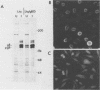
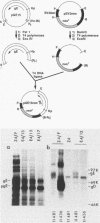
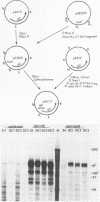
Abstract
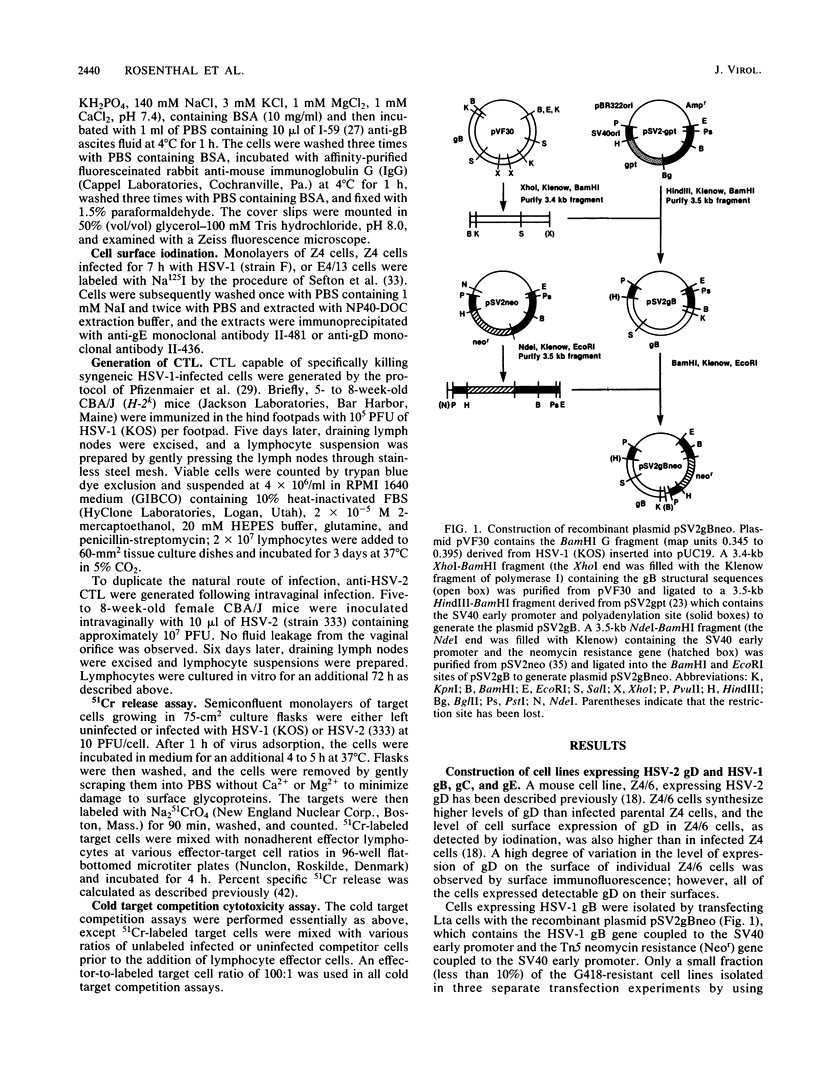
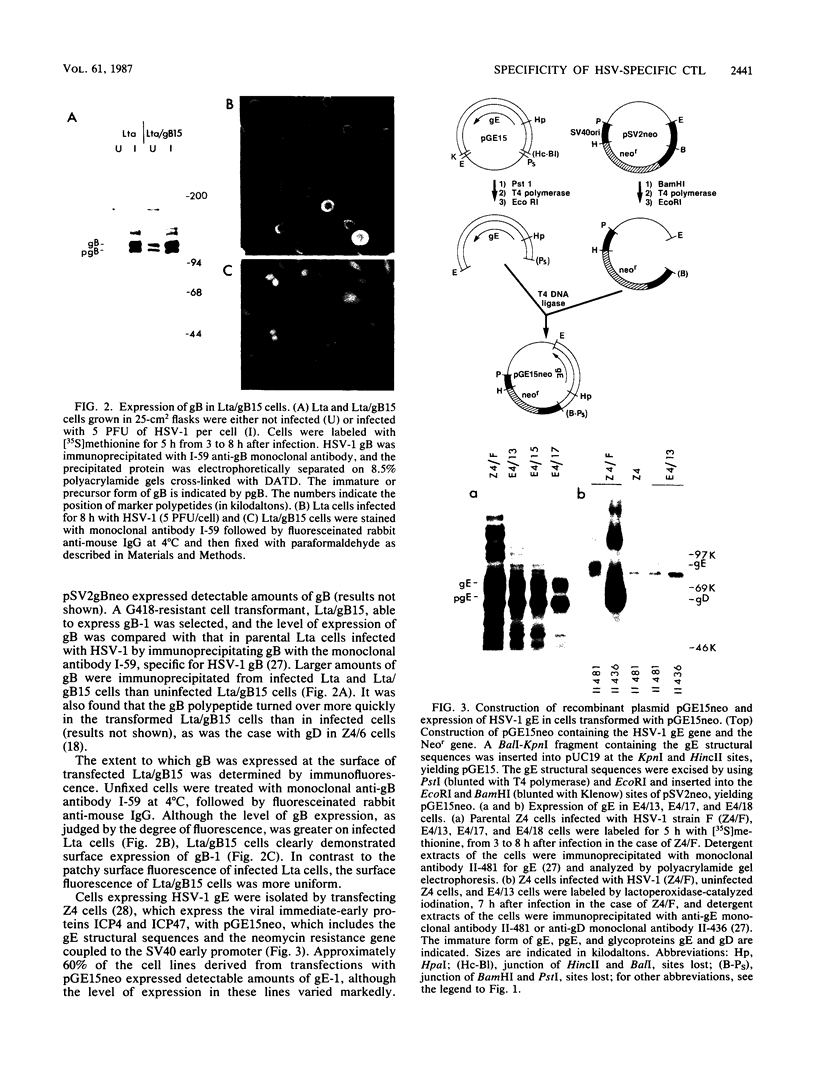
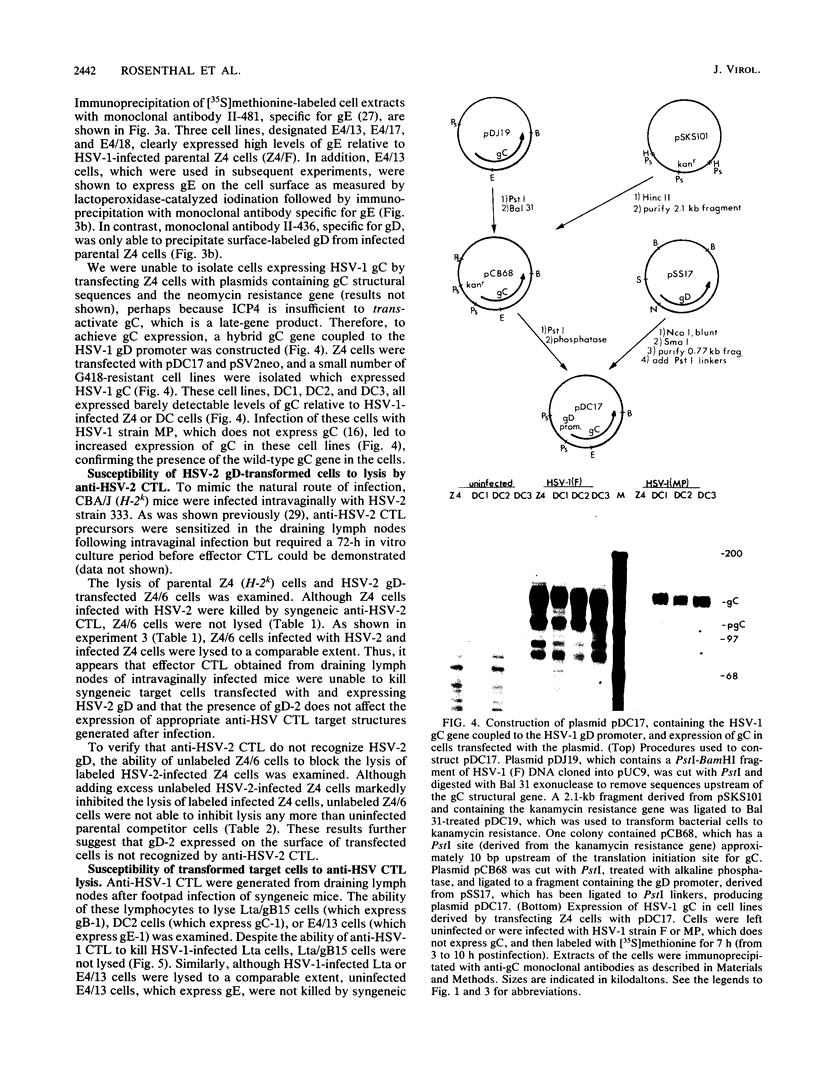
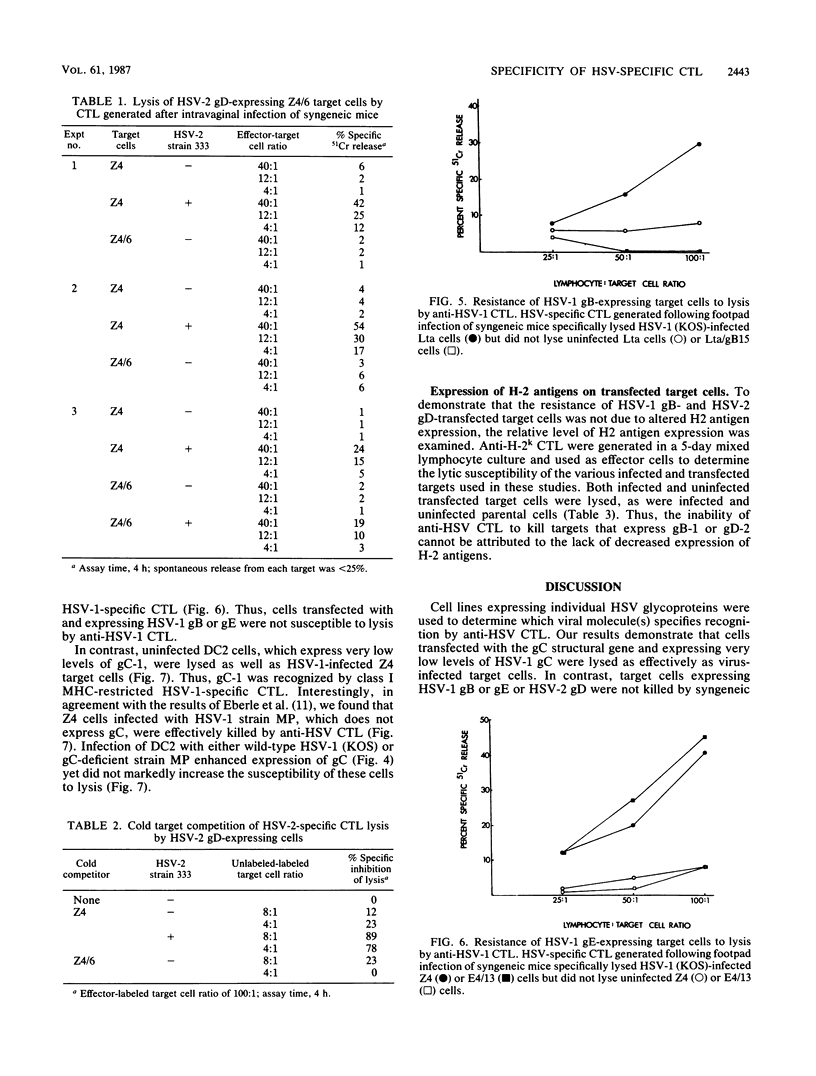
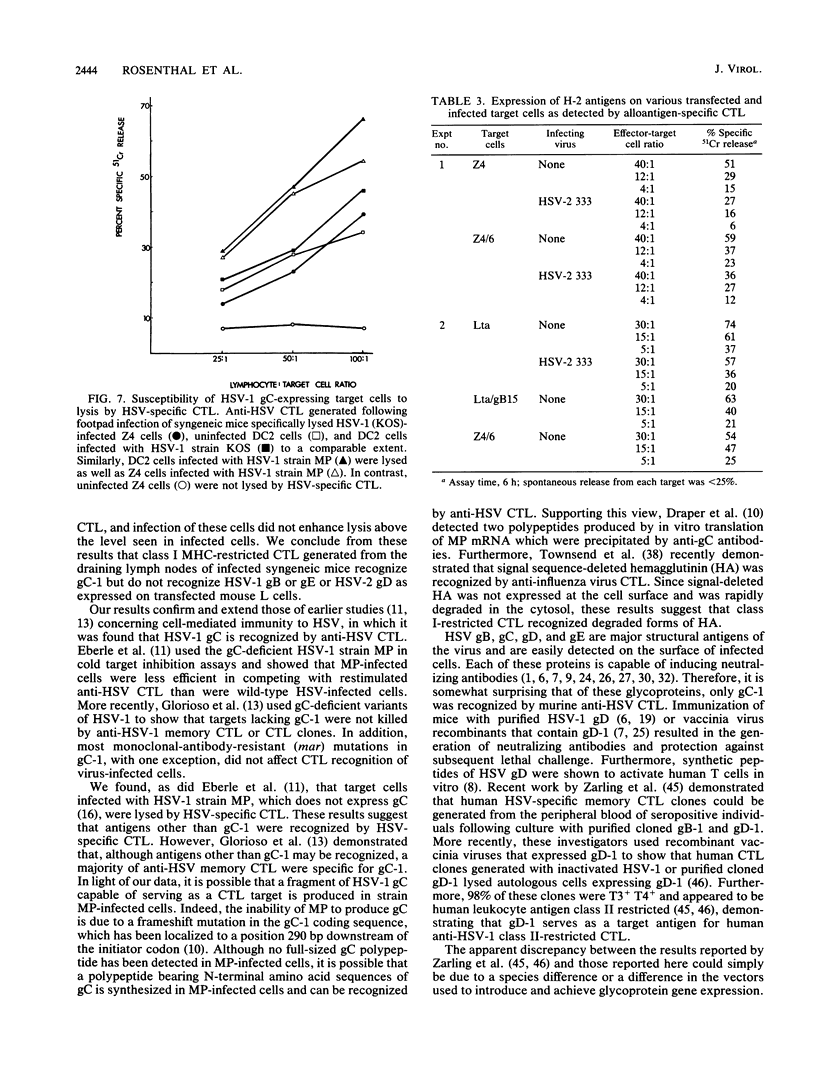
To determine which viral molecule(s) is recognized by herpes simplex virus (HSV)-specific cytotoxic T lymphocytes (CTL), target cells were constructed which express individual HSV glycoproteins. A mouse L cell line, Z4/6, which constitutively expressed high levels of HSV type 2 (HSV-2) gD (gD-2) was isolated and characterized previously (D. C. Johnson and J. R. Smiley, J. Virol. 54:682-689, 1985). Despite the expression of gD on the surface of Z4/6 cells, these cells were not killed by anti-HSV-2 CTL generated following intravaginal infection of syngeneic mice. In contrast, parental Z4 or Z4/6 cells infected with HSV-2 were lysed. Furthermore, unlabeled Z4/6 cells were unable to block the lysis of HSV-2-infected labeled target cells. Cells which express HSV-1 gB (gB-1) were isolated by transfecting L cells with the recombinant plasmid pSV2gBneo, which contains the HSV-1 gB structural sequences and the neomycin resistance gene coupled to the simian virus 40 early promoter and selecting G418-resistant cell lines. One such cell line, Lta/gB15, expressed gB which was detected by immunoprecipitation and at the cell surface by immunofluorescence. Additionally, cells expressing HSV-1 gC (gC-1) or gE (gE-1) were isolated by transfecting Z4 cells, which are L cells expressing ICP4 and ICP47, with either the recombinant plasmid pGE15neo, which contains the gE structural sequences and the neomycin resistance gene, or pDC17, which contains the gC structural gene coupled to the gD-1 promoter. A number of G418-resistant cell lines were isolated which expressed gC-1 or gE-1 at the cell surface. Anti-HSV-1 CTL generated following footpad infection of syngeneic mice were unable to lyse target cells expressing gB-1 or gE-1. In contrast, target cells expressing very low levels of gC-1 were killed as well as HSV-1-infected target cells. Furthermore, infection of gC-1-transformed target cells with wild-type HSV-1 or a strain of HSV-1 that does not express gC did not result in a marked increase in susceptibility to lysis. These results suggest that murine class I major histocompatibility complex-restricted anti-HSV CTL recognize gC-1 but do not recognize gB, gD, or gE as these molecules are expressed in transfected syngeneic target cells. The results are discussed in terms of recent evidence concerning the specificity of antiviral CTL.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Carter V. C., Rice P. L., Tevethia S. S. Intratypic and intertypic specificity of lymphocytes involved in the recognition of herpes simplex virus glycoproteins. Infect Immun. 1982 Jul;37(1):116–126. doi: 10.1128/iai.37.1.116-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- DeFreitas E. C., Dietzschold B., Koprowski H. Human T-lymphocyte response in vitro to synthetic peptides of herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1985 May;82(10):3425–3429. doi: 10.1073/pnas.82.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Costa R. H., Lee G. T., Spear P. G., Wagner E. K. Molecular basis of the glycoprotein-C-negative phenotype of herpes simplex virus type 1 macroplaque strain. J Virol. 1984 Sep;51(3):578–585. doi: 10.1128/jvi.51.3.578-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Russell R. G., Rouse B. T. Cell-mediated immunity to herpes simplex virus: recognition of type-specific and type-common surface antigens by cytotoxic T cell populations. Infect Immun. 1981 Dec;34(3):795–803. doi: 10.1128/iai.34.3.795-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Marsden H. S., McGeoch D. J. Novel herpes simplex virus type 1 glycoproteins identified by antiserum against a synthetic oligopeptide from the predicted product of gene US4. J Gen Virol. 1986 Apr;67(Pt 4):745–751. doi: 10.1099/0022-1317-67-4-745. [DOI] [PubMed] [Google Scholar]
- Glorioso J., Kees U., Kümel G., Kirchner H., Krammer P. H. Identification of herpes simplex virus type 1 (HSV-1) glycoprotein gC as the immunodominant antigen for HSV-1-specific memory cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):575–582. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R. H., Bacchetti S., Smiley J. R. Cells that constitutively express the herpes simplex virus immediate-early protein ICP4 allow efficient activation of viral delayed-early genes in trans. J Virol. 1985 May;54(2):414–421. doi: 10.1128/jvi.54.2.414-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Type-specific delayed hypersensitivity and protective immunity induced by isolated herpes simplex virus glycoprotein. J Immunol. 1983 Mar;130(3):1413–1418. [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Brandis H. Specifically immune mouse T-cells can destroy H-2 compatible murine target cells infected with herpes simplex virus types 1 or 2. Z Immunitatsforsch Immunobiol. 1977 Jul;153(2):162–173. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984 Aug 1;160(2):552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Paetkau V., Rawls W. E., Rosenthal K. L. Abrogation of anti-Pichinde virus cytotoxic T cell memory by cyclophosphamide and restoration by coinfection or interleukin 2. J Immunol. 1985 Aug;135(2):1401–1407. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Rosenthal K. L. Experiments and speculation on antiviral specificity of T and B cells. Immunol Rev. 1981;58:131–155. doi: 10.1111/j.1600-065x.1981.tb00352.x. [DOI] [PubMed] [Google Scholar]