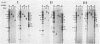Abstract
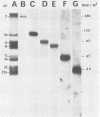
To localize the genes for the major glycoproteins of equine herpesvirus 1 (EHV-1), a library of the EHV-1 genome was constructed in the lambda gt11 expression vector. Recombinant bacteriophage expressing EHV-1 glycoprotein epitopes as fusion products with beta-galactosidase were detected by immunoscreening with monoclonal antibodies specific for each of six EHV-1 glycoproteins. Seventy-four recombinant lambda gt11 clones reactive with EHV-1 monoclonal antibodies were detected among 4 X 10(5) phage screened. Phage expressing determinants on each of the six EHV-1 glycoproteins were represented in the library. Herpesviral DNA sequences contained in lambda gt11 recombinants expressing epitopes of EHV-1 glycoproteins were used as hybridization probes for mapping insert sequences on the viral genome. Genes for five EHV-1 glycoproteins (gp2, gp10, gp13, gp14, and gp21/22a) mapped to the genome L component; only one EHV-1 glycoprotein (gp17/18) was expressed from the unique S region of the genome where genes of several major glycoproteins of other herpesviruses have been located. Two glycoproteins of EHV-1, gp13 and gp14, mapped to positions colinear with genes of major glycoproteins identified in several other alphaherpesviruses (gC- and gB-like glycoproteins, respectively). The genomic locations of other EHV-1 glycoproteins indicated the existence of major glycoproteins of EHV-1 (gp2, gp10, and gp21/22a) for which no genetic homologs have yet been detected in other herpesviruses. The results confirm the general utility of the lambda gt11 expression system for localizing herpesvirus genes and suggest that the genomic positioning of several high-abundance glycoproteins of EHV-1 may be different from that of the prototype alphaherpesvirus, herpes simplex virus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Allen G. P., O'Callaghan D. J., Randall C. C. Genetic relatedness of equine herpesvirus types 1 and 3. J Virol. 1977 Dec;24(3):761–767. doi: 10.1128/jvi.24.3.761-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. P., Yeargan M. R., Bryans J. T. Alterations in the equine herpesvirus 1 genome after in vitro and in vivo virus passage. Infect Immun. 1983 Apr;40(1):436–439. doi: 10.1128/iai.40.1.436-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bryans J. T., Allen G. P. Application of a chemically inactivated, adjuvanted vaccine to control abortigenic infection of mares by equine herpesvirus I. Dev Biol Stand. 1982;52:493–498. [PubMed] [Google Scholar]
- Bryans J. T. Herpesviral diseases affecting reproduction in the horse. Vet Clin North Am Large Anim Pract. 1980 Nov;2(2):303–312. doi: 10.1016/s0196-9846(17)30164-7. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Edson C. M., Ellis R. W., Forghani B., Gilden D., Grose C., Keller P. M., Vafai A., Wroblewska Z., Yamanishi K. New common nomenclature for glycoprotein genes of varicella-zoster virus and their glycosylated products. J Virol. 1986 Mar;57(3):1195–1197. doi: 10.1128/jvi.57.3.1195-1197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Respess R. A., Waters D. J., Thorley-Lawson D. A. Cross-reactivity between herpes simplex virus glycoprotein B and a 63,000-dalton varicella-zoster virus envelope glycoprotein. J Virol. 1985 Oct;56(1):333–336. doi: 10.1128/jvi.56.1.333-336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Keller P. M., Lowe R. S., Zivin R. A. Use of a bacterial expression vector to map the varicella-zoster virus major glycoprotein gene, gC. J Virol. 1985 Jan;53(1):81–88. doi: 10.1128/jvi.53.1.81-88.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C., Graham M. Y., Dutchik J. E., Olson M. V. A new method for purifying lambda DNA from phage lysates. DNA. 1985 Feb;4(1):39–49. doi: 10.1089/dna.1985.4.39. [DOI] [PubMed] [Google Scholar]
- Henry B. E., Robinson R. A., Dauenhauer S. A., Atherton S. S., Hayward G. S., O'Callaghan D. J. Structure of the genome of equine herpesvirus type 1. Virology. 1981 Nov;115(1):97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Bennett C. D., Ellis R. W. Identification and structure of the gene encoding gpII, a major glycoprotein of varicella-zoster virus. Virology. 1986 Jul 15;152(1):181–191. doi: 10.1016/0042-6822(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Kinchington P. R., Remenick J., Ostrove J. M., Straus S. E., Ruyechan W. T., Hay J. Putative glycoprotein gene of varicella-zoster virus with variable copy numbers of a 42-base-pair repeat sequence has homology to herpes simplex virus glycoprotein C. J Virol. 1986 Sep;59(3):660–668. doi: 10.1128/jvi.59.3.660-668.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. C., D'urso R. C., Kundel C. A., Whitbeck J. C., Bello L. J. Map location of the gene for a 130,000-dalton glycoprotein of bovine herpesvirus 1. J Virol. 1986 Nov;60(2):405–414. doi: 10.1128/jvi.60.2.405-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Thiel H. J., Schreurs C., Rziha H. J. Location of the structural gene of pseudorabies virus glycoprotein complex gII. Virology. 1986 Jul 15;152(1):66–75. doi: 10.1016/0042-6822(86)90372-7. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Pereira L., Michael N. Precise localization of genes on large animal virus genomes: use of lambda gt11 and monoclonal antibodies to map the gene for a cytomegalovirus protein family. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1266–1270. doi: 10.1073/pnas.82.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Rodgers G., Gilbert J. H., Snead R. M. Method to map antigenic determinants recognized by monoclonal antibodies: localization of a determinant of virus neutralization on the feline leukemia virus envelope protein gp70. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3675–3679. doi: 10.1073/pnas.81.12.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy C. W., Ford S. J., Porter R. C. Equine rhinopneumonitis vaccine: immunogenicity and safety in adult horses, including pregnant mares. Am J Vet Res. 1978 Mar;39(3):377–383. [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Watson R. J., Whealy M. E., Hays W. W., Enquist L. W. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein C. J Virol. 1986 May;58(2):339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Norrild B., Chan C., Pereira L. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus 2 glycoprotein lacking a known type 1 counterpart. Virology. 1984 Feb;133(1):242–247. doi: 10.1016/0042-6822(84)90447-1. [DOI] [PubMed] [Google Scholar]
- Snowden B. W., Halliburton I. W. Identification of cross-reacting glycoproteins of four herpesviruses by Western blotting. J Gen Virol. 1985 Sep;66(Pt 9):2039–2044. doi: 10.1099/0022-1317-66-9-2039. [DOI] [PubMed] [Google Scholar]
- Snowden B. W., Kinchington P. R., Powell K. L., Halliburton I. W. Antigenic and biochemical analysis of gB of herpes simplex virus type 1 and type 2 and of cross-reacting glycoproteins induced by bovine mammillitis virus and equine herpesvirus type 1. J Gen Virol. 1985 Feb;66(Pt 2):231–247. doi: 10.1099/0022-1317-66-2-231. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timmins J. G., Petrovskis E. A., Marchioli C. C., Post L. E. A method for efficient gene isolation from phage lambda gt11 libraries: use of antisera to denatured, acetone-precipitated proteins. Gene. 1985;39(1):89–93. doi: 10.1016/0378-1119(85)90112-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtinen L. W., Allen G. P., Darlington R. W., Bryans J. T. Serologic and molecular comparisons of several equine herpesvirus type 1 strains. Am J Vet Res. 1981 Dec;42(12):2099–2104. [PubMed] [Google Scholar]
- Turtinen L. W., Allen G. P. Identification of the envelope surface glycoproteins of equine herpesvirus type 1. J Gen Virol. 1982 Dec;63(2):481–485. doi: 10.1099/0022-1317-63-2-481. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Characterization and mapping of a nonessential pseudorabies virus glycoprotein. J Virol. 1986 Apr;58(1):173–178. doi: 10.1128/jvi.58.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- Yeargan M. R., Allen G. P., Bryans J. T. Rapid subtyping of equine herpesvirus 1 with monoclonal antibodies. J Clin Microbiol. 1985 May;21(5):694–697. doi: 10.1128/jcm.21.5.694-697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]