Abstract
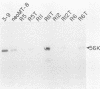
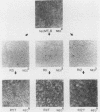
We applied the Luria and Delbruck fluctuation test to analyze high-frequency changes in the phenotype of rat cells transformed by a plasmid carrying the polyomavirus middle T (pmt) gene. All of the transformed cell lines analyzed were capable of switching to the normal state with rates ranging from 10(-3) to 10(-2) per cell per generation. Analysis of both middle T antigen and middle T transcripts indicated that the reversion occurred by a mechanism involving a transcriptional block of the pmt locus. Cell lines containing two separate loci reverted with a lower rate, suggesting that phenotypic switching in these cells involved two independent events affecting each locus. The flat revertants mutated to the transformed state with rates in the range of 10(-5) to 5 X 10(-5) per cell per generation. To determine whether changes in pmt expression would affect neighboring sequences, we transfected a hybrid plasmid carrying pmt linked to the neo marker and selected either for morphological transformants or for G418-resistant cells. Although their coordinate regulation was not absolute, both genes were usually subject to the same changes, reflected by loss and reacquisition of transcriptional activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouchard L., Vass-Marengo J., Bastin M. Expression of the malignant phenotype in rat fibroblasts transfected with the polyomavirus transforming genes. Virology. 1986 Nov;155(1):1–12. doi: 10.1016/0042-6822(86)90162-5. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara M. M., Drevon C., Harcourt S. A., Steingrimsdottir H., James M. R., Burke J. F., Arlett C. F., Lehmann A. R. Inactivation of a transfected gene in human fibroblasts can occur by deletion, amplification, phenotypic switching, or methylation. Mol Cell Biol. 1987 Apr;7(4):1459–1464. doi: 10.1128/mcb.7.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Searle S., Gillespie D. A., Bissell M., Wyke J. A. Expression of integrated Rous sarcoma viruses: DNA rearrangements 5' to the provirus are common in transformed rat cells but not seen in infected but untransformed cells. EMBO J. 1986 Apr;5(4):707–711. doi: 10.1002/j.1460-2075.1986.tb04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas C., Bastin M. Malignant transformation of rat cells by the polyomavirus middle T gene. Virology. 1985 Oct 30;146(2):233–245. doi: 10.1016/0042-6822(85)90007-8. [DOI] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Dulbecco R. Characterization of polyoma virus T antigen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1259–1263. doi: 10.1073/pnas.74.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Shibuya M., Samarut J., Hanafusa H. Revertants and partial transformants of rat fibroblasts infected with Fujinami sarcoma virus. J Virol. 1984 May;50(2):325–334. doi: 10.1128/jvi.50.2.325-334.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzig K. J., Robbins K. C., Aaronson S. A. Cellular regulation of mammalian sarcoma virus expression: a gene regulation model for oncogenesis. Cell. 1979 Apr;16(4):875–884. doi: 10.1016/0092-8674(79)90102-8. [DOI] [PubMed] [Google Scholar]
- Roginski R. S., Skoultchi A. I., Henthorn P., Smithies O., Hsiung N., Kucherlapati R. Coordinate modulation of transfected HSV thymidine kinase and human globin genes. Cell. 1983 Nov;35(1):149–155. doi: 10.1016/0092-8674(83)90217-9. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. B., Hayday A., Courtneidge S., Fried M. A frameshift at a mutational hotspot in the polyoma virus early region generates two new proteins that define T-antigen functional domains. Cell. 1986 Feb 14;44(3):477–487. doi: 10.1016/0092-8674(86)90469-1. [DOI] [PubMed] [Google Scholar]