate-buffered saline (PBS) or murine tPA (1 μM, estimated final concentration 0.01 μM; Molecular Innovations, Southfield, MI), or a combination of murine tPA and the receptor associated protein (RAP; 9 μM, estimated final concentration 0.09 μM), an antagonist of LRP. RAP was kindly provided by Dr Dudley K. Strickland from the University of Maryland (Baltimore, MD). For the vascular permeability studies, immediately after MCAO, WT mice were injected into the third ventricle with 2 μL of either wortmannin (0.1 mM; Sigma-Aldrich, St Louis, MO) or vehicle (control) at bregma −2 mm, mediolateral 0 mm, and dorsoventral 2 mm,26 followed by the intravenous injection of 0.2 mL/100 g body weight of 2% Evans blue dye. With this dose of wortmannin, the estimated final concentration in the cerebrospinal fluid is 1 μM. The intracortical and intraventricular injections were performed more than 15 minutes with a microsyringe pump controller (World Precision Instruments, Sarasota, FL) attached to a syringe holder (World Precision Instruments). After the end of the infusion, the needle was left in place for 5 minutes to avoid reflow. Six hours after reperfusion and the intravenous injection of Evans blue dye, animals were transcardially perfused and the extravasation of Evans blue dye was quantified from the absorbance at 620 nm each supernatant minus the background calculated from the baseline absorbance between 500 and 740 nm and divided by the wet weight of each hemisphere.5 Animal studies were approved by the Emory Institutional Animal Care and Use Committee.
Definition of areas of interest, immunohistochemistry, and TUNEL staining
Three areas of interest (AOIs) were previously defined in the zone of ischemic penumbra by magnetic resonance imaging parameters as described elsewhere.8,13 In brief, each coronal section was divided into 16 square areas (150 mm2 each) that involved the necrotic core and the area of ischemic penumbra, and comparable areas in the nonischemic hemisphere.8,13 AOI-1 and -3 were localized in the fronto- and temporo-parietal lobes, whereas AOI-2 corresponded to the necrotic zone in the parietal lobe. For the immunohistochemistry studies, 20 frozen brain sections 10 μm each were obtained 1 hour after reperfusion in WT and tPA−/− mice and costained with the nuclear marker 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA) and antibodies against NeuN (1:1000 dilution; Chemicon International, Temecula, CA) and Akt phosphorylated at serine 473 (Cell Signaling Technology, Danvers, MA). TdT-mediated dUTP nick end labeling (TUNEL) staining was performed with the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit S7111 (Millipore, Billerica, MA) following instructions provided by the manufacturer. To determine the number of TUNEL-positive cells, images were digitized in a Zeiss Axioplan 2 microscope (20-fold objective) with a Zeiss AxioCam and imported into AxioVision. Images were then viewed at 150% of the original 20× images with an Image MetaMorph Software. The number of TUNEL-positive cells was expressed as a percentage of the total number of cells in each field. Each experiment was repeated 8 times. Statistical analysis was performed with a 1-way analysis of variance test.
Cell cultures
Astrocytes and neurons were cultured from 1-day-old (astrocytes) and E17 (neurons) WTC57BL/6J mice as described elsewhere.9 For astrocytic cultures, cells were dissociated into a single-cell suspension by trituration through a Pasteur pipette and plated onto either 12-mm glass coverslips or 6-well plates coated with 0.05 mg/mL poly-D-lysine and grown in Dulbecco modified Eagle medium (Invitrogen) supplemented with 25 mM glucose, 10% heat-inactivated horse serum, 10% heat-inactivated fetal bovine serum, 2 μM glutamine, 10 U/mL penicillin, and 10 μg/mL streptomycin. To prepare neuronal cultures, cortical tissue was dissected, transferred into Hanks balanced salt solution containing 5% penicillin/streptomycin and 10 μM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and incubated in trypsin containing 0.02% DNase at 37°C for 15 minutes. Tissue was then triturated and the supernatant resuspended in Neurobasal media containing 2 μM l-glutamine, 2% B27, and 5% fetal bovine serum. Cells were plated onto poly-L-lysine-coated coverslips and after 3 days received serum-free media. Cultures were treated with serum free media for 24 hours, washed with PBS 3 times, and then incubated under oxygen-glucose deprivation (OGD) conditions for 1 hour. To induce OGD conditions, the culture medium was replaced by glucose-free Earle balanced salt solution previously saturated with 95% N2/5% CO2 at 37°C. Cultures were then placed in an anaerobic chamber (Billups-Rothenberg, Del Mar, CA) equipped with inlet and outlet valves, and equilibrated for 15 minutes with a continuous flux of gas (5% CO2/95% N2). With this setting, the concentration of oxygen in the media drops to less than 1%. The chamber was then sealed and placed in an incubator at 37°C. A less than 5% oxygen concentration was confirmed with an oxymeter before starting the experiments. In preliminary studies, we used a colorimetric assay to quantify the release of lactate dehydrogenase in the media of cultured astrocytes and neurons (Oxford Biomedical Research, Oxford, MI). We found that exposure to astrocytes and neurons to OGD conditions for 1 hour results in less than 1% (astrocytes) and 29% (± 3%; neurons) release of lactate dehydrogenase into the media compared with cultures treated with the cell-lysing reagent provided by the manufacturer (data not shown). As control, a similar group of cells was kept under normoxic conditions. To study the effect of the interaction between tPA and LRP on Akt phosphorylation, a subset of astrocytes and neurons cultured from WT mice were incubated with 5 μL either murine tPA (1 μM; final concentration 0.01 μM), or a combination of murine tPA and RAP (9 μM; final concentration 0.09 μM).
Immunogold electron microscopy
Immunogold electron microscopy for phosphorylated Akt was performed as previously described.27 Wild-type and tPA−/− mice underwent MCAO. One hour later, animals were anesthetized with chloral hydrate (400 mg/kg) and perfused transcardially with 150 mL 3% freshly depolymerized paraformaldehyde and 0.15% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2-7.4) at a flow rate of 8 mL/minute. Brains were removed and further fixed in the same fixative for 1 hour at 4°C and sectioned coronally at 50 μm using a Vibratome (Technical Products International, St Louis, MO). The sections were then collected and washed thoroughly with phosphate buffer. To improve reagent penetration, the sections were treated with phosphate buffer containing 0.05% Triton X-100 followed by incubation in a blocking solution containing 5% normal goat serum, 5% bovine serum albumin, and 0.1% coldwater fish skin gelatin followed by incubation with an antibody that detects Akt phosphorylated at serine 473. Thin sections were counterstained with saturated uranyl acetate and lead citrate and examined with a Hitachi H-7500 transmission electron microscope.
Western blot analysis
The whole ischemic hemisphere, as well as astrocytes and neuronal cultures, were homogenized as described elsewhere8,9,28,29 and separated on a 4% to 12% acrylamide gel before transfer to a nitrocellulose membrane. Phosphorylated Akt (serine 473) and total Akt were detected with polyclonal antibodies purchased from Cell Signaling Technology (1:1000 dilution). A subset of brain extracts from WT and tPA−/− mice was probed 6 and 24 hours after MCAO with a monoclonal antibody directed against poly(ADP-ribose)polymerase (BD Biosciences PharMingen, San Diego, CA). For the loading control, membranes were probed with an anti-β-actin antibody (Sigma-Aldrich). Each observation was repeated 6 times. The mean density of the band in the media was quantified with the National Institutes of Health Image Analyzer System, and results were presented as a mean density of the band. Statistical analysis was performed with the Student t test.
Immunoprecipitation studies
For immunoprecipitation studies, we followed a previously described protocol.30 Briefly, brain extracts were homogenized in ristocetin-induced platelet aggregation lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 50 mM sodium fluoride, 5 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing a protease and phosphatase inhibitor mixture (Roche Diagnostics, Mannheim, Germany), followed by centrifugation at 13 000g and determination of the protein concentration. Samples containing the same amount of total protein were incubated with 11H4 anti-LRP antibody (detects the last 11 amino acid of LRP's intracellular domain; kindly provided by Dr Dudley K. Strickland), followed by incubation with protein A/G plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). The beads were collected by centrifugation and washed 5 times with ice-cold buffer (50 mM Tris, 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM ethylenediaminetetraacetic acid, 10 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride, 0.1 mg aprotinin/mL, and 0.1 mg leupeptin/mL) and boiled with 1.5× reducing SDS sample buffer for 5 minutes, separated on 10% SDS polyacrylamide gel, and analyzed by Western blotting using the anti-pAkt and anti-LRP 11H4 antibodies described above.
Results
tPA induces Akt phorphorylation in the ischemic brain
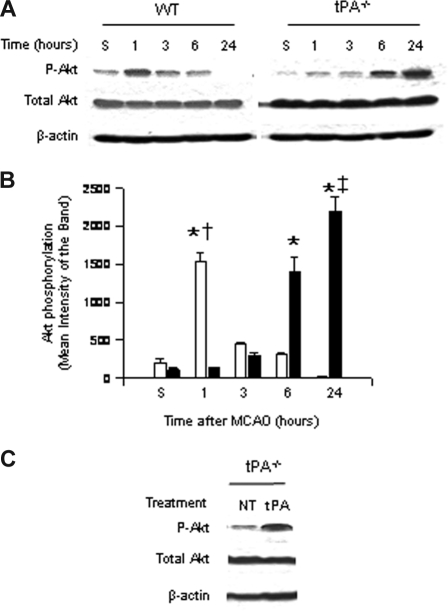
To study the role of endogenous tPA on Akt phosphorylation, we performed Western blot analysis with an antibody that detects Akt phosphorylated at serine 473 in brain extracts of WT and tPA-deficient (tPA−/−) mice 1 to 24 hours after MCAO. Our results indicate that the onset of the ischemic insult induces a rapid and transient increase in Akt phosphorylation in WT mice 1 hour after MCAO that is not observed in tPA−/− animals. Six hours after MCAO, pAkt expression in WT mice is virtually absent in glial cells and significantly decreased in neurons. In contrast, at the same time point in tPA−/− mice, there is a significant increase in the expression of neuronal pAkt (Figure 1A,B).
Figure 1.
Effect of tPA deficiency on Akt phosphorylation in the ischemic brain. (A) Representative Western blot analysis for Akt phosphorylated at serine 473 (pAkt) and total Akt in brain extracts from wild-type (WT) and tPA-deficient (tPA−/−) mice 1 to 24 hours after middle cerebral artery occlusion (MCAO). S denotes sham-operated mice. (B) Mean density of the band of 6 immunoblots for pAkt in wild-type (□) and tPA−/− mice (■) 1 to 24 hours after MCAO. n = 6. Lines depict SD. *P < .001 relative to either WT or tPA−/− sham-operated mice. †P < .001 compared with tPA−/− mice 1 hour after MCAO. ‡P < .001 relative to WT mice 24 hours after MCAO. (C) Representative Western blot analysis for pAkt in brain extracts from tPA−/− mice 1 hour after middle cerebral artery occlusion (MCAO) and either no treatment (NT) or the intracerebral injection of tPA (tPA).
Akt phosphorylation is observed in AOI-1 and -2, which correspond to the border zone between the necrotic core and the area of ischemic penumbra (defined as diffusion-perfusion mismatch); although decreased, there is still energy supply. The expression of neuronal pAkt is observed mainly in the area of ischemic penumbra, with few pAkt-positive neurons in the necrotic core. In contrast, the expression of glial pAkt is almost exclusively seen in the area of penumbra, where the leading edge of vasogenic edema is observed. Likewise, the intracerebral injection of murine tPA into the ischemic hemisphere of tPA−/− mice after MCAO increased the expression of phosphorylated Akt to levels similar to those found in WT mice (Figure 1A,C).
Six hours after MCAO, pAkt expression in WT mice is virtually absent in glial cells and significantly decreased in neurons. In contrast, at the same time point in tPA−/− mice, there is a significant increase in the expression of neuronal pAkt.
Effect of tPA deficiency on Akt phosphorylation in neurons
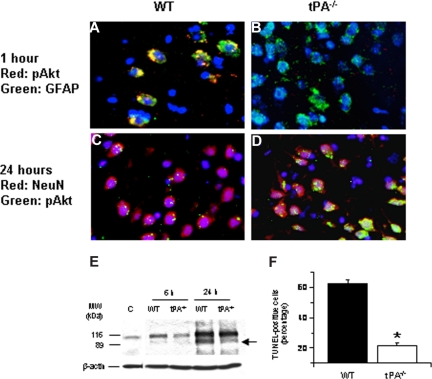
To study the effect of tPA deficiency on neuronal Akt phosphorylation, we performed immunohistochemical analysis for neuronal pAkt in WT and tPA−/− mice 24 hours after MCAO. Our results demonstrate that, 24 hours after the onset of the ischemic insult, there is a significant increase in neuronal pAkt in each AOI that is not observed in WT mice (Figure 2A-D). In addition, at that time point, we did not observe Akt phosphorylation in glial cells. Because pAkt has been considered as a neuroprotective response in the ischemic brain,16,19 we decided to study whether the increase in the expression of neuronal pAkt observed in tPA−/− mice 24 hours after MCAO had an effect on ischemic cell death. Poly(ADP-ribose) polymerase-1 (PARP-1) is a key mediator of ischemic cell death.31 Indeed, the ischemic insult induces not only an increase in the expression of PARP-1 but also its cleavage by caspases.32–34 Therefore, the cleavage of PARP-1 is considered as a marker of apoptotic cell death.35 We performed Western blot analysis in brain extracts from WT and tPA-deficient (tPA−/−) mice 6 and 24 hours after MCAO with an antibody that detects PARP-1. Our results indicate that the onset of the ischemic insult is followed by an increase in the expression of PARP-1 in WT mice (116-kDa band) that is significantly attenuated in tPA−/− animals (Figure 2E). More importantly, 24 hours after MCAO, we observed an 89-kDa band in WT but not in tPA−/− mice corresponding to the size of PARP-1–cleaved automodification and catalytic domains. To better characterize our results, we counted the number of TUNEL-positive neurons in each one of the 3 AOIs defined as described in “Definition of areas of interest, immunohistochemistry, and TUNEL staining” in WT and tPA−/− mice 24 hours after MCAO. We found that the number of TUNEL-positive neurons decreased from 62% (± 2.6%) in WT mice to 21.5% (± 1.63%) in tPA−/− mice (Figure 2F, n = 8; P < .005).
Figure 2.
Effect of tPA deficiency on ischemic cell death. (A-D) Immunohistochemical analysis of neuronal pAkt in the area of interest-2 (AOI-2) 1 (A,B) and 24 (C,D) hours after middle cerebral artery occlusion (MCAO) in wild-type (A,C) and tPA−/− (B,D) mice. Blue indicates 4′,6-diamidino-2-phenylindole and red indicates pAkt in panels A and B and NeuN in panels C and D. Green indicates GFAP in panels A and B and pAkt in panels C and D. Original magnification, A-D, 40×. Images were visualized using a Leica DMRBE microscope (Leica, Houston, TX) equipped with a 100×/1.30 numeric aperture (NA) and a LeicaDC500 camera. Images were processed using software provided by the camera manufacturer. (E) Representative Western blot analysis of PARP-1 cleavage in brain extracts from wild-type (WT) and tPA−/− mice 6 and 24 hours after MCAO. C denotes a control WT animal. The arrow indicates an approximately 89-kDa PARP-1 cleavage product. Each observation was repeated 4 times. (F) Mean percentage of TUNEL-positive cells in the area of ischemic penumbra in WT and tPA−/− mice 24 hours after MCAO; n = 7. *P < .005 compared with WT mice.
Effect of tPA deficiency on Akt phosphorylation in astrocytes
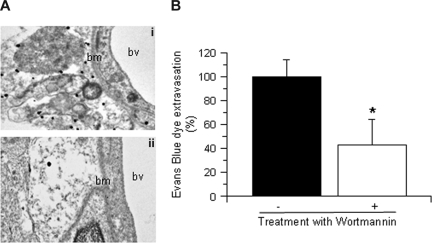
To study the role of tPA on Akt phosphorylation in glial cells, we performed immunohistochemical analysis for pAkt and GFAP 1 hour after MCAO. Our results demonstrated a significant increase in pAkt expression in glial cells surrounding the blood vessels in each AOI inWT but not in tPA−/− mice (data not shown). To further characterize this observation, we performed immunogold electron microscopy analysis for pAkt in brain sections from WT and tPA−/− mice 1 hour after MCAO. We found that cerebral ischemia induces a rapid increase in pAkt in perivascular astrocytes in WT mice (Figure 3Ai) that is not observed in tPA−/− mice (Figure 3Aii). Because one of the main functions of perivascular astrocytes is the regulation of the permeability of the NVU, we decided to study the effect of Akt inhibition on the permeability of the BBB. Wild-type mice underwent the intraventricular injection of either wortmannin (0.1 mM) or vehicle control (100% dimethyl sulfoxide) followed by the intravenous injection of Evans blue dye and MCAO as described in “Animal model of cerebral ischemia and quantification of Evans blue dye extravasation.” We found that, compared with vehicle-treated WT mice (Figure 3B filled bar), treatment with wortmannin resulted in a 57% (± 18%) decrease in the extravasation of Evans blue dye (Figure 3B open bar; n = 6; P < .005).
Figure 3.
tPA induces Akt phosphorylation in perivascular astrocytes. (A) Representative micrographs of immunogold electron microscopy analysis for pAkt in perivascular astrocytes in the area of interest-2 (AOI-2) of wild-type (i) and tPA−/− mice (ii) 1 hour after MCAO. Bv indicates blood vessel; and bm, basement membrane. Each observation was repeated 4 times. (B) Mean Evans blue dye extravasation in wild-type mice 6 hours after reperfusion and the intraventricular injection of either vehicle (control, ■) or wortmannin (□). Results are given as a percentage compared with control-treated mice. Lines depict SD; n = 8. *P < .005 compared with control-treated mice.
tPA mediates cerebral ischemia-induced Akt phosphorylation via a plasminogen-independent mechanism
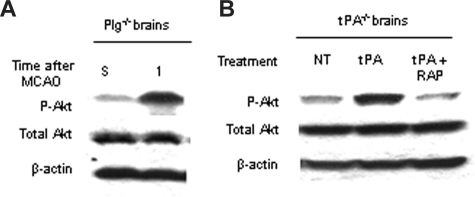
To study whether the effect of tPA on Akt phosphorylation in the early phases of cerebral ischemia is mediated by plasminogen, we performed Western blot analysis for pAkt and total Akt in brain extracts from plasminogen-deficient (Plg−/−) mice 1 hour after MCAO. Our results indicate that MCAO induces an increase in Akt phosphorylation in Plg−/− mice comparable with that found in WT animals (Figure 4A). Because we have previously described that the LRP mediates the effect of tPA on perivascular astrocytes,8 we decided to study whether whether LRP mediates the effect of tPA on Akt phosphorylation. We performed Western blot analysis for Akt phosphorylated at serine 473 in brain extracts of tPA−/− mice 1 hour after MCAO and either the injection of murine tPA (1 μM) alone or a combination of murine tPA and the RAP (9 μM), an LRP antagonist. We found that treatment with tPA induced a significant increase in the expression of pAkt that was attenuated when tPA was coadministered with RAP (Figure 4B).
Figure 4.
tPA induces Akt phosphorylation via a plasminogen-independent mechanism. (A,B) Representative Western blot analysis of pAkt and total Akt in brain extracts from Plg−/− mice (A) and tPA−/− animals (B) 1 hour after MCAO. A subset of tPA−/− mice was either left untreated (NT) or injected directly into the ischemic tissue with murine tPA (tPA) or a combination of tPA and RAP (tPA + RAP). S denotes sham-operated animal. Each observation was repeated 3 times.
The effect of tPA of Akt phosphorylation is cell type–specific
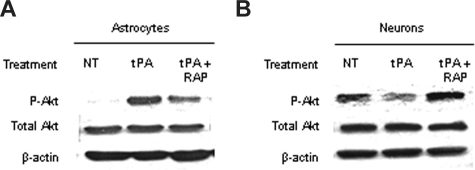
Because Akt is found in neurons and astrocytes, we decided to study the effect of tPA on Akt phosphorylation in each cell type. To accomplish this goal, we performed a Western blot analysis in extracts from WT astrocytes and neurons exposed to OGD conditions for 1 hour either without treatment or after incubation with either tPA alone or a combination of tPA and RAP. We found that incubation with tPA induced a significant increase in pAkt in astrocytes exposed to OGD conditions and that this effect was attenuated on cotreatment with tPA and RAP (Figure 5A). In contrast, incubation with tPA decreased pAkt in neurons, and this effect was inhibited by cotreatment with RAP (Figure 5B).
Figure 5.
The effect of the interaction between tPA and LRP on pAkt is cell type– specific. (A,B) Representative Western blot analysis of pAkt and total Akt in extracts from astrocytic (A) and neuronal (B) cultures after 1 hour of exposure to oxygen-glucose deprivation (OGD) conditions and incubation with either tPA or a combination of tPA and the receptor-associated protein (RAP). RAP is a molecular chaperone that inhibits the binding of LRP to its ligands. NT indicates no treatment. Each observation was repeated 4 times.
tPA induces the interaction between LRP's intracellular domain and pAkt after MCAO
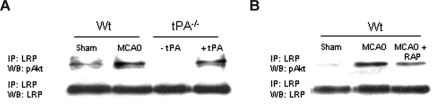
In earlier studies, we demonstrated that, during cerebral ischemia, tPA induces a rapid increase in the expression of LRP in perivascular astrocytes followed by an increase in the permeability of the BBB.8 Because tPA also mediates the expression of pAkt in perivascular astrocytes in the early phases of the ischemic insult (Figure 3A), we decided to study whether LRP's intracellular domain (LRP-ICD) and Akt interact during cerebral ischemia. We performed coimmunoprecipitation studies using antibodies against LRP-ICD and pAKt in brain extracts from WT and tPA−/− mice 1 hour after MCAO. A subset of tPA−/− mice was either left untreated (− tPA in Figure 6A) or injected directly into the ischemic tissue with tPA (+ tPA). Our results indicate that MCAO induces the interaction of LRP-ICD and pAkt and that this interaction is prevented either by genetic deficiency of tPA (Figure 6A) or by treatment with RAP (Figure 6B).
Figure 6.
LRP's intracellular domain and pAkt interact in perivascular astrocytes. (A) Coimmunoprecipitation studies in brain extracts from Wt and tPA−/− mice 1 hour after MCAO. tPA−/− were either left untreated (−tPA) or injected directly into the ischemic tissue with tPA (1 μM;+tPA). (B) Coimmunoprecipitation studies in brain extracts from Wt mice 1 hour after MCAO. Animals were either left untreated (MCAO) or injected into the ischemic tissue with RAP (9 μM; MCAO + RAP).
Discussion
The NVU is a dynamic structure assembled by endothelial cells, neurons, the basal lamina, and astrocytic end-feet processes.36 The main function of the NVU is the regulation of the passage of substances from the intravascular space into the brain.37 One of the most important regulators of this barrier function of the NVU, also known as the BBB, is the interaction between astrocytic end-feet processes and the perivascular basal lamina.38–40 Indeed, approximately 95% of the NVU is embraced by astrocytic end-feet processes,41 and detachment of astrocytes from the basal lamina results in changes in the composition of the basement membrane,38 redistribution of interendothelial tight junctions proteins, and increase in the permeability of the BBB.42 Cerebral ischemia has a profound impact on the composition and permeability of the BBB.37,43 Indeed, as early as 1 to 2 hours after the onset of the ischemic insult, there is detachment of astrocytic end-feet processes from the basal lamina with development of edema.44–46 Our work indicates that the increase in the permeability of the BBB observed during cerebral ischemia is not the consequence of a disorganized “breakdown” of the BBB but instead is the result of a carefully regulated cell signaling event activated on the interaction between tPA and the LRP in perivascular astrocytes.
LRP is a member of the LDL receptor gene family assembled by a 515-kDa heavy chain noncovalently bound to an 85-kDa light chain containing a transmembrane and a cytoplasmic domain.11 LRP has been implicated not only in the internalization of multiple ligands,11,47–49 but also in cellular signal transduction pathways12 and neurotransmission.50 In addition, recent work has suggested that LRP also plays a role in cell death.28,51 LRP is found in the human and murine CNS in neurons and in perivascular astrocytic end-feet processes.52 In neurons, LRP has been found to mediate events, such as long-term potentiation50 and calcium influx via N-methyl-D-aspartate receptors.53 LRP is also expressed in smooth muscle cells (SMCs), and specific deletion of the LRP gene from vascular SMCs on a background of LDL receptor deficiency causes SMC proliferation, increased susceptibility to cholesterol-induced atherosclerosis, and aneurysm formation.54 The role of LRP in perivascular astrocytic end-feet processes is less well understood. Our earlier studies indicate that the onset of the ischemic insult is followed by an increase in the expression of LRP in perivascular astrocytes and that treatment with either the RAP or anti-LRP antibodies after MCAO decreases the magnitude of the ischemic edema.8
tPA is a serine proteinase that is found in both the intravascular space and in the CNS. In the intravascular space, tPA's substrate is plasminogen24 and its main function is as a thrombolytic enzyme. In contrast, the substrate of tPA in the CNS has not yet been well defined,55,56 and its presence in the brain has been linked with modulation of cerebrovascular tone57 and regulation of the barrier function of the NVU.5,8,13,58 We have previously demonstrated, using in vivo zymography assay studies, that early after the onset of the ischemic insult there is an increase in tPA activity in the NVU5 in the same areas where we observed an increase in LRP expression.8 There is a growing body of evidence indicating that tPA increases in the permeability of the BBB5,59,60 and that intravenously administered tPA may cross from the intravascular space into the ischemic brain.61,62 Therefore, it is plausible to postulate that both blood-borne and astrocytic-derived tPA interact with LRP in astrocytic end-feet processes. Indeed, our earlier studies indicate that tPA induces shedding of LRP's ectodomain from perivascular astrocytes into the vascular basement membrane in areas of the NVU with detachment of astrocytic end-feet processes from the underlying glia limitans and development of cerebral edema.8
Akt (PKB) is the cellular homolog of the viral oncogene v-Akt.63 Activation of Akt occurs through its phosphorylation (pAkt), which is dependent of phosphoinositide 3 kinase (PI3K).63 The critical step for the activation of Akt is its transition from the cytosol to the plasma membrane, which is accomplished by the binding of Akt to phosphatidylinositol 3,4,5 triphosphate and phosphatidylinositol 3,4 biphosphate.63–65 A relation between tPA and pAkt has been previously suggested. However, whereas some studies indicate that treatment with tPA induces a rapid increase in pAkt expression,66 other reports demonstrate that the intravenous administration of tPA in an animal model of embolic stroke decreases the expression of pAkt.19 Here we demonstrate that the effect of tPA on pAkt is cell type–specific and varies according to the stage of the ischemic insult. A relation between LRP and pAkt has been demonstrated in mouse embryonic fibroblasts and Schwann cells.51 Our data indicate that tPA induces Akt phosphorylation in perivascular astrocytes and that this effect is inhibited by the RAP. This suggests that the effect of tPA on Akt phosphorylation is mediated by LRP.
MCAO is followed by a rapid and transient increase in the expression of pAkt in neurons and perivascular astrocytes in the ischemic hemisphere. However, whereas the increase in neuronal pAkt is considered a neuroprotective response to the ischemic insult,16,19,20,67 the role of pAkt in perivascular astrocytes is unknown. Our results indicate that, in response to the ischemic insult, tPA−/− mice have not only a significant decrease in the early expression of pAkt in perivascular astrocytes (1 hour after MCAO) but also a sustained increase in neuronal pAkt at later time points (6 and 24 hours after MCAO). We acknowledge that the use of wortmannin, a specific inhibitor of the phosphoinositide 3-kinase/Akt pathway, may have multiple effects in this pathway. However, the fact that treatment with wortmannin resulted in a significant decrease in the permeability of the BBB in the early phases of the ischemic insult suggests that Akt in perivascular astrocytes plays a role in the regulation of the permeability of the BBB during ischemic conditions. Nevertheless, because small molecules injected into the cerebrospinal fluid may cross through the basement membrane of the NVU, we cannot rule out at this moment an effect of wortmannin on Akt phosphorylation in endothelial cells. Taken together, our data indicate that the interaction between tPA and LRP in perivascular astrocytes activates a cell signaling event that results in phosphorylation of Akt and leads to an increase in the permeability of the BBB.
Because we found an increase in neuronal pAkt in tPA−/− mice at 6 and 24 hours after MCAO that was not detected in WT animals, we decided to study whether this difference had an impact on cell death. We found that, compared with WT animals, tPA−/− mice had a 41% decrease in ischemic cell death. This could be explained not only by an increase in neuronal pAkt 6 and 24 hours after MCAO but also by attenuation of an early increase in pAkt in perivascular astrocytes, which has a deleterious effect on BBB permeability. These observations are supported by our in vitro studies indicating that the effect of the interaction between tPA and LRP increases the expression of pAkt in astrocytes and decreases pAkt in neurons.
The role of pAkt in astrocytes is not well understood. However, there is evidence indicating that Akt phosphorylation in astrocytes is followed by NF-κB activation and increase in the expression of matrix metalloproteinase-9,68 which is known to have a deleterious effect on the permeability of the NVU.69,70 In addition, in previous studies, we demonstrated that the interaction between tPA and LRP in perivascular astrocytes induces NF-κB activation with increase in the expression of inducible nitric oxide synthetase,13 which also has a deleterious effect on the permeability of the NVU.71–73 Taken together with the results reported here, we postulate that, in the early phases of the ischemic insult, the interaction between tPA and LRP induces Akt phosphorylation in perivascular astrocytes, with NF-κB activation and activation of NF-κB–regulated genes known to have a deleterious effect on the permeability of the NVU. This possibility is supported by data from other laboratories using in vitro systems indicating that phosphorylation of Akt under hypoxic conditions mediates the deleterious effect of reactive oxygen species on the integrity and permeability of tight junctions proteins.21,23
Based on these observations, we hypothesize that the beneficial effect of tPA deficiency during cerebral ischemia is the combined result of inhibition of Akt phosphorylation in perivascular astrocytes (where it has a deleterious effect on the permeability of the BBB) and increase in pAkt in neurons (where it has a neuroprotective role).
In conclusion, based on our previous results5,8,13,28 and the data reported herein, we propose a model where in response to the ischemic insult there is an increase in blood-borne and astrocyte-derived tPA around the NVU. The interaction between this tPA and LRP in astrocytic end-feet processes results in cleavage of LRP's ectodomain, which is shed into the basement membrane. This results in phosphorylation of Akt by LRP's intracellular domain, pAkt-induced NF-κB activation, detachment of astrocytic end-feet processes from the glia limitans, and increase in the permeability of the NVU.
Acknowledgments
The authors thank Ms Hong Yi from the Emory University School of Medicine Electronic Microscopy Core Facility for her assistance with the electron microscopy studies.
This work was supported in part by National Institutes of Health grant NS49478 (M.Y.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A., C.Z., R.P., and Xiaohui Zhang performed research; Xiumei Zhang participated in the design of the research plan; and M.Y. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manuel Yepes, Department of Neurology and Center for Neurodegenerative Disease, Whitehead Biomedical Research Building, 615 Michael Street, Suite 505J, Atlanta, GA 30322; e-mail: myepes@emory.edu.
References
- 1.Bugge TH, Xiao Q, Kombrinck KW, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Wang YF, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 4.Yepes M, Sandkvist M, Wong MK, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- 5.Yepes M, Sandkvist M, Moore EG, et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 7.Cinelli P, Madani R, Tsuzuki N, et al. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol Cell Neurosci. 2001;18:443–457. doi: 10.1006/mcne.2001.1028. [DOI] [PubMed] [Google Scholar]
- 8.Polavarapu R, Gongora MC, Yi H, et al. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yepes M, Moore E, Brown SA, et al. Progressive ankylosis (Ank) protein is expressed by neurons and Ank immunohistochemical reactivity is increased by limbic seizures. Lab Invest. 2003;83:1025–1032. doi: 10.1097/01.lab.0000075640.49586.e6. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 11.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-κB pathway activation. Am J Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo HR, Hattori H, Hossain MA, et al. Akt as a mediator of cell death. Proc Natl Acad Sci U S A. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara T, Fujimura M, Noshita N, et al. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1:17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noshita N, Sugawara T, Lewen A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003;34:1513–1518. doi: 10.1161/01.STR.0000072986.46924.F4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang ZG, Liu XS, Hozeska-Solgot A, Chopp M. The PI3K/Akt pathway mediates the neuroprotective effect of atorvastatin in extending thrombolytic therapy after embolic stroke in the rat. Arterioscler Thromb Vasc Biol. 2007;27:2470–2475. doi: 10.1161/ATVBAHA.107.150748. [DOI] [PubMed] [Google Scholar]
- 20.Ohba N, Kiryu-Seo S, Maeda M, et al. Transgenic mouse overexpressing the Akt reduced the volume of infarct area after middle cerebral artery occlusion. Neurosci Lett. 2004;359:159–162. doi: 10.1016/j.neulet.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Kilic E, Kilic U, Wang Y, et al. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- 22.Schreibelt G, Kooij G, Reijerkerk A, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 23.Vogel C, Bauer A, Wiesnet M, et al. Flt-1, but not Flk-1, mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol. 2007;212:236–243. doi: 10.1002/jcp.21022. [DOI] [PubMed] [Google Scholar]
- 24.Bugge TH, Kombrinck KW, Flick MJ, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 25.Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. Academic Press: San Diego, CA; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- 27.Yi H, Leunissen J, Shi G, Gutekunst C, Hersch S. A novel procedure for pre-embedding double immunogold-silver labeling at the ultrastructural level. J Histochem Cytochem. 2001;49:279–284. doi: 10.1177/002215540104900301. [DOI] [PubMed] [Google Scholar]
- 28.Polavarapu R, Jie A, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol. 2008;172:1355–1362. doi: 10.2353/ajpath.2008.070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yepes M, Brown SA, Moore EG, et al. A soluble Fn14-Fc decoy receptor reduces infarct volume in a murine model of cerebral ischemia. Am J Pathol. 2005;166:511–520. doi: 10.1016/S0002-9440(10)62273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton CS, Loukinova E, Mikhailenko I, et al. Platelet-derived growth factor receptor-beta (PDGFR-beta) activation promotes its association with the LDL receptor-related protein (LRP): evidence for co-receptor function. J Biol Chem. 2005;280:27872–27878. doi: 10.1074/jbc.M505410200. [DOI] [PubMed] [Google Scholar]
- 31.Plesnila N, Zhu C, Culmsee C, et al. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:458–466. doi: 10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Eliasson MJ, Sampei K, Mandir AS, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 33.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 35.Koh DW, Dawson TM, Dawson VL. Mediation of cell death by poly(ADP-ribose) polymerase-1. Pharmacol Res. 2005;52:5–14. doi: 10.1016/j.phrs.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 37.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 38.Rieckmann P, Engelhardt B. Building up the blood-brain barrier. Nat Med. 2003;9:828–829. doi: 10.1038/nm0703-828. [DOI] [PubMed] [Google Scholar]
- 39.Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48:1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- 40.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 41.Vorbrodt AW, Dobrogowska DH. Molecular anatomy of intercellular junctions in brain endothelial and epithelial barriers: electron microscopist's view. Brain Res Brain Res Rev. 2003;42:221–242. doi: 10.1016/s0165-0173(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 42.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda S, Fini CA, Mabuchi T, et al. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagaya M, Haring HP, Stuiver I, et al. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 46.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 47.Herz J, Kowal RC, Ho YK, Brown MS, Goldstein JL. Low density lipoprotein receptor-related protein mediates endocytosis of monoclonal antibodies in cultured cells and rabbit liver. J Biol Chem. 1990;265:21355–21362. [PubMed] [Google Scholar]
- 48.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci U S A. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strickland DK, Ashcom JD, Williams S, et al. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- 50.Zhuo M, Holtzman DM, Li Y, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campana WM, Li X, Dragojlovic N, et al. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf BB, Lopes MB, VandenBerg SR, Gonias SL. Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am J Pathol. 1992;141:37–42. [PMC free article] [PubMed] [Google Scholar]
- 53.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 55.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 56.Yepes M, Sandkvist M, Coleman TA, et al. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest. 2002;109:1571–1578. doi: 10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nassar T, Akkawi S, Shina A, et al. The in vitro and in vivo effect of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Zhang L, Yepes M, et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 59.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 60.Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 61.Benchenane K, Berezowski V, Ali C, et al. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- 62.Benchenane K, Berezowski V, Fernandez-Monreal M, et al. Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke. 2005;36:1065–1070. doi: 10.1161/01.STR.0000163050.39122.4f. [DOI] [PubMed] [Google Scholar]
- 63.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 64.Kandel ES, Skeen J, Majewski N, et al. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HY, Hwang IY, Im H, Koh JY, Kim YH. Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J Neurochem. 2007;101:1236–1247. doi: 10.1111/j.1471-4159.2007.04417.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao H, Shimohata T, Wang JQ, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SD, Yang SI, Kim HC, Shin CY, Ko KH. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res. 2007;1186:12–20. doi: 10.1016/j.brainres.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 69.Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 72.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 73.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]