Abstract
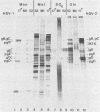
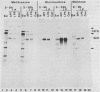
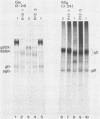
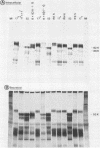
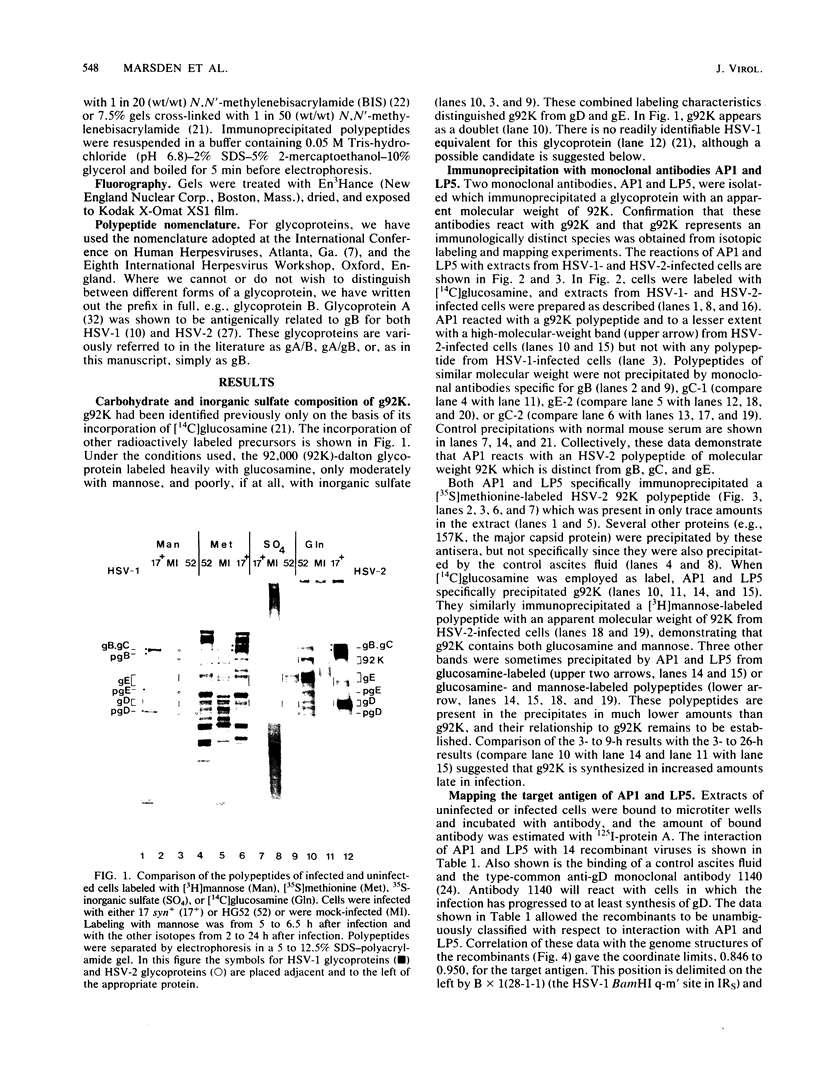
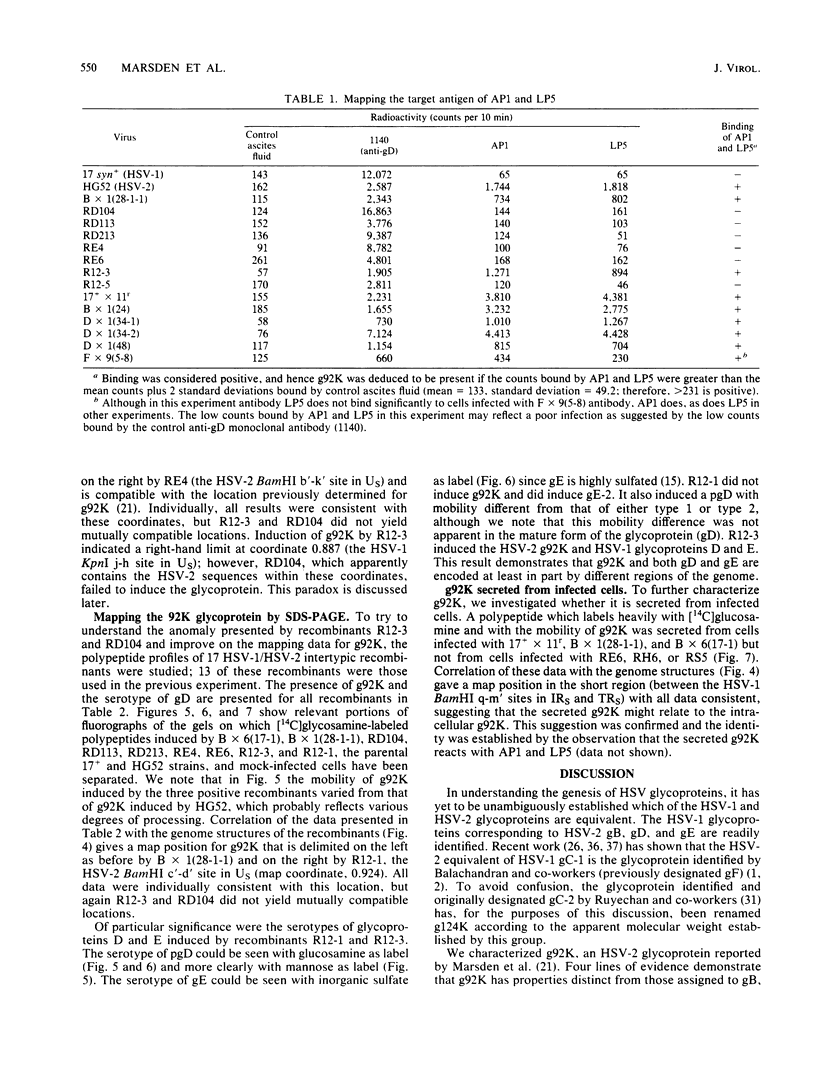
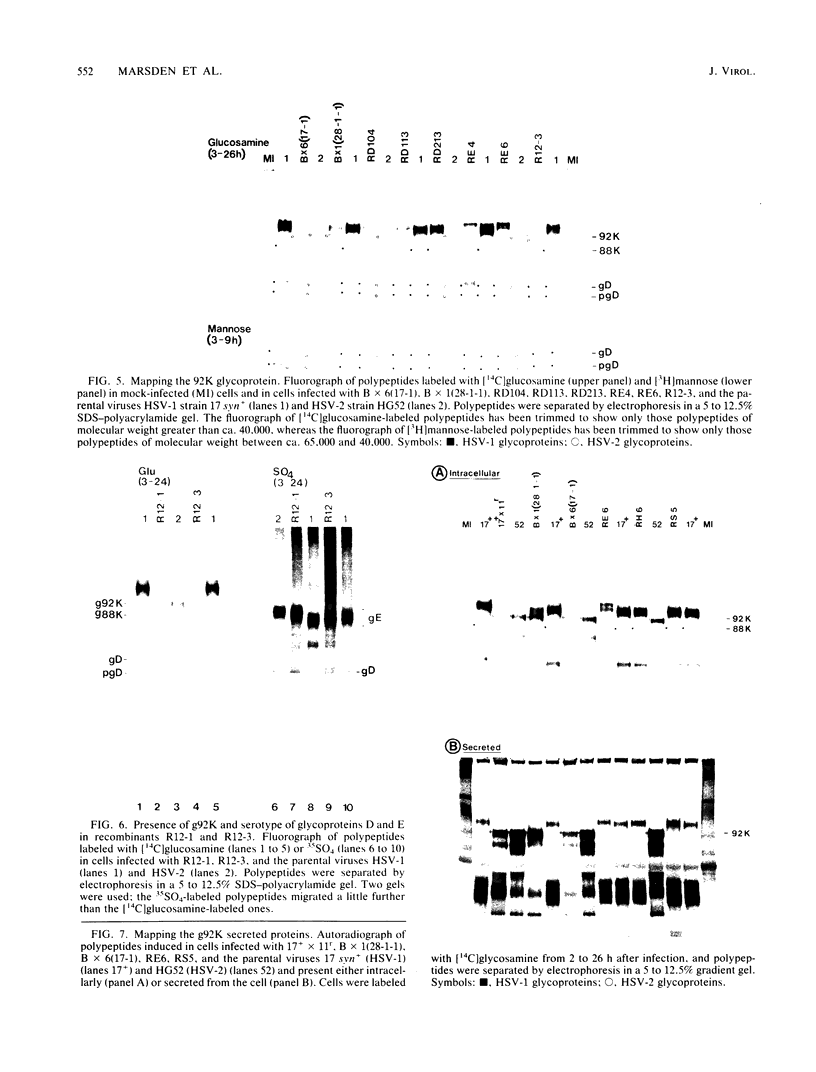
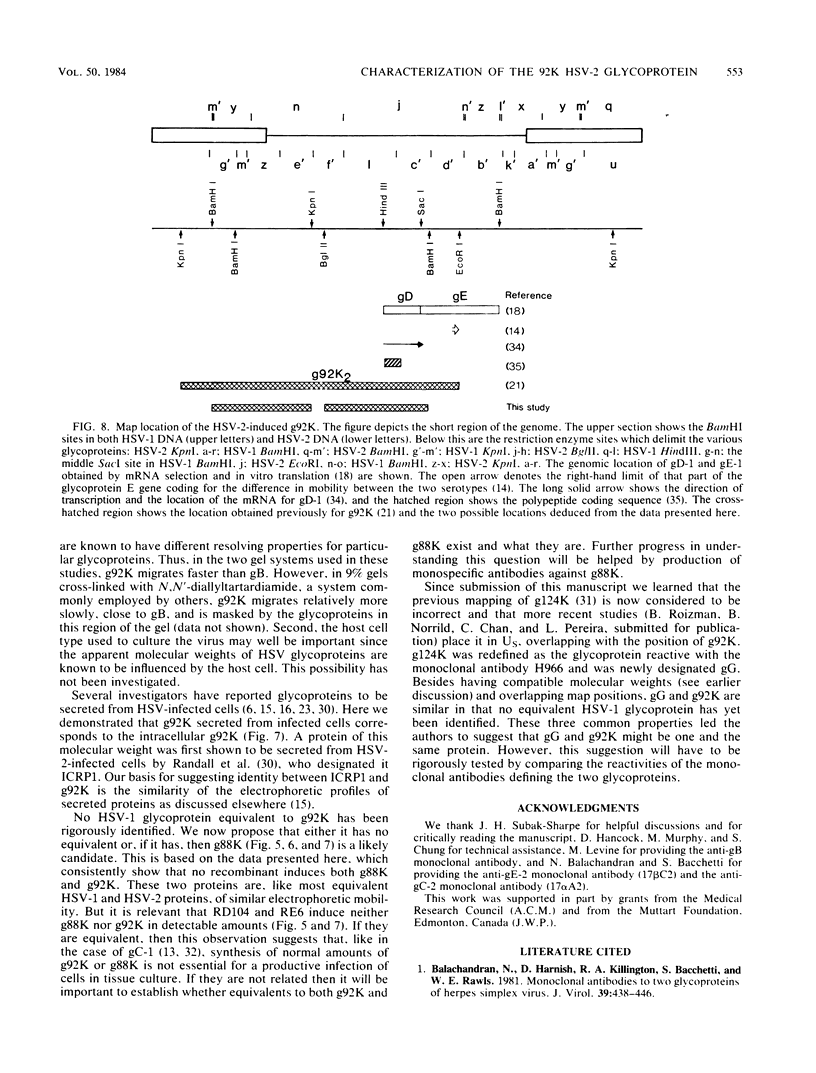
Evidence is presented showing that the 92,000-dalton glycoprotein (g92K) induced by herpes simplex virus (HSV) type 2 has properties distinct from those assigned to any other HSV glycoprotein. First, the carbohydrate composition and extent of sulfation differ from those of glycoproteins D and E. Second, two clonally unrelated monoclonal antibodies, AP1 and LP5, shown in this paper to specifically immunoprecipitate g92K, do not react with any of the known processed forms of glycoproteins B, C, D, and E. Third, by using HSV type 1/HSV type 2 intertypic recombinants and a simple radioimmunoassay, the target antigen of the two monoclonal antibodies was shown to map in the same region as g92K (0.846 to 0.924). Fourth, the intertypic recombinant R12-3 was shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of infected cells to induce the HSV type 2 g92K and HSV type 1 gD and GE, whereas R12-1, which did not induce g92K, induced HSV-2 gE and an altered gD, providing genetic evidence that g92K is encoded, at least in part, by a different region of the genome from that encoding gD and gE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Wilkie N. M., Timbury M. C. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 2 by marker rescue. J Gen Virol. 1981 Jan;52(Pt 1):121–133. doi: 10.1099/0022-1317-52-1-121. [DOI] [PubMed] [Google Scholar]
- Chen A. B., Ben-Porat T., Whitley R. J., Kaplan A. S. Purification and characterization of proteins excreted by cells infected with herpes simplex virus and their use in diagnosis. Virology. 1978 Dec;91(2):234–242. doi: 10.1016/0042-6822(78)90372-0. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Hilgers J. A radioimmunoassay for virus antibody using binding of 125I-labelled protein A. J Gen Virol. 1979 May;43(2):395–401. doi: 10.1099/0022-1317-43-2-395. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Marsden H. S., Wilkie N. M. One functional copy of the long terminal repeat gene specifying the immediate-early polypeptide IE 110 suffices for a productive infection of human foetal lung cells by herpes simplex virus. J Gen Virol. 1981 Jul;55(Pt 1):179–191. doi: 10.1099/0022-1317-55-1-179. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W. Intertypic recombinants of herpes simplex viruses. J Gen Virol. 1980 May;48(1):1–23. doi: 10.1099/0022-1317-48-1-1. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope R. G., Marsden H. S. Processing of glycoproteins induced by herpes simplex virus type 1: sulphation and nature of the oligosaccharide linkages. J Gen Virol. 1983 Sep;64(Pt 9):1943–1953. doi: 10.1099/0022-1317-64-9-1943. [DOI] [PubMed] [Google Scholar]
- Hope R. G., Palfreyman J., Suh M., Marsden H. S. Sulphated glycoproteins induced by herpes simplex virus. J Gen Virol. 1982 Feb;58(Pt 2):399–415. doi: 10.1099/0022-1317-58-2-399. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Erickson J. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. X. Proteins excreted by cells infected with herpes simplex virus, types 1 and 2. Virology. 1975 Mar;64(1):132–143. doi: 10.1016/0042-6822(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Lang J., Davison A. J., Hope R. G., MacDonald D. M. Genomic location and lack of phosphorylation of the HSV immediate-early polypeptide IE 12. J Gen Virol. 1982 Sep;62(Pt 1):17–27. doi: 10.1099/0022-1317-62-1-17. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Buckmaster A., Hancock D., Buchan A., Fuller A., Minson A. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus type 2. J Gen Virol. 1982 Dec;63(2):297–305. doi: 10.1099/0022-1317-63-2-297. [DOI] [PubMed] [Google Scholar]
- Norrild B., Vestergaard B. F. Immunoelectrophoretic identification and purification of herpes simplex virus antigens released from infected cells in tissue culture. Intervirology. 1979;11(2):104–110. doi: 10.1159/000149020. [DOI] [PubMed] [Google Scholar]
- Palfreyman J. W., Haarr L., Cross A., Hope R. G., Marsden H. S. Processing of herpes simplex virus type 1 glycoproteins: two-dimensional gel analysis using monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):873–886. doi: 10.1099/0022-1317-64-4-873. [DOI] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Norrild B., Roizman B. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5202–5206. doi: 10.1073/pnas.78.8.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Davison A. J., Marsden H. S., Timbury M. C., Subak-Sharpe J. H., Wilkie N. M. Recombinants between herpes simplex virus types 1 and 2: analyses of genome structures and expression of immediate early polypeptides. J Virol. 1978 Nov;28(2):499–517. doi: 10.1128/jvi.28.2.499-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R. E., Killington R. A., Watson D. H. Glycoproteins with type common and type specific antigenic sites excreted from cells infected with herpes simplex virus types 1 and 2. J Gen Virol. 1980 Jun;48(Pt 2):297–310. doi: 10.1099/0022-1317-48-2-297. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbury M. C. Temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1971 Nov;13(2):373–376. doi: 10.1099/0022-1317-13-2-373. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Colberg-Poley A. M., Marcus-Sekura C. J., Carter B. J., Enquist L. W. Characterization of the herpes simplex virus type 1 glycoprotein D mRNA and expression of this protein in Xenopus oocytes. Nucleic Acids Res. 1983 Mar 11;11(5):1507–1522. doi: 10.1093/nar/11.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Showalter S. D., Bladen S. V., Heilman C. J., Jr, Hampar B. Herpes simplex virus type 2 glycoprotein gF and type 1 glycoprotein gC have related antigenic determinants. J Virol. 1983 Jul;47(1):185–192. doi: 10.1128/jvi.47.1.185-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]