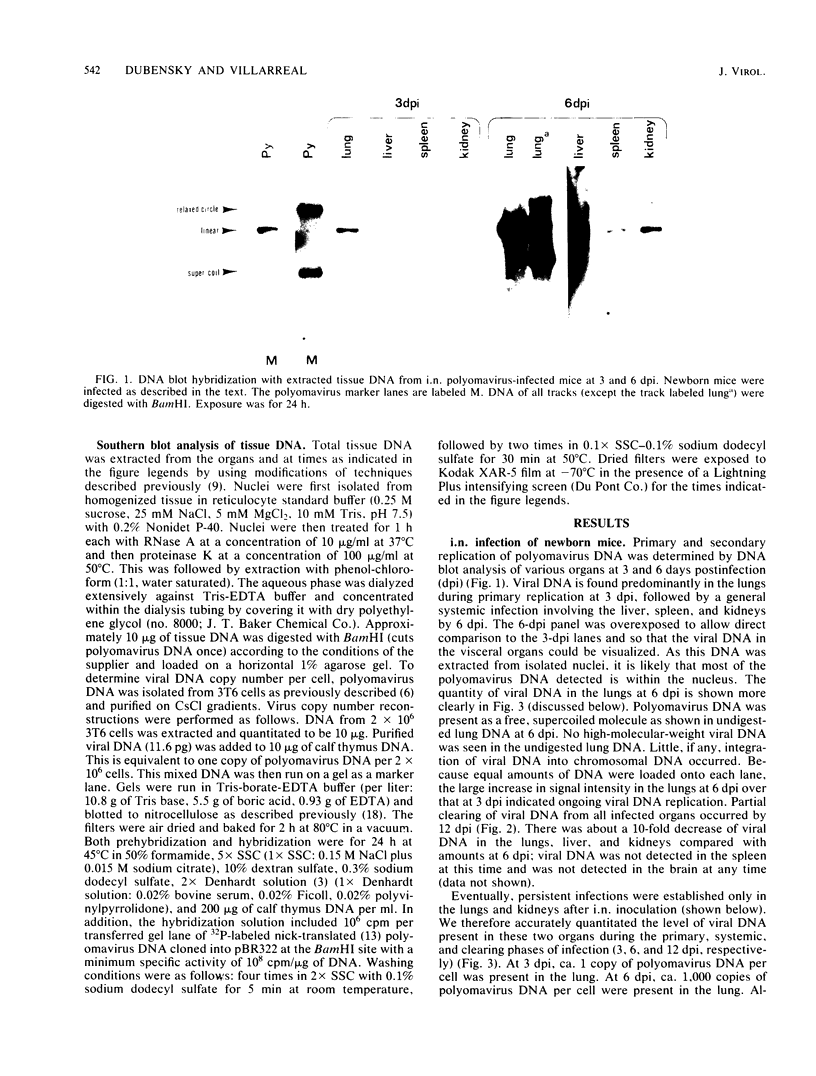
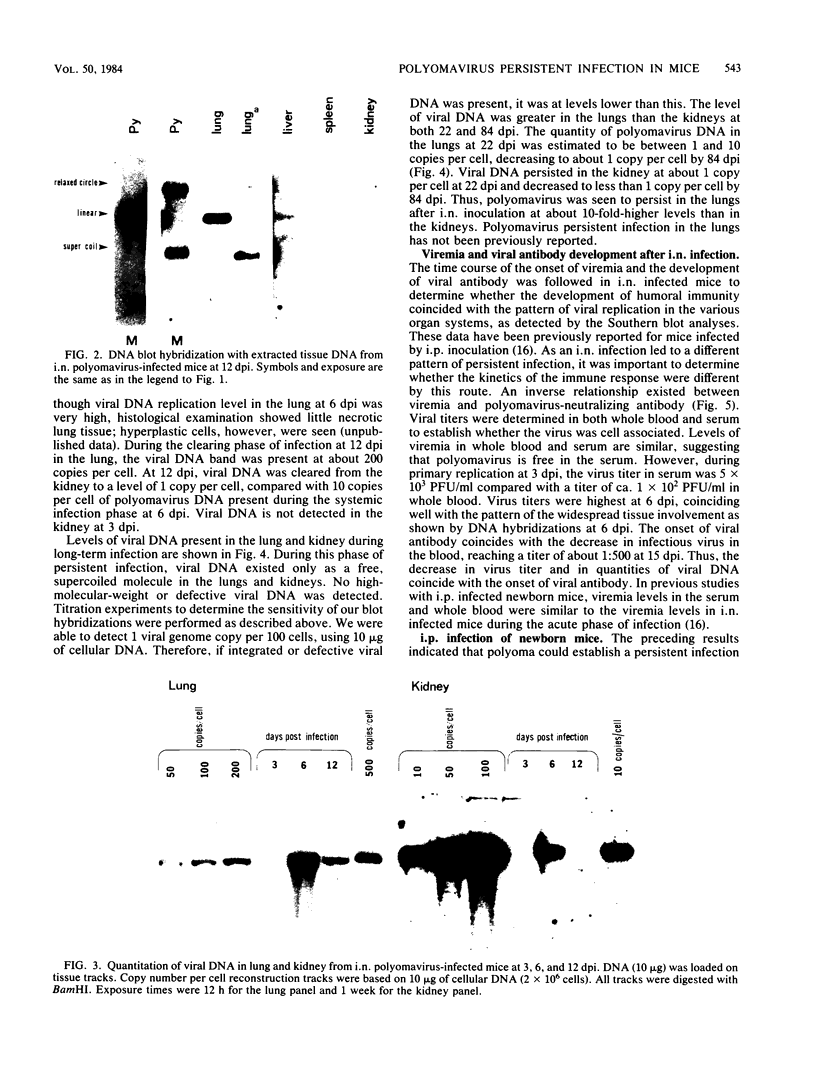
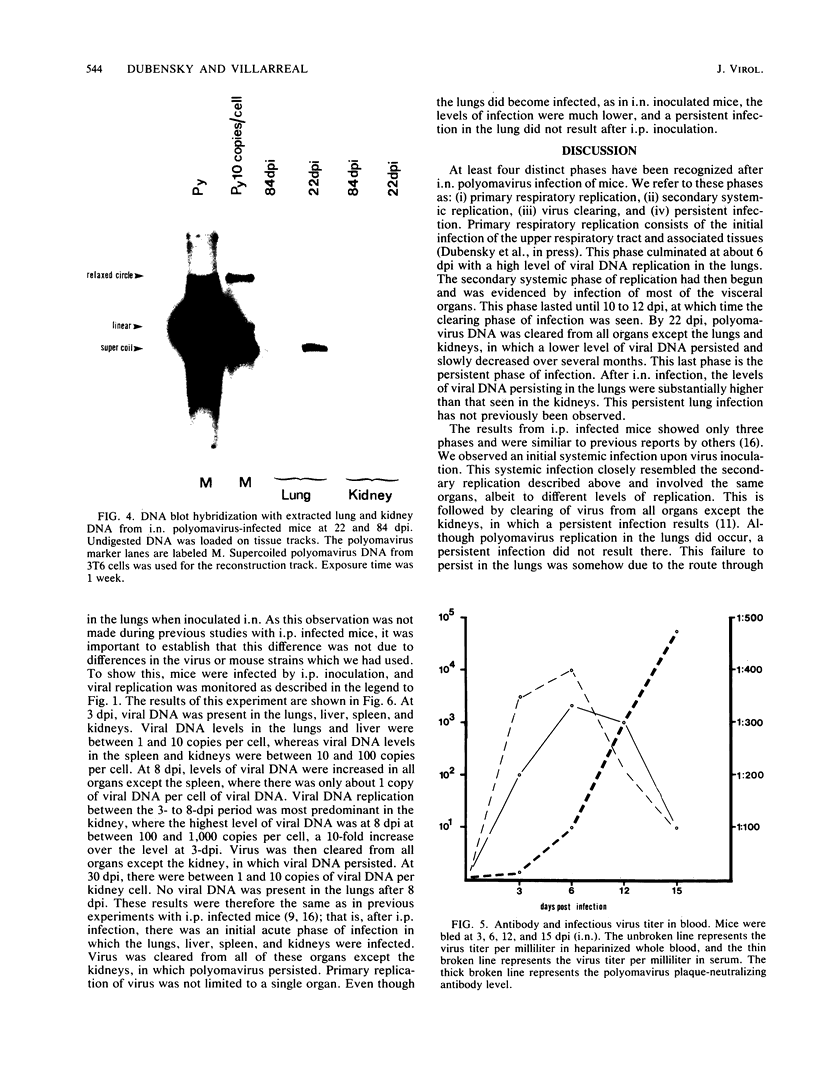
Abstract
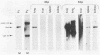
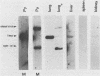
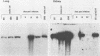
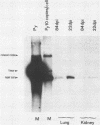
Using DNA blot analysis, we monitored the course of polyomavirus infection in mice receiving an intranasal inoculation and compared this with the course of infection in mice receiving an intraperitoneal inoculation. Intranasal infection was characterized by an initial primary replication phase in the respiratory tract, followed by a systemic infection of the visceral organs. At 12 days postinfection, there was partial clearing of viral DNA in all organs; by 22 days postinfection, viral DNA persisted only in the lungs and kidneys, and the level of DNA slowly decreased during the next 3 months. Lungs have been a previously unrecognized site for polyomavirus persistent infection. In contrast to intranasal infection, intraperitoneal infection of mice was characterized by only three phases: an initial systemic phase in which viral DNA was found in the same respiratory and visceral organs as during intranasal infection, clearing of the virus from the organs, and ultimately, a persistent infection in the kidneys but not in the lungs. Thus, different organs became persistently infected when mice were inoculated via these different routes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Gaugas J. M., Allison A. C., Chesterman F. C., Rees R. J., Hirsch M. S. Immunological control of polyoma virus oncogenesis in mice. Br J Cancer. 1973 Jan;27(1):10–17. doi: 10.1038/bjc.1973.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Law L. W. Studies of the significance of tumor antigens in induction and repression of neoplastic diseases: presidential address. Cancer Res. 1969 Jan;29(1):1–21. [PubMed] [Google Scholar]
- McCance D. J. Growth and persistence of polyoma early region deletion mutants in mice. J Virol. 1981 Sep;39(3):958–962. doi: 10.1128/jvi.39.3.958-962.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Reactivation of polyoma virus in kidneys of persistently infected mice during pregnancy. Infect Immun. 1979 Sep;25(3):998–1002. doi: 10.1128/iai.25.3.998-1002.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Transplacental transmission of polyoma virus in mice. Infect Immun. 1977 Oct;18(1):196–202. doi: 10.1128/iai.18.1.196-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Growth curves of polyoma virus in mice and hamsters. Natl Cancer Inst Monogr. 1960 Sep;4:189–209. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P. The epidemiology of mouse polyoma virus infection. Bacteriol Rev. 1961 Mar;25:18–31. doi: 10.1128/br.25.1.18-31.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- STEWART S. E., EDDY B. E., BORGESE N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. J Natl Cancer Inst. 1958 Jun;20(6):1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Pizer L. I., Moorhead J. W. Tolerance and suppression of immunity to herpes simplex virus: different presentations of antigens induce different types of suppressor cells. Infect Immun. 1983 May;40(2):514–522. doi: 10.1128/iai.40.2.514-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]