Abstract
The yeast Saccharomyces cerevisiae has a limited life-span, which is measured by the number of divisions that individual cells complete. Among the many changes that occur as yeasts age are alterations in chromatin-dependent transcriptional silencing. We have genetically manipulated histone deacetylases to modify chromatin, and we have examined the effect on yeast longevity. Deletion of the histone deacetylase gene RPD3 extended life-span. Its effects on chromatin functional state were evidenced by enhanced silencing at the three known heterochromatic regions of the genome, the silent mating type (HM), subtelomeric, and rDNA loci, which occurred even in the absence of SIR3. Similarly, the effect of the rpd3Δ on life-span did not depend on an intact Sir silencing complex. In fact, deletion of SIR3 itself had little effect on life-span, although it markedly accelerated the increase in cell generation time that is observed during yeast aging. Deletion of HDA1, another histone deacetylase gene, did not result in life-span extension, unless it was combined with deletion of SIR3. The hda1Δ sir3Δ resulted in an increase in silencing, but only at the rDNA locus. Deletion of RPD3 suppressed the loss of silencing in rDNA in a sir2 mutant; however, the silencing did not reach the level found in the rpd3Δ single mutant, and RPD3 deletion did not overcome the life-span shortening seen in the sir2 mutant. Deletion of both RPD3 and HDA1 caused a decrease in life-span, which resulted from a substantial increase in initial mortality of the population. The expression of both of these genes declines with age, providing one possible explanation for the increase in mortality during the life-span. Our results are consistent with the loss of rDNA silencing leading to aging in yeast. The functions of RPD3 and HDA1 do not overlap entirely. RPD3 exerts its effect on chromatin at additional sites in the genome, raising the possibility that events at loci other than rDNA play a role in the aging process.
INTRODUCTION
Individual cells of the budding yeast Saccharomyces cerevisiae undergo a finite number of cell divisions (Mortimer and Johnston, 1959; Muller et al., 1980). Thus, the life-span of this unicellular eukaryote can be defined as the total number of times the cell divides or the number of daughter cells it produces before dying. Yeast aging is accompanied by many morphological and physiological changes (reviewed in Jazwinski, 1996), including increased cell generation time (Egilmez and Jazwinski, 1989) and sterility (Muller, 1985; Smeal et al., 1996). One of the hallmarks of mammalian cellular senescence is the gradual loss of telomere DNA sequences, as cultures exhaust population doublings. This telomere attrition has been proposed to play a causal role in cellular senescence (Harley et al., 1990; Harley, 1991; Allsopp et al., 1992). In fact, inactivation of telomerase activity in human cells hastened cellular senescence and was accompanied by shortened telomeres (Feng et al. 1995). In contrast, constitutive activation of telomerase maintained telomere length and postponed cellular senescence (Bodnar et al., 1998). In yeast, aging cells do not suffer telomere shortening (D’mello and Jazwinski, 1991); however, another age-related event is associated with telomeres. This is the loss of transcriptional silencing at least at one telomere (Kim et al., 1996). Loss of silencing also has been described at the silent mating type (HM) loci of old yeast (Smeal et al., 1996).
Transcriptional silencing is a manifestation of chromosomal position effect, in which a euchromatic gene translocated to a heterochromatic region is expressed in a portion of a cell population, resulting in a mosaic or variegated phenotype (Spofford, 1976; Henikoff, 1990). In the yeast genome, the HM loci, telomeres, and the rDNA locus are known to exhibit transcriptional silencing (Klar et al., 1981; Nasmyth et al., 1981; Gottschling et al., 1990; Bryk et al., 1997; Smith and Boeke, 1997; Smith et al., 1998). Efficient transcriptional silencing at the HM loci and telomeres requires a number of genes. These include SIR1, SIR2, SIR3, SIR4, and RAP1 and genes encoding histones H3 and H4 (Laurenson and Rine, 1992; Loo and Rine, 1995). Increased copy numbers of SIR3, but not of SIR2 or SIR4, resulted in spreading of silenced telomeric domains (Renauld et al., 1993; Hecht et al., 1996; Strahl-Bolsinger et al., 1997). This observation implies that the SIR3 gene product may be a limiting, major structural component of the silencing machinery. SIR2, which is known to suppress meiotic and mitotic recombination involving rDNA repeats (Gottlieb and Esposito, 1989), enhanced rDNA silencing in a dosage-dependent manner (Bryk et al., 1997; Fritze et al., 1997; Smith and Boeke, 1997; Smith et al., 1998). To the contrary, SIR4 inhibited rDNA silencing (Smith and Boeke, 1997; Smith et al., 1998).
Transcriptional silencing is also affected by modification of the core histones by acetylation or deacetylation. Specific histone domains required for efficient silencing are localized to the N-terminal tails of H3 and H4 (Park and Szostak, 1990; Aparicio et al., 1991; Johnson et al., 1992; Thompson et al., 1994). These H3/H4 silencing domains have been shown, genetically and physically, to interact with Sir3p or Sir4p (Johnson et al., 1990; Hecht et al., 1995, 1996). Acetylation of the core histones is reversibly catalyzed by histone acetyltransferases and deacetylases. Yeast RPD3 and HDA1 encode histone deacetylases: mutations in these genes lead to histone hyperacetylation and enhanced transcriptional silencing at HM and the subtelomeric loci examined (Sussel et al., 1995; Rundlett et al., 1996; Vannier et al., 1996). RPD3 is also known to affect transcription of various other genes, including repression of HO, TRK2, STE6, PHO5, SPO13, and IME2 (reviewed by Grunstein, 1997; Struhl, 1998). Mammalian histone deacetylase genes HDAC1 and HDAC2, both of which are homologous to yeast RPD3 (Taunton et al., 1996; Yang et al., 1996), also mediate transcriptional repression in association with a number of corepressors (reviewed by Grunstein, 1997; Struhl, 1998).
The attenuated silencing observed in old yeast cells suggests a possible connection between chromatin-dependent transcriptional silencing and yeast aging. As an approach to gaining more insight into a possible connection between chromatin functional state and aging, we deleted RPD3, HDA1, SIR2, and SIR3 either singly or in combination, and examined the life-span of the mutants. Chromatin changes were monitored by examination of transcriptional state. Our data show a correlation between life-span and chromatin-dependent transcriptional silencing at the rDNA locus in yeast.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Yeast strains used in this study are listed in Table 1. The diploid YPK4.7 was constructed by “self mating” of a haploid derivative of YPH501 (Sikorski and Hieter, 1989), which was performed by inducing the HO gene (Herskowitz and Jensen, 1991). Haploid segregants of YPK4.7 show no significant differences in mean life-span (Kirchman and Jazwinski, unpublished results).
Table 1.
Strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| Diploid | ||
| YPK4.7 | MATa/MATα ura3-52/ura3-52 lys2-801amber/lys2-801amber ade2-101ochre/ade2-101ochre trp1-Δ63/trp1-Δ63 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 | Kirchman et al., 1999 |
| YSK631 | YPK4.7 with +/rpd3Δ::URA3 +/hda1Δ::HIS3 | This study |
| YSK668 | YPK4.7 with +/rpd3Δ::URA3 +/sir3Δ::LEU2 | This study |
| Haploid | ||
| YSK661 | MATα rpd3Δ::URA3;haploid segregant of YSK631 | This study |
| YSK662 | MATa rpd3Δ::URA3 hda1Δ::HIS3; haploid segregant of YSK631 | This study |
| YSK663 | MATα; haploid segregant of YSK631 | This study |
| YSK664 | MATα hda1Δ::HIS3; haploid segregant of YSK631 | This study |
| YSK710 | MATα rpd3Δ::URA3 sir3Δ::LEU2; haploid segregant of YSK668 | This study |
| YSK711 | MATa sir3Δ::LEU2; haploid segregant of YSK668 | This study |
| YSK712 | MATa; haploid segregant of YSK668 | This study |
| YSK713 | MATα rpd3Δ::URA3; haploid segregant of YSK668 | This study |
| YSK770 | YSK663 with sir3Δ::LEU2 | This study |
| YSK771 | YSK664 with sir3Δ::LEU2 | This study |
| DY2126 | MATα ade2 can1 his3 leu2 trp1 ura3 hmr::TRP1 | Jiang and Stillman, 1996 |
| YSK694 | DY2126 with hda1Δ::HIS3 | This study |
| YSK696 | DY2126 with rpd3Δ::URA3 | This study |
| YSK726 | DY2126 with sir3Δ::LEU2 | This study |
| YSK730 | DY2126 with hda1Δ::HIS3 sir3Δ::LEU2 | This study |
| YSK728 | DY2126 with rpd3Δ::URA3 sir3Δ::LEU2 | This study |
| YDS21U | MATa ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 URA3::TelVR (2.1 kb from Tel) | Rundlett et al., 1996 |
| YSK831 | YDS21U with hda1Δ::HIS3 | This study |
| YSK830 | YDS21U with rpd3Δ::TRP1 | This study |
| YSK829 | YDS21U with sir3Δ::LEU2 | This study |
| YSK833 | YDS21U with hda1Δ::HIS3 sir3Δ::LEU2 | This study |
| YSK832 | YDS21U with rpd3Δ::TRP1 sir3Δ::LEU2 | This study |
| M1 | MATα his3-Δ200 leu2-Δ1 ura3-167 trp1-Δ63 met15-Δ1 RDN1::Ty1-MET15 | Smith and Boeke, 1997 |
| M9 | Same as M1 except that Ty1-MET15 is inserted in a non-rDNA locus | Smith and Boeke, 1997 |
| JS218 | M1 with sir2::HIS3 | Smith and Boeke, 1997 |
| YSK757 | M1 with sir3Δ::LEU2 | This study |
| YSK753 | M1 with hda1Δ::pRS405 (LEU2) | This study |
| YSK781 | M1 with sir3Δ::LEU2 hda1Δ::pRS403 (HIS3) | This study |
| YSK755 | M1 with rpd3Δ::pRS404 (TRP1) | This study |
| YSK779 | JS218 with rpd3Δ::pRS306 (URA3) | This study |
| YCYL11 | M9 with rpd3Δ::pRS404 (TRP1) | This study |
| YSK783 | M1 with sir3Δ::LEU2 rpd3Δ::pRS406 (URA3) | This study |
| YPK9 | MATa ura3-52 lys2-801amber ade2-101ochre trp1-Δ63 his3-Δ200 leu2-Δ1 | From YPK4.7 (Kirchman et al., 1999) |
| YAB11 | YPK9 with sir2::URA3 | This study |
| YAB12 | YPK9 with rpd3Δ::pRS306 (URA3) | This study |
| YAB13 | YAB11 with rpd3Δ::pRS403 (HIS3) | This study |
| X2180-1A | MATa SUC2 mal1 mel1 gal2 CUP1 | The Berkeley Yeast Genetic Stock Center |
Genes of interest were disrupted by the “γ transformation” procedure (Sikorski and Hieter, 1989). The rpd3 deletants were created by replacing 63% of the coding region from the HindIII site (+467) to the EcoRI site (+1292) with pRS306 (the appropriate derivative, pMV130, was provided by Richard Gaber, Northwestern University) (Vidal and Gaber, 1991), pRS403, or pRS404. The pRS series of plasmids has been described (Sikorski and Hieter, 1989). (Life-spans were not dependent on the selectable marker.) In strain YAB11, sir2 has an insertion of a PCR-amplified fragment containing URA3 at the BglII site in the coding region at + 1023 from the first nucleotide of the translation initiation codon. The hda1 deletants were constructed by replacing 70% of the coding region spanning positions + 137 and + 2627 with pRS403 or pRS405. To delete SIR3, an ∼3-kb SacI–HindIII fragment isolated from plasmid pAR78 (provided by Scott G. Holmes, Princeton University) was used to replace most of the coding region with LEU2. All the deletions/disruptions were confirmed by Southern blot analysis.
Media and Genetic Methods
Yeast media were prepared as described (Rose et al., 1990). The synthetic medium containing Pb2+ (Cost and Boeke, 1996) or 5-fluoroorotic acid (Gottschling et al., 1990) was prepared as described. Standard genetic methods were used for mating, sporulation, and tetrad analysis (Rose et al., 1990). Transcriptional silencing of TRP1 at the hmr locus or URA3 inserted 2.1 kb from the right telomere of chromosome V was determined quantitatively by plating serial dilutions of cells, as described previously (Gottschling et al., 1990; Sussel et al., 1995). In the presence of Ura3p, 5-fluoroorotic acid is converted to a toxic compound. Student’s t test was used to assess the significance of differences in silencing, except when the colony-forming units were very low. In that case, the Poisson 95% central confidence intervals were compared.
Life-span Determination
Life-spans of yeast cells were determined as described elsewhere (Kim et al., 1998). Briefly, cells were grown in liquid YPG medium (1% yeast extract, 2% peptone, 3% glycerol) to suppress growth of petite yeasts. Exponentially growing cells were spotted on standard YPD plates (1% yeast extract, 2% peptone, 2% glucose, 2% agar) at low density. An appropriate number of individual cells were randomly picked under a microscope and aligned in isolated areas with a micromanipulator. After incubation of the plates at 30°C, virgin cells (new buds) were separated from their mother cells and left at the original spot, and the mother cells were discarded. The life-spans of these virgin cells were determined by recording the total number of daughter cells produced and removed. Mother cells were scored dead when budding ceased completely and they lost refractility. The nonparametric Wilcoxon signed rank test was performed to assess significance of differences in life-span.
Northern Blot Analysis and Quantitation of mRNA Levels
Age-synchronized cell populations of X2180-1A were prepared by rate-zonal sedimentation in sucrose density gradients (Egilmez et al., 1990; Kim et al., 1998). Total RNA was isolated from cells of different ages using glass beads and hot acidic phenol (Ausubel et al., 1993). To detect age-dependent changes in mRNA levels, Northern hybridization with DNA probes and quantitation of mRNA levels were performed as described (Sun et al., 1994).
RT-PCR Analysis of mRNA Levels
To examine age-dependent expression of RPD3, RT-PCR analysis was performed because of the low levels of RPD3 mRNA. Total RNA was isolated from age-synchronized cell populations as described above. In 0.5-ml microcentrifuge tubes, 1 μg of the RNA preparation was digested with 1 U of RNase-free DNase I (amplification grade, Life Technologies-BRL, Gaithersburg, MD) in a total volume of 10 μl, as recommended by the manufacturer. After DNase I treatment, 0.1 μl of the reaction containing 0.1 μg of DNase I-treated RNA was run on an agarose gel alongside the same amount of untreated RNA control to ensure that DNase I treatment did not result in RNA degradation. At the same time, to ensure that DNA present in the RNA preparations was completely digested by DNase I, 0.1 μg of DNase I-treated RNA was subjected to PCR analysis with the same primers used for RT-PCR (see below for the PCR reaction conditions). The DNase I-treated RNA was precipitated with 2.5 volumes of 100% ethanol and resuspended in 9 μl of diethylpyrocarbonate-treated water.
To synthesize first-strand cDNA, 1 μl (0.5 μg) of oligo d(T)12–18 (Life Technologies-BRL) was added to the tubes containing 9 μl of the DNase I-treated RNA. After 10-min incubation at 70°C, the tubes were chilled in an ice slurry. After brief centrifugation, the following ingredients were added to each tube containing the RNA and oligo d(T): 4 μl of 5× first-strand buffer (Life Technologies-BRL; 250 mM Tris-HCl, pH 8.3, 375 mM KCl, 15 mM MgCl2), 2 μl of 0.1 M dithiothreitol, 1 μl of 10 mM each dATP, dGTP, and dTTP mix, 1 μl of 0.1 mM dCTP, and 1 μl (∼10 μCi) of [α-32P] dCTP (3000 Ci/mmol). After incubation of the reaction mixture for 2 min at 42°C, 1 μl (200 U) of Superscript II RNase H-Reverse Transcriptase (Life Technologies-BRL) was added to each tube. The first-strand cDNA synthesis was performed for 50 min at 42°C. The reaction was stopped by incubating the tubes for 15 min at 70°C.
To quantitate the amount of cDNA synthesized in each tube, 3 μl of the cDNA synthesis reaction were mixed with the same volume of sequencing stop buffer (90% deionized formamide, 20 mM EDTA, pH 8.0, 0.05% bromophenol blue, 0.05% xylene cyanol). The tubes were heated for 3 min at 90°C, and the samples were loaded onto a 6% polyacrylamide, 7 M urea sequencing gel, alongside 32P-labeled size marker DNAs. The amount of cDNA synthesized in each sample was quantitated by phosphorimaging.
After normalization of the amount of cDNA present in each tube, 1 μl of the cDNA sample was diluted 1:10 in water to obtain a 0.1× cDNA sample in addition to the 1× cDNA sample. For each cDNA sample, 1.25 μl of 0.1×, 2.5 μl of 0.1×, 5 μl of 0.1×, 1 μl of 1×, and 2 μl of 1× cDNA sample were added to five separate, fresh tubes. After deionized water was added to each tube to bring the volume up to 37 μl, the following ingredients were added: 5 μl of 10× PCR buffer (200 mM Tris-HCl, pH 8.4, 500 mM KCl, 25 mM MgCl2), 1 μl of 10 mM each dATP, dTTP, dGTP, and dCTP mix, 1 μl of each RPD3 primer at 50 mM concentration, 1 μl (10 μCi) of [α-32P] dCTP (3000 Ci/mmol), and 1 μl (5 U) of Taq polymerase (Promega, Madison, WI). The primers were 5′-(+470 from the first nucleotide of the ATG start codon) GGTGGTGGCTCTATGGAAGGA-3′ and 3′-GGATCCCTACGGCTTCTAAA CCC (+1305)-5′. PCR amplification using this primer pair generates a 836-bp product specific to RPD3. After 5-min incubation at 94°C, the PCR amplification continued for 30 cycles, each cycle consisting of 1.5 min at 94°C, 1.5 min at 54°C, and 2.5 min at 72°C. The PCR products were separated on a 6% nondenaturing polyacrylamide gel. After the gel was dried, quantitation of DNA bands amplified from the RPD3 cDNA was performed by phosphorimaging, using the PhosphorImager and ImageQuaNT Software (Molecular Dynamics, Sunnyvale, CA).
Quantitation of Extrachromosomal rDNA Circles
Yeast cells were grown to an OD600 of 0.8, and DNA was extracted from harvested cells using the Easy DNA kit (Invitrogen, San Diego, CA). DNA (20 μg) was electrophoresed for 20 h at 1 V/cm in an 0.6% agarose gel containing 40 mM Tris-acetate, 1 mM EDTA, pH 8.0, in the absence of ethidium bromide. The separated DNA was transferred to a Nytran membrane (Schleicher and Schuell, Keene, NH). rDNA was detected by hybridization with a 32P-labeled DNA probe and quantitated by phosphorimaging. Normalization for DNA loading was performed with an actin (ACT1) DNA probe. The identity of rDNA circles was further confirmed by two-dimensional gel electrophoresis in the presence of chloroquine, as described (Sinclair and Guarente, 1997).
RESULTS
Deletion of RPD3 Results in Life-span Extension
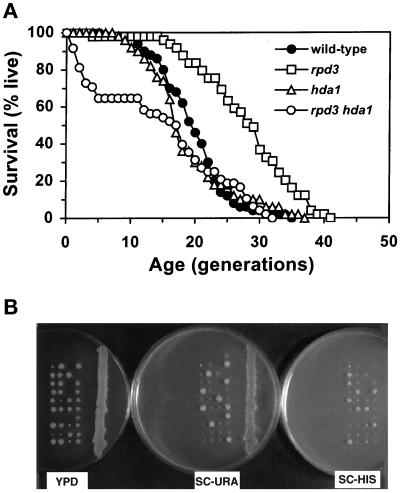
We first examined the effect of genes encoding histone deacetylases on yeast replicative life-span. RPD3 and HDA1 were deleted from a diploid strain (YPK4.7). After sporulation and tetrad dissection, germinated rpd3Δ, hda1Δ, and rpd3Δ hda1Δ segregants were examined for their life-spans (Figure 1A). The average life-span of the rpd3Δ segregant was extended by 41% compared with the wild-type control. The hda1Δ segregant showed little change compared with the wild-type control. The rpd3Δ hda1Δ double mutant had a significantly shorter mean life-span because of high initial mortality.
Figure 1.
Effects of histone deacetylase mutants on life-span and colony-forming ability. (A) Survival curves of wild-type (YSK663), hda1Δ (YSK664), rpd3Δ (YSK661), and hda1Δ rpd3Δ (YSK662) segregants of YSK631. The percentages of live cells are plotted as a function of age in generations. Mean life-span (and the number of cells analyzed) was 19.8 (50) for the wild-type, 18.9 (50) for the hda1Δ, 27.9 (49) for the rpd3Δ, and 14.8 (48) for the hda1Δ rpd3Δ strains. The rpd3Δ and hda1Δ rpd3Δ strains were significantly different from the wild-type in life-span (the P values were 0.000001 and 0.0078, respectively). (B) Tetrads obtained from sporulated YSK631 [+/rpd3Δ:: URA3, +/hda1Δ:: HIS3] were dissected on a YPD plate. After 3-d incubation at 30°C, the germinated colonies were replica-plated onto complete synthetic medium lacking uracil (SC-URA) or histidine (SC-HIS). All of the segregants that formed smaller colonies on the YPD plate were Ura+ and His+, indicating that the hda1Δ rpd3Δ double-mutant segregants had difficulty in normal colony formation.
Most of the rpd3Δ hda1Δ mother cells that had stopped cell division either early or late were attached to a large bud. The budding pattern was frequently random during the life-span, after the initial drop in survival. The increase in frequency of random budding is an age-related phenotype (Jazwinski et al., 1998). Consistent with the shorter mean life-span associated with high initial mortality, the germinated rpd3Δ hda1Δ segregants formed much smaller colonies, compared with the other segregants (Figure 1B). These results suggest that RPD3 and HDA1 share some essential function, but their effects on yeast aging are different. Similar results were obtained with the same segregants from other tetrads. Deletion of HOS1 or HOS2, both of which share sequence homology with RPD3 and HDA1 (Rundlett et al., 1996), showed no effect on life-span (our unpublished results).
Deletion of SIR3 from the hda1Δ Strain Results in Life-span Extension
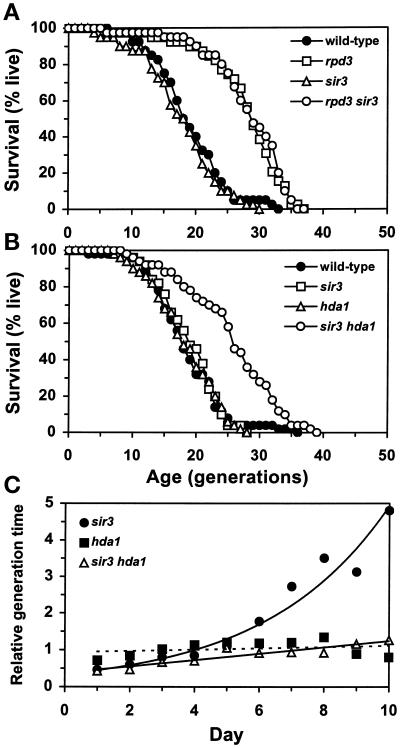
To determine whether the life-span extension shown by the rpd3Δ segregants is mediated by the Sir silencing complex, SIR3 was deleted from a diploid heterozygous for rpd3Δ, and meiotic segregants were examined (Figure 2A). The rpd3Δ sir3Δ segregant showed as much life-span extension as the rpd3Δ alone, whereas the sir3Δ segregant was virtually the same as the wild-type control in life-span. This result indicates that life-span extension by RPD3 deletion does not require the intact Sir silencing complex needed for efficient silencing at telomeres and at HM loci.
Figure 2.
Effects of SIR3 deletion on life-span and senescence. (A) Survival curves of wild-type (YSK712), rpd3Δ (YSK713), sir3Δ (YSK711), and rpd3Δ sir3Δ (YSK710) segregants of YSK668. Mean life-span (and the number of cells analyzed) was 19.1 (40) for the wild-type, 27.9 (40) for the rpd3Δ, 17.8 (40) for the sir3Δ, and 28.4 (40) for the rpd3Δ sir3Δ strains. The rpd3Δ and rpd3Δ sir3Δ strains differed from wild-type in life-span (P < 0.00001). (B) Survival curves of wild-type (YSK663), hda1Δ (YSK664), sir3Δ (YSK770), and hda1Δ sir3Δ (YSK771) strains. Mean life-span (and the number of cells analyzed) was 18.6 (50) for the wild-type, 18.3 (50) for the hda1Δ, 19.0 (50) for the sir3Δ, and 25.6 (50) for the hda1Δ sir3Δ strains. The life-span of the hda1Δ sir3Δ strain was significantly longer than that of the control, the sir3Δ, or the hda1Δ strains (P = 0.000002, 0.000028, and 0.000002, respectively). (C) Change in generation time of the hda1Δ, sir3Δ, or hda1Δ sir3Δ mother cells relative to the wild-type control during the life-spans shown in B. From the day life-span determination was started, the total number of buds generated by the wild-type mother cells was divided by the total number of buds generated by the hda1Δ, sir3Δ, or hda1Δ sir3Δ mother cells. The resulting number corresponds to an estimate of the average generation time of each mutant strain relative to that of the wild type. The relative generation times were calculated until day 10. By this time, all of the strains completed >96% of their life-spans.
SIR3 was also deleted from the hda1Δ strain, and the life-span of the hda1Δ sir3Δ strain was analyzed (Figure 2B). Interestingly, the average life-span of the double mutant was extended by as much as 38%, whereas either single mutant showed little change as observed before. While measuring the life-spans, we noticed differences among different strains in the length of time taken for mother cells to generate consecutive buds (generation time) (Figure 2C). The mother cells of the sir3Δ strain had shorter generation times at early ages than the wild-type control. With age, however, they exhibited an exponential increase in generation time, compared with the control. The generation time of the hda1Δ strain remained close to that of the wild-type control throughout the life-span. The generation time of the hda1Δ sir3Δ double mutant was initially as short as that of the sir3Δ single mutant but did not increase with age at the same rapid rate. Therefore, the synthetic life-span extension phenotype of the hda1Δ sir3Δ strain is the result of generation of more daughter cells for a prolonged time, compared with either single mutant or the control. The exponential increase in generation time in the sir3Δ strain represents an acceleration of an aging phenotype (Egilmez and Jazwinski, 1989). To our knowledge, this is the second example in which an age-related phenotype has been separated from longevity. In the other case, an earlier than usual increase in cell size was obtained when life-span was extended by other means (Chen et al., 1990).
Transcriptional Silencing in the rpd3Δ and hda1Δ sir3Δ Strains
We next wanted to determine whether the average life-spans of the deletion mutants for RPD3, HDA1, or SIR3 correlate with chromatin changes. For this purpose, transcriptional silencing of each mutant was examined at HMR, at a subtelomeric site, and at RDN1. At the silent mating-type locus, silencing was significantly increased in the rpd3Δ strain but not in the hda1Δ strain (Table 2). At the subtelomeric locus, both the rpd3Δ and hda1Δ strains showed increased silencing, with the effect of rpd3Δ being greater than that of hda1Δ (Table 3), as observed previously (Rundlett et al., 1996). At both loci examined, silencing was abolished by deletion of SIR3, as expected.
Table 2.
Effect of deletion of RPD3, HDA1, and SIR3 on HMR silencing
| Strain (all hmr::TRP1) | No. of cultures | Frequency of Trp+ colonies (mean ± SD) | Relative frequency |
|---|---|---|---|
| DY2126 (control) | 8 | 5.19 ± 2.74 × 10−6 | 1.00 |
| YSK694 (hda1Δ) | 8 | 6.12 ± 3.97 × 10−6 | 1.18 |
| YSK696 (rpd3Δ) | 11 | 2.08 ± 1.40 × 10−6* | 0.40 |
| YSK726 (sir3Δ) | 9 | 1.03 ± 0.08 | 1.98 × 105 |
| YSK730 (hda1Δ sir3Δ) | 9 | 0.98 ± 0.08 | 1.89 × 105 |
| YSK728 (rpd3Δ sir3Δ) | 12 | 0.92 ± 0.08** | 1.77 × 105 |
P < 0.05 compared with the control.
P < 0.01 compared with the sir3Δ strain.
Table 3.
Effect of deletion of RPD3, HDA1, and SIR3 on telomeric silencing
| Strain (all URA3::TelVR) | No. of cultures | Frequency of 5-fluoroorotic acid-resistant colonies (mean ± SD) | Relative frequency |
|---|---|---|---|
| YDS21U (control) | 8 | 0.049 ± 0.046 | 1.00 |
| YSK831 (hda1Δ) | 8 | 0.210 ± 0.063** | 4.29 |
| YSK830 (rpd3Δ) | 8 | 0.631 ± 0.147** | 12.88 |
| YSK829 (sir3Δ) | 7 | 9.27 ± 7.47 × 10−8 | 1.89 × 10−6 |
| YSK833 (hda1Δ sir3Δ) | 8 | 12.84 ± 18.07 × 10−8 | 2.62 × 10−6 |
| YSK832 (rpd3Δ sir3Δ) | 8 | 34.81 ± 55.04 × 10−8* | 7.10 × 10−6 |
P < 0.05 compared with the sir3Δ strain.
P < 0.01 compared with the control.
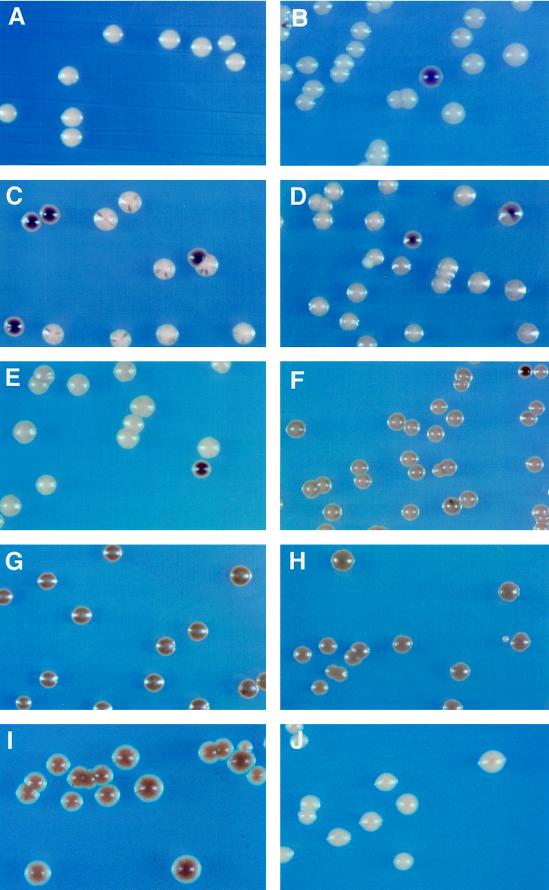
For rDNA silencing, we used the color assay of colonies arising from cells containing MET15 integrated in the RDN1 locus (Cost and Boeke, 1996; Smith and Boeke, 1997). As shown previously, rDNA silencing was dependent on SIR2, as judged by the lighter background colony colors in the sir2Δ strain, compared with the intermediate brown colors of the control colonies (Figure 3, compare A, B, and C). In addition, the more frequent appearance of dark brown colonies or colony sectors in the sir2Δ strain, which results from mitotic recombination leading to complete loss of MET15, indicates that SIR2 is also required for suppression of mitotic recombination involving rDNA repeats. In contrast, colonies of the rpd3Δ or the rpd3Δ sir3Δ strains showed uniformly intensified brown colors, indicating that both rDNA silencing and recombinational suppression were increased by deletion of RPD3 (Figure 3, G and H). Colony colors of the hda1Δ or the sir3Δ strains were not substantially different from those of the control, although colony colors of the sir3Δ strain appeared to be slightly intensified (Figure 3, D and E), as reported previously (Smith et al., 1998). Interestingly, colonies of the hda1Δ sir3Δ double mutant developed in as uniformly intense brown colors as the rpd3Δ mutant (Figure 3F). This indicates that deletion of both HDA1 and SIR3 increased rDNA silencing and recombinational suppression to an extent similar to the increase by RPD3 deletion. Deletion of RPD3 had little effect on transcription of MET15 located outside the RDN1 locus (Figure 3J).
Figure 3.
Effect of various deletions on rDNA silencing. Cells from (A) M9 (Ty1-MET15 inserted in a non-rDNA locus), (B) M1 (RDN1::Ty1-MET15), (C) JS218 (M1 with sir2:: HIS3), (D) YSK757 (M1 with sir3Δ:: LEU2), (E) YSK753 (M1 with hda1Δ:: LEU2), (F) YSK781 (M1 with sir3Δ:: LEU2, hda1Δ:: HIS3), (G) YSK755 (M1 with rpd3Δ:: TRP1), (H) YSK783 (M1 with sir3Δ:: LEU2, rpd3Δ:: URA3), (I) YSK779 (M1 with rpd3Δ:: URA3, sir2:: HIS3), and (J) YCYL11 (M9 with rpd3Δ:: TRP1) were streaked on modified YPD agar medium containing Pb2+. The plates were incubated for 1 wk at 30°C. MET15+ cells grown on Pb2+ medium form white colonies (A), but met15 mutant cells develop dark brown colonies on the same medium. In the control M1 (B), the Ty1-MET15 is located upstream of the 5S rDNA in the RDN1 locus (Smith and Boeke, 1997).
Deletion of RPD3 Partially Suppresses the Silencing Defect in the sir2Δ and sir3Δ Strains
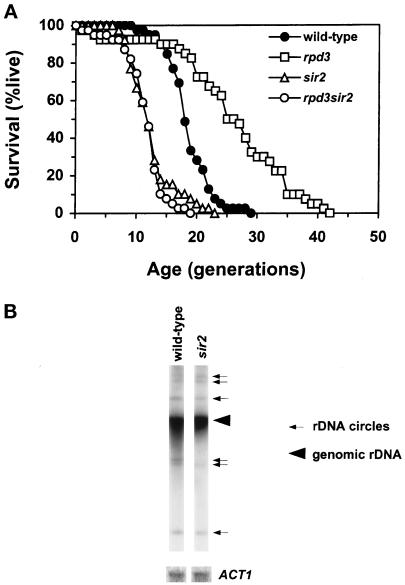
We noticed that the polar budding of mother cells occurred less frequently in the rpd3Δ sir3Δ double mutant than in the sir3Δ single mutant. In contrast, the hda1Δ sir3Δ double mutant maintained a polar budding pattern. This suggests suppression of the HM silencing defect of the sir3Δ by deletion of RPD3, but not by deletion of HDA1. In fact, the sir3Δ rpd3Δ strain showed a slight but significant increase in HMR silencing compared with the sir3Δ strain (Table 2). This small increase in silencing in a sir3Δ rpd3Δ strain was observed at the subtelomeric locus as well (Table 3). Deletion of RPD3 was also able to partially suppress the increased mitotic recombination in the sir2Δ strain at the RDN1 locus, as indicated by less frequent appearance of dark brown-colored colonies and sectors, and to enhance rDNA silencing. The uniformly brown colony colors of the rpd3Δ sir2Δ double mutant were nearly, but not quite, as intense as those of the rpd3Δ single mutant (Figure 3I). This indicates that deletion of RPD3 can overcome the loss of silencing and increased mitotic recombination in rDNA caused by deletion of SIR2 (Figure 3, compare B and C). The sir2Δ, however, prevents the rpd3Δ from maximally enhancing silencing of rDNA (Figure 3, compare G and I). We also determined the life-span of the sir2 mutant, in which rDNA silencing is severely abated (Bryk et al., 1997; Fritze et al., 1997; Smith and Boeke, 1997; Smith et al., 1998). The mean life-span of the sir2 mutant was significantly reduced, compared with the wild-type control (Figure 4A). The deletion of RPD3 did not suppress this decline in life-span in the sir2Δ strain (Figure 4A), despite its enhancement of rDNA silencing.
Figure 4.
Effects of SIR2 deletion on life-span and ERC generation. (A) Survival curves of wild-type control (YPK9), sir2 (YAB11), rpd3Δ (YAB12), and rpd3Δ sir2 (YAB13). Mean life-span (and the number of cells analyzed) was 18.7 (39) for the wild-type, 25.8 (40) for the rpd3Δ, 12.5 (39) for the sir2, and 11.6 (39) for the rpd3Δ sir2 strains, respectively. The rpd3Δ, sir2, and rpd3Δ sir2 strain life-spans differed from wild-type (P ≪ 0.0001). There is no statistical difference between the life-spans of the sir2 and rpd3Δ sir2 strains (P = 0.8). (B) ERC generation in the wild-type and sir2 strains used in A. The large arrowhead indicates genomic rDNA, whereas the smaller arrows point to extrachromosomal rDNA.
It has been shown recently that extrachromosomal rDNA circles (ERCs) accumulate in old yeast cells, presumably through recombination at RDN1 and amplification, and that induction of ERCs can cause yeast aging (Sinclair and Guarente, 1997). Deletion of SIR2 increases recombination at RDN1 (Gottlieb and Esposito, 1989; Smith and Boeke, 1997). We compared the relative amounts of ERCs present in wild-type and sir2 mutant cells and found that their amount in sir2 mutants did not exceed that present in the wild-type control (Figure 4B). This suggests that ERC production is not the cause of the curtailed life-span in the sir2 mutant.
Changes in RPD3, HDA1, and SIR3 mRNA Levels with Age
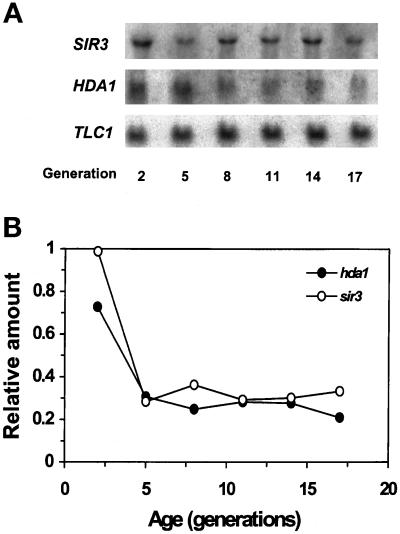
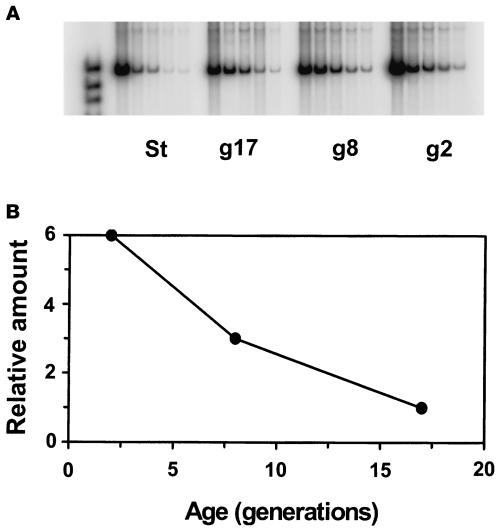
To obtain more insight into how RPD3, HDA1, and SIR3 affect life-span, we determined their expression patterns as a function of age. The mRNA levels of HDA1 and SIR3, normalized to the level of TLC1 RNA, which remains relatively constant with age, dropped sharply from generation 2 to 5 and after this remained low in older cells (Figure 5). For RPD3, RT-PCR analysis was performed because we encountered difficulty detecting its mRNA on Northern blots. The results indicate that the amount of RPD3 mRNA also decreases with age (Figure 6). A substantial decrease in SIR1 mRNA levels was also observed in older cells (our unpublished results). These patterns of decrease in gene expression were always reproducible in at least three determinations in each case. It has been shown previously that a decrease in transcript levels is not characteristic of the majority of genes during the yeast life-span (Egilmez et al., 1989).
Figure 5.
SIR3 and HDA1 mRNA levels in age-synchronized cells. (A) Northern blot. RNA was prepared from 2, 5, 8, 11, 14, and 17 generation-old cells. Total RNA (10 μg) from each RNA sample was loaded in each lane of a gel. The same blot was used repeatedly by hybridizing with one DNA probe at a time and stripping it. The probe DNA to detect 5.8S rRNA (158 bp) was prepared by labeling the 5′ end of an oligonucleotide (5′-CATTTCGCTGCGTTCTTCATC-3′) with 32P. The DNA probe to detect TLC1 RNA (1.3 kb) was the 1.28-kb XhoI fragment isolated from pBlue61 (Singer and Gottschling, 1994). The DNA probe for HDA1 mRNA (2.5 kb) was the 1.8-kb XhoI– XbaI fragment isolated from pskB93 (Rundlett et al., 1996). The DNA probe specific to SIR3 mRNA (3 kb) was the 2.3-kb ClaI–XhoI fragment isolated from pJR517 (provided by J. Rine, University of California, Berkeley, CA). (B) Quantitation of mRNA. The amount of mRNA present in each lane was quantitated by phosphorimaging and normalized to the amount of TLC1 RNA present in the same lane to obtain age-specific relative amounts of individual mRNA species. The amount of TLC1 RNA, which is a component of the yeast telomerase, remains relatively constant compared with 5.8S rRNA.
Figure 6.
RT-PCR analysis of the RPD3 mRNA levels in age-synchronized cells. (A) Gel electrophoresis of PCR products. RNA was prepared from young (2-generation old, g2), middle-aged (8-generation old, g8), old (17-generation old, g17), and mixed-age cells from a stationary culture (St). After equalization of the amount of first-strand cDNA synthesized from each of these RNA samples, twofold serial dilutions of each were prepared and subjected to PCR (see MATERIALS AND METHODS). (B) Quantitation of PCR products. The amount of the PCR amplification products obtained for each dilution was quantitated in each lane in A by phosphorimaging. This was plotted against the template concentration. The slopes of the linear regressions obtained are plotted as a function of age to portray the change in mRNA levels during the life-span. The r2 values for the linear regressions were 0.948, 0.981, and 0.958 for the 2-, 8-, and 17-generation–old cells, respectively.
DISCUSSION
Histone Deacetylase Genes Play a Role in Determining Yeast Life-span
Deletion of RPD3 or HDA1 (in the presence of a sir3Δ) results in a substantial increase in yeast life-span. This suggests that the acetylation profile of the core histones, which determines the degree of accessibility of the DNA in chromatin, is a determinant of yeast longevity. Indeed, the deletion of these deacetylase genes causes changes in chromatin, as evidenced by alterations in transcriptional silencing at three known heterochromatic loci in yeast.
It would be a mistake, however, to interpret the data solely in terms of the changes in gene activity at HM, telomeres, and rDNA, which were assayed to confirm that the deletion of the deacetylases resulted in predictable functional consequences. It is known that RPD3 impinges on the expression of at least several yeast genes outside these heterochromatic loci, and it is likely that HDA1 similarly affects several genes. Apart from these local, gene-specific effects, these deacetylases may exert more global effects on larger chromatin domains. They may also be more generally involved in chromatin remodeling. Nevertheless, it is interesting to explore the potential role of chromatin changes at HM, telomeres, and rDNA in yeast aging that our results support.
Increased Heterochromatic Silencing by rpd3Δ and Partial Suppression of Silencing Defect by rpd3Δ
Hyperacetylation of the core histones is expected to “loosen” chromatin assembly, resulting in decreased silencing (Wolffe, 1996; Grunstein, 1997). In fact, silent heterochromatic regions of metazoan genomes are generally hypoacetylated compared with those of euchromatic regions (reviewed by Grunstein, 1997). Mutation in RPD3, however, increases silencing despite its hyperacetylation effect on all of the N-terminal lysine residues of histones H3 and H4 examined (Sussel et al., 1995; Rundlett et al., 1996; Vannier et al., 1996). It has been speculated that hyperacetylation might result in increased transcription of SIR3 or other genes involved in silencing, hence enhanced silencing (Rundlett et al., 1996; Grunstein, 1997). Our data, however, indicate that this may not be the case; deletion of RPD3 from the sir3Δ or sir2Δ strains resulted in partial yet significant restoration of silencing. (After this article was submitted, Smith et al. [1999] reported that an rpd3 mutation enhances silencing of rDNA and at the HM locus in a sir3 mutant, in agreement with our findings.) Moreover, it is not SIR4 whose transcription could be induced by RPD3 deletion, because increase in SIR4 dosage results in reduced rDNA silencing (Smith et al., 1998). Other arguments can be made for the rpd3Δ effect on telomeric silencing not being the result of SIR4 induction. Alternatively, what is more important in determining the intensity of heterochromatic silencing may be the actual acetylation patterns of the core histones, as suggested by Vannier et al. (1996). Accordingly, increased silencing by deletion of RPD3 might be a consequence of the altered histone acetylation pattern, associated with hyperacetylation.
rDNA Silencing and Yeast Aging
Our data support a positive correlation between yeast aging and loss of rDNA silencing. Deletion of RPD3 or deletion of both HDA1 and SIR3 showed increased rDNA silencing and extended life-span. On the other hand, mutation in SIR2 showed decreased rDNA silencing and shortened life-span. In addition, the SIR4–42 allele, which extends life-span (Kennedy et al., 1995), also increases rDNA silencing (Smith et al., 1998); however, increased rDNA silencing alone may not be sufficient for life-span extension because deletion of SIR4 did not increase life-span yet it increased rDNA silencing (Kennedy et al., 1995; Smith and Boeke, 1997; Smith et al., 1998). Deletion of SIR4 may invoke some other cellular responses that can compromise life-span extension resulting from increased rDNA silencing. These other processes may involve silencing at loci other than RDN1. These other loci may include subtelomeric genes. Loss of silencing at the silent mating-type loci does not in itself appear to be a cause of yeast aging (Kennedy et al., 1995). The notion that telomeric silencing may be a culprit in yeast aging seems at first blush to be difficult to reconcile with the results with the rpd3Δ and hda1Δ. Although the effects of the former on telomeric silencing and life-span are consistent with such an interpretation, the results with the latter are certainly not. It is important to keep in mind, however, that the physiological functions of these two genes are not completely overlapping (Figure 2), although both encode histone deacetylases. Thus, the mechanisms of aging in which they are involved may differ. RPD3 may function in both rDNA and telomeric silencing, whereas HDA1 may impinge on rDNA silencing alone.
One possibility may be that the loss of silencing at HM and telomeres during aging results in age changes, such as sterility (Smeal et al., 1996) and increased generation time (Egilmez and Jazwinski, 1989; Figure 2), that themselves do not affect the life-span. For an effect on life-span to be observed, events at the rDNA locus either alone or in conjunction with the HM and telomere changes may be required. Some of the latter age changes may even have a salutary effect, such as the increase in stress resistance on loss of telomeric silencing (Kennedy et al., 1995).
In evaluating the physiological significance of the enhanced silencing afforded by rpd3Δ or hda1Δ, the magnitude of the actual silencing should be kept in mind. The silencing increases seen in a rpd3Δ sir3Δ strain, although significant, do not approach wild-type levels at HM and telomeres, yet life-span is extended. This focuses attention on rDNA. Life extension is correlated with an increase in rDNA silencing in the rpd3Δ strain (with or without the sir3Δ) and the hda1Δ sir3Δ strain; however, the assay is not easy to quantitate. Furthermore, this assay monitors the activity of an integrated RNA polymerase II-dependent gene and not rRNA transcription. Thus, the physiological significance of the silencing changes is not entirely clear. Nevertheless, the state of rDNA chromatin appears to be important for life-span.
The lack of extension of life-span in the rpd3 mutant in the presence of the sir2 mutation (Figure 4A) may be due to a threshold effect. The enhancement of rDNA silencing seen in the double mutant did not quite approach that seen in the rpd3 single mutant (Figure 3, G and I). Alternatively, the deletion of RPD3 may exert an effect on life-span outside the RDN1 locus. This effect may require concomitant events at RDN1. It is noteworthy that the extension of life-span by deletion of RPD3 is associated with silencing of rDNA well beyond that in the wild type (Figure 3, B and G). Similar silencing is seen in the rpd3Δ sir3Δ and hda1Δ sir3Δ strains (Figure 3, F and H), in which extension of life-span is also observed (Figures 1 and 2).
rDNA Silencing and Generation of ERCs
Recently, accumulation of ERCs has been proposed as a cause of yeast aging (Sinclair and Guarente, 1997). This proposal is based on two major observations. First, sgs1 mutant mother cells accumulated ERCs more rapidly and displayed a shorter life-span. Second, induction of ERCs from plasmids resulted in a shorter life-span of cells harboring the plasmids. The life-span–shortening effect of the sir2 mutant correlated with a loss of rDNA silencing but not with ERC production (Figure 4), although recombination at the rDNA locus increases in sir2. This implies that generation of ERCs might have little to do with chromatin structural changes related to rDNA silencing and that ERCs are not an obligatory feature of aging. Further analysis of generation and accumulation of ERCs in age-synchronized cell populations will help provide more insight into the mutual relationships of rDNA silencing, ERC generation, and yeast aging. It is relevant to note, however, that petite yeasts, which have a substantially larger amount of extrachromosomal rDNA (Conrad-Webb and Butow, 1995), also have a longer life-span than their parent grande strains (Kirchman et al., 1999).
The Low Levels of RPD3 and HDA1 mRNA As a Possible Cause of Aging
The decrease in RPD3 mRNA level in older cells seems to be contradictory to the observation that deletion of RPD3 resulted in life-span extension. It is possible, of course, that the life-span would be shorter were it not for the decrease in RPD3 expression. The HDA1 mRNA level was also lower in old cells than in young cells, although deletion of HDA1 alone had no effect on life-span; however, because the hda1Δ rpd3Δ double mutant had a significantly shorter life-span with high initial mortality, we consider that the lower levels of both RPD3 and HDA1 mRNA species in old cells may be a potential contributor to normal aging. Deletion of either gene alone exhibited different effects on silencing and transcription (Rundlett et al., 1996) and probably, as a consequence, on life-span, because their functions are somewhat different. Their common function involved in life-span maintenance may be revealed when the levels of both gene products are low.
The relatively low level of SIR3 mRNA in old cells provides a plausible explanation for the decrease in telomeric and HM silencing in older cells (Kim et al., 1996; Smeal et al., 1996), because Sir3p is a limiting factor for the silencing effect exerted by the Sir complex (Renauld et al., 1993; Hecht et al., 1996; Strahl-Bolsinger et al., 1997). An alternative but not mutually exclusive explanation for the loss of telomeric silencing with age is the relocalization of the Sir3p and Sir4p to the nucleolus that occurs in old cells (Kennedy et al., 1997). The loss of HM silencing (Smeal et al., 1996), and therefore the increase in sterility (Muller, 1985; Smeal et al., 1996), of old mother cells may be accounted for by a substantial decrease in the SIR1 mRNA level in older cells.
Possible Consequences of Alterations in rDNA Chromatin
The studies described here implicate histone deacetylases and chromatin changes as determinants of yeast longevity. These chromatin changes can impinge on various cellular processes that are dependent on accessibility to DNA. Plausible candidates, suggested by the studies, are alterations in transcriptional status. In fact, chromatin-dependent silencing of rDNA appears to most readily explain the observations. Gene-specific regulatory effects at loci throughout the yeast genome may also be involved, but they are more difficult to assess, as are other effects of chromatin structural changes. If indeed transcription of rDNA is the process critical for life-span, what are the ramifications? A proper supply of rRNA might be necessary to guarantee longevity. In fact, the deletion of SIR2 curtails life-span (Figure 4A) and has been suggested to yield excessive production of rRNA (Smith and Boeke, 1997). In contrast, the rpd3Δ (or deletion of HDA1 and SIR3) extends life-span and may tighten control to prevent undue rRNA transcription.
There are data consistent with the model sketched above. During yeast aging, there is an increase in cellular rRNA content that does not keep up with the increase in cell volume (Motizuki and Tsurugi, 1992; Jazwinski, 1996). Concomitantly, a decline in protein synthesis rate occurs, which may be the cause of the increase in generation time and ultimately death. These excessive rRNA levels may not be matched by ribosomal protein synthesis, resulting in defective ribosome assembly. The rpd3Δ may mitigate this imbalance to maintain protein synthesis rates longer. This scenario does not take into account, of course, the action of the deacetylases and Sir proteins at other locations, which may further complicate their effects on longevity.
ACKNOWLEDGMENTS
We thank Ashley Schneider in this laboratory for help in construction of strain YAB13. We thank those who provided plasmids and yeast strains (P. A. Kirchman, R. Gaber, M. Grunstein, L. Pillus, D. J. Stillman, S. Holmes, and J. Boeke). We thank B. Villeponteau for stimulating discussion. This work was supported by grants from the National Institute on Aging of National Institutes of Health (United States Public Health Service). A.B. was the recipient of a postdoctoral fellowship from the Ministry of Education and Culture of Spain.
REFERENCES
- Allsopp R, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1993. [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:225–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- Chen JB, Sun J, Jazwinski SM. Prolongation of the yeast life span by the v-Ha-RAS oncogene. Mol Microbiol. 1990;4:2081–2086. doi: 10.1111/j.1365-2958.1990.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Conrad-Webb H, Butow RA. A polymerase switch in the synthesis of rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:2420–2428. doi: 10.1128/mcb.15.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost GJ, Boeke JD. A useful colony color phenotype associated with the yeast selectable/counterselectable marker MET15. Yeast. 1996;12:939–941. doi: 10.1002/(SICI)1097-0061(199608)12:10%3C939::AID-YEA988%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- D’mello NP, Jazwinski SM. Telomere length constancy during aging of Saccharomyces cerevisiae. J Bacteriol. 1991;173:6709–6713. doi: 10.1128/jb.173.21.6709-6713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egilmez NK, Chen JB, Jazwinski SM. Specific alterations in transcript prevalence during the yeast life span. J Biol Chem. 1989;264:14312–14317. [PubMed] [Google Scholar]
- Egilmez NK, Chen JB, Jazwinski SM. Preparation and partial characterization of old yeast cells. J Gerontol. 1990;45:B9–B17. doi: 10.1093/geronj/45.1.b9. [DOI] [PubMed] [Google Scholar]
- Egilmez NK, Jazwinski SM. Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae. J Bacteriol. 1989;171:37–42. doi: 10.1128/jb.171.1.37-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider GW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Position effect variegation after 60 years. Trends Genet. 1990;6:422–426. doi: 10.1016/0168-9525(90)90304-o. [DOI] [PubMed] [Google Scholar]
- Herskowitz I, Jensen RE. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Longevity-assurance genes and mitochondrial DNA alterations: yeast and filamentous fungi. In: Schneider EL, Rowe JW, editors. Handbook of the Biology of Aging. 4th ed. San Diego: Academic Press; 1996. pp. 39–54. [Google Scholar]
- Jazwinski SM, Kim S, Lai C-Y, Benguria A. Epigenetic stratification: the role of individual change in the biological aging process. Exp Gerontol. 1998;33:571–580. doi: 10.1016/s0531-5565(98)00029-1. [DOI] [PubMed] [Google Scholar]
- Jiang YW, Stillman DJ. Epigenetic effects on yeast transcription caused by mutations in an actin-related protein present in the nucleus. Genes Dev. 1996;10:604–619. doi: 10.1101/gad.10.5.604. [DOI] [PubMed] [Google Scholar]
- Johnson LM, Fisher-Adams G, Grunstein M. Identification of a nonbasic domain in the histone H4 N-terminus required for repression of the yeast silent mating loci. EMBO J. 1992;11:2201–2209. doi: 10.1002/j.1460-2075.1992.tb05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Kayne PS, Kahn ES, Grunstein M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1990;87:6286–6290. doi: 10.1073/pnas.87.16.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Austriaco NR, Jr, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, Pak SM, Laroche T, Gasser SM, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Kim S, Kirchman PA, Benguria A, Jazwinski SM. Experimentation with the yeast model. In: Yu BP, editor. Methods in Aging Research. Boca Raton: CRC Press; 1998. pp. 191–213. [Google Scholar]
- Kim S, Villeponteau B, Jazwinski SM. Effect of replicative age on transcriptional silencing near telomeres in Saccharomyces cerevisiae. Biochem Biophy Res Comm. 1996;219:370–376. doi: 10.1006/bbrc.1996.0240. [DOI] [PubMed] [Google Scholar]
- Kirchman PA, Kim S, Lai C-Y, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJS, Strathern JN, Broach JR, Hicks JB. Regulation of transcription in expressed and unexpressed mating type cassettes of yeast. Nature. 1981;289:239–244. doi: 10.1038/289239a0. [DOI] [PubMed] [Google Scholar]
- Laurenson P, Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S, Rine J. Silencing and heritable domains of gene expression. Annu Rev Cell Dev Biol. 1995;11:519–548. doi: 10.1146/annurev.cb.11.110195.002511. [DOI] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- Motizuki M, Tsurugi K. The effect of aging on protein synthesis in the yeast Saccharomyces cerevisiae. Mech Ageing Dev. 1992;64:235–245. doi: 10.1016/0047-6374(92)90081-n. [DOI] [PubMed] [Google Scholar]
- Muller I. Parental age and the life span of zygotes of Saccharomyces cerevisiae. Antonie Leeuwenhoek J Microbiol Serol. 1985;51:1–10. doi: 10.1007/BF00444223. [DOI] [PubMed] [Google Scholar]
- Muller I, Zimmermann M, Becker D, Flomer M. Calendar life span versus budding life span of Saccharomyces cerevisiae. Mech Ageing Dev. 1980;12:47–52. doi: 10.1016/0047-6374(80)90028-7. [DOI] [PubMed] [Google Scholar]
- Nasmyth KA, Tatchell K, Hall BD, Astell CR, Smith M. A position effect in the control of transcription at yeast mating type loci. Nature. 1981;289:244–250. doi: 10.1038/289244a0. [DOI] [PubMed] [Google Scholar]
- Park EC, Szostak JW. Point mutations in the yeast histone H4 gene prevent silencing of the silent mating type locus HML. Mol Cell Biol. 1990;10:4932–4934. doi: 10.1128/mcb.10.9.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld H, Aparicio OM, Zierath P, Billington BL, Chhablani SK, Gottschling DE. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- Rundlett SE, Carmen AA, Kobayashi R, Bavykin S, Turner BM, Grunstein M. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc Natl Acad Sci USA. 1996;93:14503–14508. doi: 10.1073/pnas.93.25.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles: a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Singer MS, Gottschling DE. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- Smeal T, Claus J, Kennedy B, Cole F, Guarente L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell. 1996;84:633–642. doi: 10.1016/s0092-8674(00)81038-7. [DOI] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Pillus L, Boeke JD. Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics. 1998;149:1205–1219. doi: 10.1093/genetics/149.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Caputo E, Boeke JD. A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol Cell Biol. 1999;19:3184–3197. doi: 10.1128/mcb.19.4.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spofford JB. Position-effect variegation. In: Ashburner M, Novitski E, editors. The Genetics and Biology of Drosophila. 1C. New York: Academic Press; 1976. pp. 955–1018. [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Sun J, Kale SP, Childress AM, Pinswasdi C, Jazwinski SM. Divergent roles of RAS1 and RAS2 in yeast longevity. J Biol Chem. 1994;269:18638–18645. [PubMed] [Google Scholar]
- Sussel L, Vannier D, Shore D. Suppressors of defective silencing in yeast: effects on transcriptional repression at the HMR locus, cell growth and telomere structure. Genetics. 1995;141:873–888. doi: 10.1093/genetics/141.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Ling X, Grunstein M. The histone H3 amino terminus is required for both telomeric and silent mating locus repression in yeast. Nature. 1994;369:245–247. doi: 10.1038/369245a0. [DOI] [PubMed] [Google Scholar]
- Vannier D, Balderes D, Shore D. Evidence that the transcriptional regulators SIN3 and RPD3, and a novel gene (SDS3) with similar functions, are involved in transcriptional silencing in S. cerevisiae. Genetics. 1996;144:1343–1353. doi: 10.1093/genetics/144.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Gaber RF. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP. Histone deacetylase: a regulator of transcription. Science. 1996;272:371–372. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]
- Yang WM, Inouye C, Zeng Y, Bearss D, Seto E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc Natl Acad Sci USA. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]