Abstract
Outcomes of 159 young patients with inherited metabolic disorders (IMDs) undergoing transplantation with partially HLA-mismatched unrelated donor umbilical cord blood were studied to investigate the impact of graft and patient characteristics on engraftment, overall survival (OS), and graft-versus-host disease (GVHD). Patients received myeloablative chemotherapy (busulfan, cyclophosphamide, ATG) and cyclosporine-based GVHD prophylaxis. Infused cell doses were high (7.57 × 107/kg) because of the patients' young age (median, 1.5 years) and small size (median, 12 kg). Median follow-up was 4.2 years (range, 1-11 years). The cumulative incidences of neutrophil and platelet engraftment were 87.1% (95% confidence interval [CI], 81.8%-92.4%) and 71.0% (95% CI, 63.7%-78.3%). A total of 97% achieved high (> 90%) donor chimerism. Serum enzyme normalized in 97% of patients with diseases for which testings exist. Grade III/IV acute GVHD occurred in 10.3% (95% CI, 5.4%-15.2%) of patients. Extensive chronic GVHD occurred in 10.8% (95% CI, 5.7%-15.9%) of patients by 1 year. OS at 1 and 5 years was 71.8% (95% CI, 64.7%-78.9%) and 58.2% (95% CI, 49.7%-66.6%) in all patients and 84.5% (95% CI, 77.0%-92.0%) and 75.7% (95% CI, 66.1%-85.3%) in patients with high (80-100) performance score. In multivariate analysis, favorable factors for OS were high pretransplantation performance status, matched donor/recipient ethnicity, and higher infused colony forming units.
Introduction
Inherited metabolic disorders (IMDs), in particular the lysosomal and peroxisomal storage diseases, cause progressive organ failure and death early in life.1 In the past 25 years, nearly a thousand patients with these types of storage disorders, including mucopolysaccharidosis (MPS) type I (Hurler syndrome), other MPS, adrenoleukodystrophy (ALD), metachromatic leukodystrophy (MLD), Krabbe disease, and others have received allogeneic hematopoietic stem cell transplantation (HSCT) with bone marrow from matched or mismatched related donors who were either carriers or noncarriers of the disease, resulting in clinical benefit in many of them.2–16 The benefit is primarily derived from the replacement of missing enzyme produced by donor cells circulating in the blood and also from engraftment of donor-derived glial cells in the brain.16–19 However, many children with IMDs who could benefit from HSCT do not have a matched bone marrow donor. Recent reports demonstrate successful use of banked unrelated donor umbilical cord blood transplantation (UCBT) for the treatment of malignant and nonmalignant diseases.20–29 Large inventories of UCB units are available in public banks for transplantation in those lacking bone marrow donors.
Due to the rarity of IMD, there has never been a large series of patients who underwent transplantation with UCB. We now describe the results of 159 consecutive young pediatric patients (92 of whom were previously reported for short-term outcomes22,26,30,31) with IMDs who underwent transplantations with UCB at a single center. This series is unique for several reasons. It represents the first publication describing a larger population of small, young patients without a malignant diagnosis receiving UCBT after uniform cytoreduction, graft-versus-host disease (GVHD) prophylaxis and supportive care who were then followed for up to 11 years after transplantation. It also allows analysis of the impact of cell dose, HLA matching, and graft characteristics, comparing traditional parameters like total nucleated cell (TNC) dose to more complex measurements such as CD34 and colony-forming units (CFUs), in a population of patients receiving high-dose, partially HLA-mismatched UCB grafts where relapse of a malignant disorder was not a competing risk.
Methods
Patients
Between August 1995 and April 2007, 159 consecutive young children with IMDs referred to Duke University Medical Center were treated with unrelated donor UCBT. These patients lacked HLA-matched, related bone marrow donors who were not carriers of the disease. Diagnoses were confirmed by enzyme or substrate analysis in the peripheral blood or skin fibroblasts.32 In addition, DNA mutation analyses were performed whenever possible. All patients were enrolled in a Duke University Medical Center Institutional Review Board (IRB)–approved protocol or treatment plan for transplantation. Written informed consent was obtained for all patients according to the Declaration of Helsinki. Data on 67 of these patients have not been previously reported. Limited and partial data on 92 of 159 patients with a much shorter follow-up have been published previously in the Cord Blood Transplantation (COBLT) study26 and as part of disease specific reports for neonatal Krabbe,22 Hurler,31 and ALD.30
Donor selection
Unrelated cord blood units (CBUs) from 8 U.S. public banks were selected for transplantation after matching by intermediate-resolution HLA-A and HLA-B and high-resolution HLA-DRB1 typing. The CBU with the highest number of nucleated cells that matched at least 3 of 6 HLA loci was selected. Molecular matching at HLA-DRB1 was favored and at least one antigen from each locus (A, B, DRB1) was matched. Units were screened for the genetic disease for which the patient was undergoing transplantation to avoid selection of a carrier donor. The unit with the highest enzyme activity was selected whenever possible.33 Precryopreservation CBU characteristics, including total nucleated cell (TNC) and CD34 content and clonal hematopoietic progenitor cells (colony forming units [CFUs]), were obtained from the cord blood banks supplying the donor units.
Conditioning regimen
All patients had central venous catheters placed prior to UCBT. Patients received myeloablative conditioning with 16 doses of busulfan given orally (n = 127) or intravenously (n = 32) every 6 hours over 4 days (days −9 to −6) with phenytoin prophylaxis against seizures, followed by cyclophosphamide 50 mg/kg/dose for 4 days intravenously with mesna prophylaxis against hemorrhagic cystitis (days −5 to −2), and equine antithymocyte globulin (ATG) 30 mg/kg/dose intravenously daily for 3 days (days −3 to −1). Busulfan pharmacokinetics was studied after the first dose, and subsequent doses were adjusted to maintain a steady state of 600 to 900 ng/mL. None of the patients received radiation therapy.
Graft analysis and transplantation procedure
The cryopreserved units of cord blood were thawed and washed as described by Rubinstein et al.34 The TNCs, CD34+ cells, CD3+ cells, and CFUs were enumerated; ABO and Rh typing, viability, and bacterial and fungal cultures were performed after thawing. After washing and resuspension in a volume of dextran/albumin solution not to exceed 5 mg/kg of the patient's body weight, the cord blood unit was infused through the patient's central venous line over 15 to 30 minutes.
GVHD prophylaxis and treatment
GVHD prophylaxis was cyclosporine and methylprednisolone in 125 patients who underwent transplantation between 1995 and 2004 and cyclosporine and mycophenolate mofetil in the 34 patients who underwent transplantation after 2004. Cyclosporine was given for 9 months and then tapered if there was no active GVHD. Methylprednisolone or mycophenolate mofetil was continued for 2 to 3 months in patients without ongoing GVHD. The severity of acute GVHD (aGVHD) was scored according to the standard criteria.35 Patients with grade 1 aGVHD were treated with topical therapies. Those with grades 2 to 4 GVHD were treated with methylprednisolone or switched from cyclosporine to tacrolimus plus daclizumab.
Supportive care
Patients were nursed in reverse isolation rooms on a dedicated transplant unit with high-efficiency particulate air filtration system. Standard prophylaxis against viral pathogens and Pneumocystis carinii was used.26,31 Patients who underwent transplantation before 1999 received prophylaxis against fungal infections with low-dose amphotericin-B while those who underwent transplantation after 2000 received voriconazole. Empiric broad spectrum antibiotic therapy was started with the first fever. Intravenous immunoglobulin (500 mg/kg per dose) was given weekly until day 100 and then monthly until discontinuation of GVHD therapy and documentation of antibody production. Veno-occlusive disease prophylaxis was continuous infusion heparin (100 U/kg per day) from day −10 to day 28. Patients received TPN, transfusions of leukocyte-depleted and -irradiated packed red cells and platelets, and granulocyte colony stimulating factor 10 μg/kg intravenously daily from day 1 until their white blood cell (WBC) count was greater than 5 × 109/L (5 000/μL). Patients with MPS syndromes underwent tonsillectomy, adenoidectomy, and pressure equalization (PE) tube placement before transplantation. Patients with MPS were also evaluated for increased intracranial pressure by magnetic resonance imaging (MRI) or computed tomography (CT) scan and measurement of opening and closing pressures via lumbar puncture before initiation of chemotherapy for cytoreduction. If increased intracranial pressure was detected, a ventriculo-peritoneal shunt was placed approximately 2 weeks before transplantation. Patients with feeding problems had G-tubes placed as needed.
Posttransplantation evaluation
Donor cell chimerism and enzyme levels were measured at engraftment, day 100, every 3 months during the first posttransplantation year and yearly thereafter. Chimerism was confirmed by restriction fragment length polymorphism, microsatellite markers, HLA, or XY fluorescent in situ hybridization (FISH). Blood enzyme levels were measured when possible (eg, the enzyme cannot be measured in the blood of patients with Sanfilippo A or ALD). In addition to standard pretransplantation organ function studies,31 patients were evaluated by other relevant specialists (ophthalmology, cardiology, audiology, pulmonology, radiology, dentistry, and orthopedics), and tested with a disease-specific panel of blood tests, imaging studies (eg, brain/spine MRI), and neurophysiologic studies, including electroencephalogram, brainstem auditory evoked response, visual evoked response, and nerve conduction studies. All children underwent neurodevelopmental assessments with standardized testing at the Program for Neurodevelopmental Function in Rare Disorders, Center for the Study of Development and Learning at the University of North Carolina at Chapel Hill before transplantation, every 3 to 6 months for the first year, every 6 months for the second to third year, and yearly thereafter.
Statistical analysis
Neutrophil engraftment was defined as the first day of 3 consecutive days of an absolute neutrophil count of 500 donor cells/mm3 or more; platelet engraftment was defined as the day of achieving an untransfused platelet count of 50 000/mm3 or more for 7 days. Acute GVHD was scored in all patients within 100 days,35 and the chronic GVHD (cGVHD) at the highest level per standard criteria.36 The probabilities of neutrophil and platelet engraftment, aGVHD, and cGVHD were estimated using the cumulative incidence function method.37 Neutrophil engraftment was assessed in patients surviving to day 14 treating death without the event as a competing risk, while autologous reconstitution was censored. For platelet engraftment, aGVHD, and cGVHD, patients were evaluable after surviving to day 14, and death without the event was the competing event, while patients were censored on the last day of follow-up. Differences between subgroups were compared using the Gray K-Sample test.38 The probability of overall survival was calculated with the use of the Kaplan-Meier estimator,39 and differences between groups were compared using the log-rank statistics.40 Cox proportional-hazards regression was used to create prognostic models with multiple variables.41 Multivariate models were constructed using forward stepwise selection with statistical significance based on a P value of .05 or less; all variables met the proportional hazards assumption. Results were expressed as hazard ratios, which compare the relative rate of event occurrence between covariate categories. Baseline variables, including performance status, patient age at transplantation, recipient and donor sex, disease status at transplantation, date of transplantation, total cell dose before cryopreservation and reinfusion, reinfused CD34 dose, reinfused CD3 dose, reinfused CFUs, HLA-matching, recipient and donor ethnicity, ABO matching, recipient cytomegalovirus (CMV) serostatus, and recipient weight at transplantation were considered. All P values are 2-sided. Analyses were completed using the SAS system, version 8.2, and R, version 2.1.1 (SAS, Cary, NC).
Results
Characteristics of the patients and their donors
From 1995 to 2007, 159 consecutive young pediatric patients with IMDs were treated with UCBT. The patient and donor characteristics are listed in Table 1. The median age of the patients was 1.50 years (range, 0.05-26.25 years), with 57% of patients younger than 2 years old at transplantation. The median weight of the group was 12 kg (range, 2.74-73.1 kg). Most patients were male (61%) and white (84%). Before transplantation, 19.5% of patients were CMV seropositive, a lower rate than seen in previously reported patient cohorts, likely due to the younger age of these patients. A large proportion of patients (41.5%) had a poor performance status (Lansky score < 80%). The median time between the initial patient evaluation at our center and start of cytoreduction was 34.5 days, while the median time between the date of diagnosis and start of cytoreduction was 87 days. All but 7 patients received grafts mismatched at 1 (n = 75), 2 (n = 73) or 3 (n = 4) HLA loci. A total of 88 (55%) donor-graft pairs were matched for ABO, 81 (50.9%) were matched for sex, and 123 (82%) were matched for ethnicity.
Table 1.
Characteristics of 159 patients and their CBUs
| Characteristics | Median | Range |
|---|---|---|
| Age, y | 1.50 | 0.05-26.25 |
| Weight at transplantation, kg | 12.0 | 2.74-73.10 |
| Cryopreserved TNCs, × 107/kg | 9.73 | 2.24-50.37 |
| Reinfused TNCs, × 107/kg | 7.57 | 1.49-32.40 |
| Postthaw reinfused CD34+, × 105/kg | 2.14 | 0.42-104.75 |
| Postthaw reinfused CD3+, × 106/kg | 14.15 | 3.26-100.56 |
| Total postthaw reinfused CFUs, × 104/kg | 5.74 | 0.00-105.30 |
| Primary disease | N | % |
| Hurler syndrome | 45 | 28.3 |
| Krabbe disease | 36 | 22.6 |
| Sanfilippo syndrome | 19 | 11.9 |
| Metachromatic leukodystrophy | 15 | 9.4 |
| Adrenoleukodystrophy (ALD) | 13 | 8.2 |
| Tay Sachs disease | 9 | 5.7 |
| Hunter syndrome | 6 | 3.8 |
| Lesch-Nyhan disease | 4 | 2.5 |
| Sandhoff disease | 3 | 1.9 |
| Hurler Scheie | 2 | 1.3 |
| Niemann-Pick B | 2 | 1.3 |
| Alpha mannosidosis | 1 | 0.6 |
| GM1 gangliosidosis | 1 | 0.6 |
| I-cell disease | 1 | 0.6 |
| Maroteaux-Lamy syndrome | 1 | 0.6 |
| Pelizaeus-Merzbacher disease | 1 | 0.6 |
| Performance status < 80 | 66 | 41.5 |
| Recipient CMV serology negative | 128 | 80.5 |
| HLA match | ||
| 6/6 | 7 | 4.4 |
| 5/6 | 75 | 47.2 |
| 4/6 | 73 | 45.9 |
| 3/6 | 4 | 2.5 |
| ABO matching mismatched | 71 | 44.7 |
| Recipient sex female | 62 | 39.0 |
| Unit sex female | 80 | 50.3 |
| Sex matching (recipient/unit) | ||
| F/F | 32 | 20.1 |
| F/M | 30 | 18.9 |
| M/M | 49 | 30.8 |
| M/F | 48 | 30.2 |
| Recipient race white | 133 | 83.7 |
| Donor race white | 126 | 83.4 |
| Race matching (recipient/unit) | ||
| White/white | 113 | 74.8 |
| White/other | 15 | 9.9 |
| Other/other | 10 | 6.6 |
| Other/white | 13 | 8.6 |
Characteristics of the cord blood grafts
The median TNCs per kilogram of the selected grafts (precryopreservation) was 9.73 × 107 cells/kg (range, 2.24-50.37 × 107 cells/kg) using data provided by the cord blood banks. The median doses infused from the thawed graft (measured in the Stem Cell Laboratory at Duke University at the time of thaw) were 7.57 × 107 TNC/kg (range, 1.49-32.40 × 107 TNC/kg), 2.14 × 105 CD34+ cells/kg (range, 0.42-104.75 × 105 CD34+ cells/kg) and 5.74 × 104 CFU/kg (range, 0.00-105.30 × 104 CFU/kg; Table 1).
Neutrophil and platelet engraftment
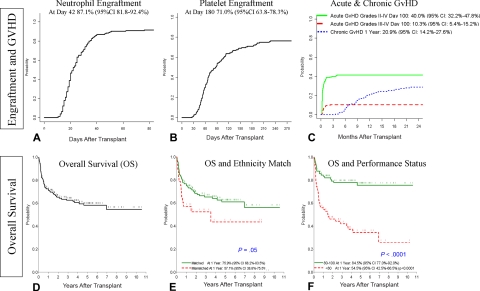
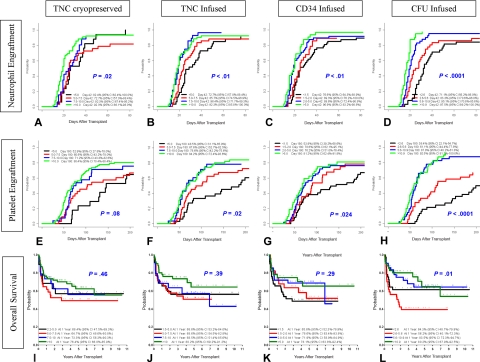
Engrafting patients (146 of 159) achieved neutrophil engraftment in a median of 22 days (range, 10-76 days). A total of 13 patients exhibited autologous reconstitution (n = 5), primary graft failures (n = 5), or significant late graft dysfunction (n = 3); 2 of these patients ultimately required second transplants. The cumulative incidence of neutrophil engraftment by day 42 was 87.1% (95% confidence interval [CI], 81.8%-92.4%; Figure 1A). In univariate analysis, neutrophil engraftment (Figure 2A-D) was influenced by patient age (P < .01), unit sex (P < .01), cryopreserved TNCs (P < .01), infused TNCs (P < .01), infused CD34 (P < .01), infused CFUs (P < .01), infused CD3 (P < .01), HLA match (P = .04), and recipient CMV serostatus (P < .01; Table 2). In multivariate analysis, patient age of 2 years or younger (P < .01), male CBU (P < .01), more than 2.1 × 105/kg of infused CD34 (P = .03), and more than 5.7 × 104/kg of infused CFUs (P < .001) were statistically significant favorable factors for neutrophil engraftment (Table 2).
Figure 1.
Probability of engraftment, GVHD, and OS, and the impact of certain patient characteristics. (A) Probability of neutrophil engraftment. (B) Probability of platelet engraftment (50 000). (C) Probability of grades II to IV aGVHD, grades III to IV aGVHD, and cGVHD. (D) Probability of OS. (E) Impact of the donor-patient ethnicity matching on the OS; P = .05 in multivariate analysis. (F) Impact of performance status (80-100 vs < 80) on the OS; P < .001 in multivariate analysis.
Figure 2.
Impact of graft characteristics on the probability of engraftment and OS. Probability plots are shown for the each of the 4 quartiles. Panels A, E, and I depict the impact of cryopreserved TNCs (× 107/kg recipient weight) on neutrophil engraftment, platelet engraftment, and OS, respectively. Panels B, F, and J depict the impact of infused TNCs (× 107/kg recipient weight) on neutrophil engraftment, platelet engraftment, and OS, respectively. Panels C, G, and K depict the impact of infused CD34 cells (× 105/kg recipient weight) on neutrophil engraftment, platelet engraftment, and OS, respectively. Panels D, H, and L depict the impact of infused CFUs (× 104/kg recipient weight) on neutrophil engraftment, platelet engraftment, and OS, respectively. P values are shown with each plot for the quartile analysis. Further analyses of the impact of the graft characteristics above and below the median value on the engraftment and OS were conducted and are presented in Tables 2–5, and in the text of the paper.
Table 2.
Results of univariate and multivariate analyses of graft and patient factors influencing neutrophil engraftment
| Variable | Univariate analysis (includes only significant variables) |
Multivariate analysis (includes only significant variables) |
Favorable in multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | ||
| Age, y | |||||||
| Older than 2 | 0.52 | 0.36-0.73 | < .01 | 0.59 | 0.42-0.84 | .003 | Patients ≤ 2 y |
| 2 or younger | 1.00 | 1.00 | |||||
| Unit sex | |||||||
| Male | 1.57 | 1.13-2.19 | < .01 | 1.67 | 1.18-2.35 | .003 | Male units |
| Female | 1.00 | 1.00 | |||||
| Cryopreserved cell dose, × 107/kg | |||||||
| More than 9.7 | 1.74 | 1.25-2.43 | < .01 | ||||
| 9.7 or less | 1.00 | ||||||
| Reinfused cell dose, × 107/kg | |||||||
| More than 7.6 | 1.78 | 1.28-2.48 | < .01 | ||||
| 7.6 or less | 1.00 | ||||||
| Reinfused CD34, × 105/kg | |||||||
| More than 2.1 | 1.76 | 1.26-2.46 | < .01 | 1.46 | 1.03-2.08 | .03 | Higher CD34 |
| 2.1 or less | 1.00 | 1.00 | |||||
| Reinfused CFUs, × 104/kg | |||||||
| More than 5.7 | 3.04 | 2.14-4.33 | < .01 | 2.55 | 1.75-3.72 | < .001 | Higher CFUs |
| 5.7 or less | 1.00 | 1.00 | |||||
| Reinfused CD3, × 106/kg | |||||||
| More than 14.2 | 1.63 | 1.16-2.28 | < .01 | ||||
| 14.2 or less | 1.00 | ||||||
| HLA match | |||||||
| 5/6 or 6/6 | 1.44 | 1.02-2.01 | .04 | ||||
| 3/6 or 4/6 | 1.00 | ||||||
| Recipient CMV serostatus | |||||||
| Positive | 0.56 | 0.36-0.86 | < .01 | ||||
| Negative | 1.00 | ||||||
Variables not found to be statistically significant in univariate analysis include performance status (< 80, ≥ 80), recipient sex, unit sex, sex matching (recipient/unit), date of transplantation (after January 1, 2001, before January 1, 2001), recipient ethnicity, unit ethnicity, ethnicity matching (recipient/unit), ABO match, and recipient weight (< 12 kg, ≥ 12 kg).
The cumulative incidence of platelet engraftment (50 000) by day 180 was 71.0% (95% CI, 63.7%-78.3%) in a median of 87 days (range, 25-379 days; Figure 1B). In univariate analysis, platelet engraftment (Figure 2E-H) was influenced by patient age (P < .01), patient sex (P = .02), cryopreserved TNCs (P = .01), infused TNCs (P < .01), infused CD34 (P = .03), infused CFUs (P < .01), and ABO match (P = .04; Table 3). In multivariate analysis, recipients aged 2 years or younger at transplantation (P = .005), female patients (P = .03), and more than 5.7 × 104/kg of infused CFUs (P < .001) were favorable factors for platelet engraft-ment (Table 3).
Table 3.
Results of univariate and multivariate analyses of graft and patient factors influencing platelet engraftment
| Variable | Univariate analysis (includes only significant variables) |
Multivariate analysis (includes only significant variables) |
Favorable in multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | ||
| Age, y | |||||||
| Older than 2 | 0.46 | 0.31-0.67 | < .001 | 0.57 | 0.38-0.85 | < .01 | Patients ≤ 2 y |
| 2 or younger | 1.00 | 1.00 | |||||
| Recipient sex | |||||||
| Male | 0.64 | 0.45-0.93 | .02 | 0.65 | 0.44-0.95 | .03 | Female recipients |
| Female | 1.00 | 1.00 | |||||
| Cryopreserved cell dose, × 107/kg | |||||||
| More than 9.7 | 1.58 | 1.10-2.28 | .01 | ||||
| 9.7 or less | 1.00 | ||||||
| Reinfused cell dose, × 107/kg | |||||||
| More than 7.6 | 1.85 | 1.28-2.66 | < .01 | ||||
| 7.6 or less | 1.00 | ||||||
| Reinfused CD34, × 105/kg | |||||||
| More than 2.1 | 1.49 | 1.04-2.15 | .03 | ||||
| 2.1 or less | 1.00 | ||||||
| Reinfused CFU, × 104/kg | |||||||
| More than 5.7 | 3.42 | 2.33-5.02 | < .001 | 2.81 | 1.87-4.21 | < .001 | Higher CFU infused |
| 5.7 or less | 1.00 | 1.00 | |||||
| Reinfused CD3, × 106/kg | |||||||
| More than 14.2 | 1.45 | 1.01-2.09 | .05 | ||||
| 14.2 or less | 1.00 | ||||||
| ABO match | |||||||
| Mismatched | 1.47 | 1.02-2.13 | .04 | ||||
| Matched | 1.00 | ||||||
Variables not found to be statistically significant in univariate analysis include performance status (< 80, ≥ 80), unit sex, sex matching (recipient/unit), date of transplantation (after 1/1/2001, before 1/1/2001), HLA match (3/6 and 4/6 versus 5/6 and 6/6), recipient ethnicity, unit ethnicity, ethnicity matching (recipient/unit), recipient CMV status, and recipient weight (<12 kg, ≥ 12 kg).
GVHD
Among the 155 evaluable patients, 48 (31%) had grade II, 8 (5.1%) had grade III, and 8 (5.1%) had grade IV aGVHD. Skin alone was involved in 32 (66.7%) patients with grade II aGVHD, while all 16 patients with grade III or IV aGVHD had a combination of skin, gut, and/or liver involvement. The cumulative incidence of grades II to IV and grades III to IV aGVHD by day 100 was 40.0% (95% CI, 32.2%-47.8%) and 10.3% (95% CI, 5.4%-15.2%), respectively (Figure 1C). In the univariate and multivariate analysis, ABO mismatch (P = .01 and P < .01) was associated with increased incidence of grades II to IV aGVHD (Table 4). However, grades III to IV aGVHD was not influenced by any variable.
Table 4.
Results of univariate and multivariate analyses of graft and patient factors influencing aGVHD grades II to IV and cGVHD
| Variable | Univariate analysis (includes only significant variables) |
Multivariate analysis (includes only significant variables) |
Favorable in multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | ||
| aGVHD grades II-IV* | |||||||
| Date of transplantation | |||||||
| After January 1, 2001 | 2.07 | 0.99-4.35 | .05 | ||||
| Before January 1, 2001 | 1.000 | ||||||
| ABO match | |||||||
| Mismatched | 1.88 | 1.14-3.08 | .01 | 1.92 | 1.17-3.15 | < .01 | ABO matched |
| Matched | 1.00 | 1.00 | |||||
| cGVHD† | |||||||
| CD34, × 105/kg | |||||||
| More than 2.1 | 0.54 | 0.29-1.02 | .06 | 0.43 | 0.22-0.82 | .01 | CD34 > 2.1 |
| 2.1 or less | 1.00 | 1.00 | |||||
| Recipient ethnicity | |||||||
| Other | 2.50 | 1.19-5.24 | .02 | 3.40 | 1.6-7.45 | .002 | White patient |
| White | 1.00 | 1.00 | |||||
| ABO match | |||||||
| Mismatched | 2.09 | 1.12-3.90 | .02 | 2.36 | 1.3-4.45 | .008 | ABO matched |
| Matched | 1.00 | 1.00 | |||||
Grades II-IV aGVHD: Variables not found to be statistically significant in univariate analysis include performance status (< 80, ≥ 80), age (> 2 years, < 2 years), recipient sex, unit sex, sex matching (recipient/unit), cryopreserved cell dose × 107/kg (> 9.7, ≤ 9.7), reinfused cell dose × 107/kg (> 7.6, ≤ 7.6), reinfused CD34 × 105/kg (> 2.1, ≤ 2.1), reinfused CFUs × 104/kg (> 5.7, ≤ 5.7), reinfused CD3 × 106/kg (> 14.2, ≤ 14.2), recipient ethnicity, unit ethnicity, ethnicity matching (recipient/unit), HLA match (5/6 and 6/6, 3/6 and 4/6), recipient CMV serostatus, and recipient weight (< 12 kg, ≥ 12 kg).
cGVHD: Variables not found to be statistically significant in univariate analysis include performance status (< 80, ≥ 80), age (> 2 years, < 2 years), recipient sex, unit sex, cryopreserved cell dose × 107/kg (> 9.7, ≤ 9.7), reinfused cell dose × 107/kg (> 7.6, ≤ 7.6), reinfused CFUs × 104/kg (> 5.7, ≤ 5.7), reinfused CD3 × 106/kg (> 14.2, ≤ 14.2), date of transplantation (after 1/1/2001, before 1/1/2001), unit ethnicity, ethnicity matching (recipient/unit), HLA match (5/6 and 6/6, 3/6 and 4/6), recipient CMV serostatus, and recipient weight (< 12 kg, ≥ 12 kg).
cGVHD developed in 42 (21 with extensive cGVHD; 21 with limited cGVHD) of the 137 patients who survived more than 90 days (30.66%). The cumulative incidence of any cGVHD was 20.9% (95% CI, 14.2%-27.6%) and 28.8% (95% CI, 21.4%-36.2%) by 1 and 2 years after transplantation, respectively (Figure 1C). Extensive cGVHD occurred in 10.8% (95% CI, 5.7%-15.9%) at 1 year and 14.4% (95% CI, 8.7%-20.1%) at 2 years. In multivariate analysis, lower infused CD34 (P = .01), nonwhite patients (P < .01), and ABO mismatching (P < .01) were associated with an increased risk of cGVHD (Table 4). A total of 7 patients developed isolated autoimmune hemolytic anemia and/or cytopenia without other features of cGVHD.
Overall survival and causes of death
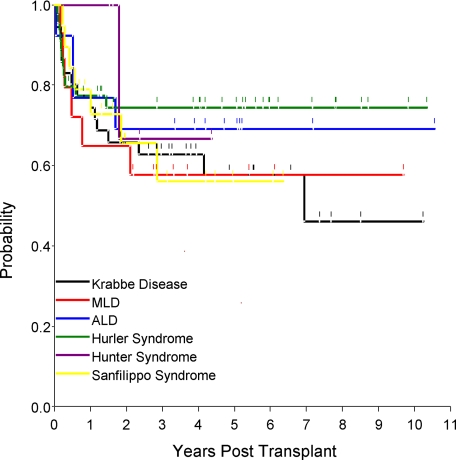
The estimated probability of overall survival (OS) at 180 days, 1 year, 3 years, and 5 years was 79.0% (95% CI, 72.6%-85.4%), 71.8% (95% CI, 64.7%-78.9%), 62.7% (95% CI, 54.8%-70.5%), and 58.2% (95% CI, 49.7%-66.6%), respectively (Figure 1D). In univariate analysis, performance status of 80 to 100 by Lansky (P < .001) and a higher infused CFUs (P < .01) were significantly associated with a higher probability of OS. In multivariate analysis, performance status (Figure 1F) of 80 to 100 (P < .001), infused CFUs greater than 5.7 × 104/kg (P = .02), and matched ethnicity (P = .05) increased OS (Table 5). In patients with high performance status (80-100), the OS at 6 months, 1 year, 3 years, and 5 years was 88.4% (95% CI, 79.6%-97.1%), 84.5% (95% CI, 77.0%-92.0%), 77.9% (95% CI, 69.1%-86.8%), and 75.7% (95% CI, 66.1%-85.3%), respectively. Kaplan-Meier estimates of probability of overall survival for various diagnoses were determined and are presented in Figure 3. The 1-year probability of overall survival (given within the parentheses after each disease) for Krabbe disease (74.5%), MLD (65.0%), ALD (76.9%), Hurler syndrome (77.3%), Hunter syndrome (100.0%), and Sanfilippo syndrome (78.9%) were similar. The 5-year survival among diseases—Krabbe disease (56.7%), MLD (57.8%), ALD (69.1%), Hurler syndrome (74.5%), Hunter syndrome (66.7%), and Sanfilippo syndrome (56.2%)—was also similar.
Table 5.
Result of univariate and multivariate analyses of graft and patient factors on overall survival (OS)
| OS Variable | Univariate analysis (includes only significant variables) |
Multivariate analysis (includes only significant variables) |
Favorable in multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | ||
| Performance status | |||||||
| Less than 80 | 3.72 | 2.17-6.36 | < .001 | 3.49 | 2.00-6.07 | < .001 | Lansky score 80-100 |
| 80 or more | 1.00 | 1.00 | |||||
| Reinfused CFUs, × 104/kg | |||||||
| More than 5.7 | 0.47 | 0.28-0.80 | .005 | 0.53 | 0.31-0.91 | .02 | CFUs > 5.7 |
| 5.7 or less | 1.00 | 1.00 | |||||
| HLA match | |||||||
| 5/6 or 6/6 | 0.62 | 0.37-1.04 | .07 | ||||
| 3/6 or 4/6 | 1.00 | ||||||
| Ethnicity matching | |||||||
| Mismatched | 1.70 | 0.93-3.10 | .09 | 1.84 | 1.00-3.38 | .05 | Matched ethnicity |
| Matched | 1.00 | 1.00 | |||||
Variables not found to be statistically significant in univariate analysis include performance status (< 80, ≥ 80), age (> 2 years, ≤ 2 years), recipient sex, unit sex, sex matching (recipient/unit), cryopreserved cell dose × 107/kg (> 9.7, ≤ 9.7), reinfused cell dose × 107/kg (> 7.6, ≤ 7.6), reinfused CD34 × 105/kg (> 2.1, ≤ 2.1), reinfused CFUs × 104/kg (> 5.7, ≤ 5.7), reinfused CD3 × 106/kg (> 14.2, ≤ 14.2), date of transplantation (after 1/1/2001, before 1/1/2001), recipient ethnicity, unit ethnicity, ethnicity matching (recipient/unit), ABO match, HLA match (5/6 and 6/6, 3/6 and 4/6), recipient CMV serostatus, and recipient weight (< 12 kg, ≥ 12 kg).
Figure 3.
Disease-specific Kaplan-Meier estimates of the probability of OS.
Of 62 total deaths, 45 (72.3%) were transplantation-related—8 (12.9%) from graft failure, 21 (33.9%) from organ failure, 13 (21.0%) from infection, and 3 (4.8%) from GVHD. Late deaths, generally related to progression or cGVHD, occurred in 11 patients. The causes of death in the study patients grouped according to high (Lansky score, 80-100) and low (Lansky score < 80) pretransplantation performance status is shown in Table 6. Of note, deaths due to progressive disease or organ failure were higher in the poor performance group. In the period between analysis of data in June 2007 and the manuscript submission, 3 additional patients have died (one each from cGVHD, central venous catheter sepsis, and progressive disease).
Table 6.
Causes of death in patients
| Primary causes of death | Performance status |
Total |
||||
|---|---|---|---|---|---|---|
| 80 to 100 |
Less than 80 |
|||||
| n | % | n | % | n | % | |
| Graft failure | 5 | 25.0 | 3 | 7.1 | 8 | 12.9 |
| Organ failure | 5 | 25.0 | 16 | 38.1 | 21 | 33.9 |
| Infection | 5 | 25.0 | 8 | 19.1 | 13 | 21.0 |
| Progressive disease | 1 | 5.0 | 9 | 21.4 | 10 | 16.1 |
| GVHD | 0 | 0.0 | 3 | 7.1 | 3 | 4.8 |
| Other | 4 | 20.0 | 3 | 7.1 | 7 | 11.3 |
| Total | 20 | 100.0 | 42 | 100.0 | 62 | 100.0 |
Grouped according to the high (Lansky score 80-100) and low (Lansky score < 80) performance status at the time of transplantation.
Clinical outcomes and follow-up
All engrafting patients, except 3, achieved donor chimerism of greater than 90%. All but 4 engrafting patients with diseases for which leukocyte or plasma enzyme level measurements exist achieved and sustained normal enzyme levels. All patients returned to our center on a yearly basis for evaluation of long-term clinical and developmental outcomes and late effects. The patients who underwent transplantation as newborns had better functional outcomes than those with early infantile forms of disease with progressive symptoms (those with poor performance status) at the time of transplantation.22 The latter group experienced disease stabilization and prolongation of life, without significant neurologic or functional improvement.22 Patients with juvenile, less rapidly progressive forms of disease, experienced greater clinical benefit, as reflected by their superior performance status, despite their higher age, at the time of transplantation. This report extends observations of clinical outcomes on 92 of 159 previously reported patients. With longer follow-up, those children benefiting in the short term have maintained improvements and continued to gain developmental milestones over 5 to 11 years after transplantation.
In this series, 45 patients with severe phenotype Hurler syndrome (MPS I) underwent transplantation and have now been followed for a median of 5.6 years (range, 1-11 years). All of the surviving patients have experienced disease stabilization and most continue to gain cognitive skills. All children of sufficient age attend school, with 81% placed in age-appropriate classes. Most of the patients with average IQ have required an aide in the classroom to help them attend to tasks. All but 2 children experienced stabilization or improvement of corneal clouding. Orthopedic problems have progressed in many children, with some requiring surgical correction. A total of 3 children had surgery for carpal tunnel syndrome, 4 for back or spine, 2 for hip problems, and 2 for knee problems. A total of 2 children have been treated with growth hormone for short stature and 2 (1 boy, 1 girl) have developed precocious puberty. A total of 2 children have also developed Hurler-associated retinal disease.
In contrast, of 19 children who underwent transplantation for MPS III, a phenotype with predominant central nervous system (CNS) involvement, 12 survived and 9 had disease stabilization with less impact in cognitive function. In the only 2 children who underwent transplantation at younger than 2 years of age, modest gains in cognitive skills continue to be observed 3 to 5 years after transplantation, although these children continue to have overall global developmental delay. Children who received transplants appear to have fewer behavioral problems and have better sleeping patterns as compared with children who did not receive transplants.
Only 2 of 5 children who underwent transplantation for infantile Tay Sachs disease are surviving long term (> 5 years). They have both stabilized, but neither gained skills after transplantation and both remain severely debilitated. One child who underwent transplantation as a newborn survived for 5 years and then died suddenly of unknown causes (autopsy denied by family). This child could sit with support, but could not stand or walk independently. One of the 2 children with juvenile Tay Sachs disease who underwent transplantation has survived 2 years. This child has stabilized motor function after transplantation.
One patient with Pelizaeus Merzbacher disease received a transplant at 9 months of age. He is now 2.5 years old and has experienced continued but slow functional gains in both cognitive and motor skills. Nerve conduction studies, which were abnormal before transplantation, have normalized. MRI shows progressive improvement in myelination. Nystagmus, involuntary movements, and ataxia have improved but not resolved. Vision and hearing are normal.
Discussion
We describe outcomes of a large series of predominantly small and young children with IMDs belonging to the lysosomal and peroxisomal disorders who underwent transplantation with UCBT at a single center after uniform cytoreduction and were followed for 1 to 11 years (median, 4.6 years). Important variables improving OS significantly were better performance status (P < .001), higher infused CFUs (P = .02), and matched ethnicity between the CBU and the recipient (P = .05). The cumulative incidence (87.1% by day 42) and speed (median, day 22) of neutrophil engraftment was higher and faster than previously reported in large cohort studies.27,28,42 Most patients (97%) achieved and sustained full donor chimerism (> 90%) and normalized enzyme levels where measurable. This high level of donor chimerism is better than those reported in the literature after unrelated bone marrow transplantation. Although detailed, disease-specific outcomes are not fully described in this report; all surviving children with good performance status at transplantation have experienced and sustained stabilization and/or improvements in cognitive and motor function after transplantation.
Only 13 (8.2%) of 159 patients in this series experienced autologous recovery or graft failure. This rate of graft failure in patients receiving transplants for IMDs compares favorably with previous reports using cord blood or bone marrow as the graft source. The low rate of graft failure may be related to improved cytoreduction with the addition of ATG to the preparative regimen. In addition, the patients in this UCBT series received relatively higher cell doses (median cryopreserved TNCs and infused TNCs of 9.73 × 107/kg and 7.57 × 107/kg, respectively) than those of varied age and size previously reported. Platelet engraftment was also accelerated, likely related to higher cell dosing.
An analysis of the impact of HLA matching on GVHD and survival could be studied in this group of patients who because of their diagnosis did not have relapse as a competing risk, and who also received very high cell doses from a single UCB graft. In this context, HLA matching approached significance (P = .07) as a predictor of OS, although it did not influence the incidence or severity of acute or chronic GVHD. It should be noted that the sample size in this series may be too small to fully appreciate the impact of HLA matching. This notion is supported by the observation that ethnic disparity between the donor and recipient was a significant predictor of chronic GVHD (P = .002) and OS (P = .05), as ethnicity may be a surrogate marker for HLA matching. Further analysis of the impact of HLA matching should continue to be examined both by analyses of high resolution matching and inclusion of other HLA loci (eg, HLA-C) in larger series of patients, perhaps through registry analyses.
Of interest are the observations that donor/recipient sex may influence UCB transplantation outcomes. In multivariate analyses in this patient cohort, neutrophil engraftment was higher in male patients and platelet engraftment was higher in patients who received a transplant from a female donor. It is also noteworthy that in multivariate analysis, boys had a better survival in the pediatric malignancy stratum of the COBLT trial. These observations may be clinically important although unproven in these smaller cohorts. One might speculate that disparities of the H-Y minor histocompatibility antigens may be responsible for these results. This hypothesis will have to be tested in a much larger series of patients to be accepted as valid.
The previously unreported observation that infused (postthaw) CFUs is the graft characteristic that best correlated with engraftment of both neutrophils and platelets as well as overall survival in multivariate analysis is very important. The fact that TNCs, viability, CD34, and CFUs were enumerated on all thawed CBUs in the same laboratory using standard operating procedures (SOPs), allowed these observations to emerge. Given the need for development of potency and release assay for cord blood, our data would suggest that postthaw CFUs could serve this purpose. Standardization and validation of the assay between cord blood banks and transplant center laboratories would be required. Studies looking at correlations between CFUs recovered from a thawed segment and CFUs from the bag are under way in our laboratory and by others, to determine whether former could be used as a product release assay in the future.
ABO mismatching was a significant predictor of both acute and chronic GVHD (P ≤ .01). This patient cohort also had a relatively higher incidence of autoimmune cytopenias, particularly in younger patients. We have seen high incidence of autoimmune cytopenia and hemolytic anemia in infants and young children undergoing UCBT.43 The mechanisms underlying the impact of ABO matching on these manifestations of GVHD is unclear, but should be the subject of future and larger studies. Most of the patients developed autoimmune hematologic problems during or after the tapering of immunosuppression (later in the course of transplantation). Alterations in thymic ontogeny by both cytoreduction and posttransplantation immunosuppressive therapy may explain the higher incidence of autoimmune problems in this younger patient cohort.
In the last 25 years, approximately 1000 HSCTs for inherited metabolic disorders have been reported.44 Most of this experience is with matched related bone marrow transplantation. A smaller number of patients have received transplantation from T cell–depleted mismatched related or matched unrelated adult bone marrow donors. Table 7 compares the outcome data from current series with those previously published for bone marrow transplantation (BMT) for Hurler and ALD. The rates of engraftment and OS at 2 years in a group of 40 patients with Hurler syndrome who receive transplants from unrelated bone marrow donors at 14 different centers were 62.5% and 49%, respectively.5 Of the survivors, approximately 30% had no donor cell engraftment. A retrospective analysis of 74 transplant recipients for Hurler syndrome from unrelated donor bone marrow or peripheral blood stem cells (PBSCs) performed at 16 centers revealed an “alive and engrafted” rate of less than 55% at a follow-up of 3.7 years, but a higher engraftment rate for UCBT patients. In a retrospective questionnaire-based analysis of 94 patients with ALD at 43 centers, of whom 52 received unrelated donor (83% bone marrow) transplantation, the probability of OS after unrelated donor transplantation was 53%.7,45 In another study of haploidentical bone marrow transplantation for Hurler disease, only 9 (35%) of 26 patients were engrafted and alive at a median follow-up of 4.6 years. In comparison, the probabilities of donor cell engraftment at 42 days and overall survival at 1, 3, and 5 years after transplantation in our group of patients who underwent unrelated UCBT were 87.1%, 79.0%, 71.8%, and 58.2%, respectively. In patients with high performance status (80-100), the OS at 1, 3, and 5 years was 84.5%, 77.9%, and 75.7%, respectively. Outcome data on a large series of patients who underwent transplantation at a single center with consistent cytoreduction and supportive care may reflect expertise in treating larger numbers of patients with these rare diagnoses. However, there may also be patient selection and referral pattern bias.
Table 7.
Outcomes of hematopoietic stem cell transplantation in patients with Hurler syndrome and adrenoleukodystrophy: comparison of our data with published reports
| Author | Center or group | Study years | Donor source (no. patients) | Median follow-up, y | OS after first transplantation, % | Engraftment, % | Patients achieving high (> 90%) donor chimerism, % |
|---|---|---|---|---|---|---|---|
| Hurler syndrome | |||||||
| Bolens et al45 | EBMT | 1994-2004 | R-BM/PBSC (49), R-CB (3), U-CB (20), U-BM/PBSC (70) | 3.3 | 57 | ||
| Peters et al5 | 14 centers | 1989-1994 | U-BM (40) | 1.3 | 49 at 2 y | 63 | |
| Peters et al6 | 13 centers | 1983-1995 | R-M-BM (carrier (13); normal (15)) | 7.3 | 75 at 5 y | 85 | 54.0 |
| R-MM-BM (carrier (23); normal (3)) | 4.6 | 53 at 5 y | 61 | 62.0 | |||
| Souillet et al46 | Lyon, France, single center | 1986-2001 | U-BM (15) | 4.7 | 82 | 3 of 17 autologous recovery or rejection | 47.1 |
| R-BM (10); R-CB (2) | 4.7 | 90 | 4 of 12 autologous recovery or rejection | 33.3 | |||
| Current study | Duke University | 1995-2007 | U-CB (45) | 5.8 | 77.3 at 1 y and 74.5 at 5 y | 88.9 | 88.9 |
| Adrenoleukodystrophy | |||||||
| Peters et al7 | 43 centers | 1982-1999 | R-M-BM (33); R-MM-BM (9) | 3.1 | 64 | 93 | |
| U-BM (40); U-CB (12) | 3.1 | 53 | 80 | ||||
| Current study | Duke University | 1995-2007 | U-CB (13) | 6 | 76.9 at 1 y and 69.1 at 5 y | 84.6 | 84.6 |
EBMT indicates European Group for Blood and Marrow Transplantation (Barcelona, Spain); R-BM, related bone marrow; R-M-BM, related matched bone marrow; R-MM-BM, related mismatched bone marrow; R-PBSC, related peripheral blood stem cell; R-CB, related cord blood; U-CB, unrelated cord blood; and U-BM, unrelated bone marrow.
Published and current data confirm that HSCT, including UCBT, is effective in the treatment for some inherited metabolic diseases. However, the procedure carries significant risks due to the effects of preparative regimens and donor-host immunologic interactions. The late effects of administration of chemotherapy at an early age and the “natural history” of these diseases in transplant recipients are not known at the present time. Development of strategies to reduce early and late toxicities of transplantation therapy, including the use of reduced intensity cytoreduction without compromising engraftment, may decrease morbidity and mortality in the future.
The mechanisms through which allogeneic HSCT correct IMDs are only partially understood. It is clear that after engraftment of nonaffected donor cells, enzyme can be replaced on a permanent basis. It is also known that donor-derived glial cells engraft in the brain, providing a sustained source of enzyme replacement in the CNS. Effects on the peripheral nervous system are less well understood and may not be as complete as those in the CNS. Differences in correction of disease in nonhematopoietic organs between different cell sources (eg, bone marrow vs UCB) have not been formally studied. There is an impression that UCBT results in improved neurocognitive and orthopedic outcomes. Demonstration of engraftment and nonhematopoietic differentiation of UCB cells in brain and heart, as well as preclinical and animal studies demonstrating propagation of cells of various lineages, including pancreas, liver, bone, cartilage, neurons, oligodendrocytes, retinal cells, and cardiac myocytes, raises the possibility that cord blood cells may serve as one of many sources of cells used in tissue repair and regeneration.
In conclusion, UCBT is an effective therapy for children with otherwise lethal inborn errors of metabolism. The rapid availability of donor cells allows the patients to proceed to transplantation within a few weeks of diagnosis. With the institution of newborn screening for Krabbe disease (New York State began the first pilot study on August 8, 2006) some infants will be diagnosed in the first month of life, before the disease has caused major neurologic damage, allowing for early transplantation therapy. Earlier transplantation in patients with IMDs when they have a better performance status is associated with the best survival and clinical outcomes. Infused CFUs is the best graft parameter predicting engraftment and OS. Cord blood is an ideal donor source for these underserved patients with orphan diseases who are young and physically small and therefore can receive a high cell dose from a single CBU and can benefit from rapid intervention.
Acknowledgments
The authors thank Sophia Avrutsky for her consistent performance and overall dedication in plating and counting CFUs for more than 20 years; Hutton Kearney for helping collect the chimerism data; Randall Fehdrau for providing the database of busulfan pharmacokinetics; Drs Jerry Thompson, Cynthia Tifft, John Hopwood, David Wenger, and Ed Kolodny for assaying enzyme levels and performing mutational analysis in these patients and their cord blood donors; the staff of the Pediatric Blood and Marrow Transplant Program for providing excellent care to these patients; and the patients and their families. We dedicate this manuscript to the memory of Dr William Krivit, who was a pioneer in this field and inspired our work.
Partial financial support was provided by the National Heart, Lung, and Blood Institute (Bethesda, MD) of the National Institutes of Health (grant no. N01-HB-67138).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.K.P. conceptualized the study, collected clinical data, analyzed data, wrote the manuscript, and performed all the coordination efforts; J.K. conceptualized the study, analyzed data, collected clinical data, edited the manuscript, and made a direct contribution in the generation of graft data; A.M. and S.C. were involved in statistical analysis, review of the data, and preparation of the manuscript; S.H.P., P.S., T.A.D., K.P., S.L., J.A., S.W., D.S., and P.L.M. collected clinical data and prepared the manuscript; and M.L.E. performed neurodevelopmental assessment and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vinod K. Prasad, PBMT Division, Box 3350, Duke University Medical Center, Durham, NC 27710; e-mail: vinod.prasad@duke.edu.
References
- 1.Vellodi A. Lysosomal storage disorders. Br J Haematol. 2005;128:413–431. doi: 10.1111/j.1365-2141.2004.05293.x. [DOI] [PubMed] [Google Scholar]
- 2.Whitley CB, Belani KG, Chang PN, et al. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genetics. 1993;46:209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 3.Vellodi A, Young E, New M, Pot-Mees C, Hugh-Jones K. Bone marrow transplantation for Sanfilippo disease type B. J Inherited Metabol Dis. 1992;15:911–918. doi: 10.1007/BF01800232. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro E, Krivit W, Lockman L, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356:713–718. doi: 10.1016/S0140-6736(00)02629-5. [DOI] [PubMed] [Google Scholar]
- 5.Peters C, Balthazor M, Shapiro EG, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 6.Peters C, Shapiro EG, Anderson J, et al. Hurler syndrome. II: outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 7.Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 8.Miano M, Lanino E, Gatti R, et al. Four year follow-up of a case of fucosidosis treated with unrelated donor bone marrow transplantation. Bone Marrow Transplant. 2001;27:747–751. doi: 10.1038/sj.bmt.1702994. [DOI] [PubMed] [Google Scholar]
- 9.Malm G, Ringden O, Winiarski J, et al. Clinical outcome in four children with metachromatic leukodystrophy treated by bone marrow transplantation. Bone Marrow Transplant. 1996;17:1003–1008. [PubMed] [Google Scholar]
- 10.Krivit W, Pierpont ME, Ayaz K, et al. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI): biochemical and clinical status 24 months after transplantation. N Engl J Med. 1984;311:1606–1611. doi: 10.1056/NEJM198412203112504. [DOI] [PubMed] [Google Scholar]
- 11.Krivit W, Shapiro E, Kennedy W, et al. Treatment of late infantile metachromatic leukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322:28–32. doi: 10.1056/NEJM199001043220106. [DOI] [PubMed] [Google Scholar]
- 12.Krivit W, Shapiro EG, Peters C, et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N Engl J Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs JR, Hugh-Jones K, Barrett AJ, et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet. 1981;2:709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- 14.Guffon N, Souillet G, Maire I, et al. Juvenile metachromatic leukodystrophy: neurological outcome two years after bone marrow transplantation. J Inherited Metabol Dis. 1995;18:159–161. doi: 10.1007/BF00711755. [DOI] [PubMed] [Google Scholar]
- 15.Aubourg P, Blanche S, Jambaque I, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322:1860–1866. doi: 10.1056/NEJM199006283222607. [DOI] [PubMed] [Google Scholar]
- 16.Prasad VK, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41:99–108. doi: 10.1038/sj.bmt.1705970. [DOI] [PubMed] [Google Scholar]
- 17.Cogle CR, Yachnis AT, Laywell ED, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004;363:1432–1437. doi: 10.1016/S0140-6736(04)16102-3. [DOI] [PubMed] [Google Scholar]
- 18.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 19.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JN, Davies SM, Defor T, et al. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Kurtzberg J, Gluckman E, et al. Umbilical cord blood hematopoietic stem and repopulating cells in human clinical transplantation. Blood Cells. 1991;17:313–329. [PubMed] [Google Scholar]
- 22.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 24.Gluckman E. Bone marrow transplantation in children with hereditary disorders [review]. Curr Opin Pediatr. 1996;8:42–44. doi: 10.1097/00008480-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 26.Martin PL, Carter SL, Kernan NA, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 30.Beam D, Poe MD, Provenzale JM, et al. Outcomes of unrelated umbilical cord blood transplantation for x-linked adrenoleukodystrophy. Biol Blood Marrow Transplant. 2007;13:665–674. doi: 10.1016/j.bbmt.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 31.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 32.Wenger DA, Williams C. Screening for lysosomal disorders. In: Hommes FA, editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. New York: Wiley-Liss; 1991. pp. 587–617. [Google Scholar]
- 33.DeGasperi R, Raghavan SS, Sosa MG, et al. Measurements from normal umbilical cord blood of four lysosomal enzymatic activities: alpha-L-iduronidase (Hurler), galactocerebrosidase (globoid cell leukodystrophy), arylsulfatase A (metachromatic leukodystrophy), arylsulfatase B (Maroteaux-Lamy). Bone Marrow Transplant. 2000;25:541–544. doi: 10.1038/sj.bmt.1702185. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 36.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 38.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 39.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 40.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 41.Cox DR. Regression models and life-tables. J Royal Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 42.Wall DA, Carter SL, Kernan NA, et al. Busulfan/melphalan/antithymocyte globulin followed by unrelated donor cord blood transplantation for treatment of infant leukemia and leukemia in young children: the Cord Blood Transplantation study (COBLT) experience. Biol Blood Marrow Transplant. 2005;11:637–646. doi: 10.1016/j.bbmt.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Page KM, Wood S, Prasad VK, Szabolcs P, Kurtzberg J. Post transplant autoimmune hemolytic anemia and other cytopenias are increased in young babies undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2007;13:64. doi: 10.1016/j.bbmt.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boelens JJ. Trends in haematopoietic cell transplantation for inborn errors of metabolism [review]. J Inherited Metabol Dis. 2006;29:413–420. doi: 10.1007/s10545-005-0258-8. [DOI] [PubMed] [Google Scholar]
- 45.Boelens JJ, Wynn RF, O'Meara A, et al. Outcomes of hematopoietic stem cell transplantation for Hurler's syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40:225–233. doi: 10.1038/sj.bmt.1705718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souillet G, Guffon N, Maire I, et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]