Abstract
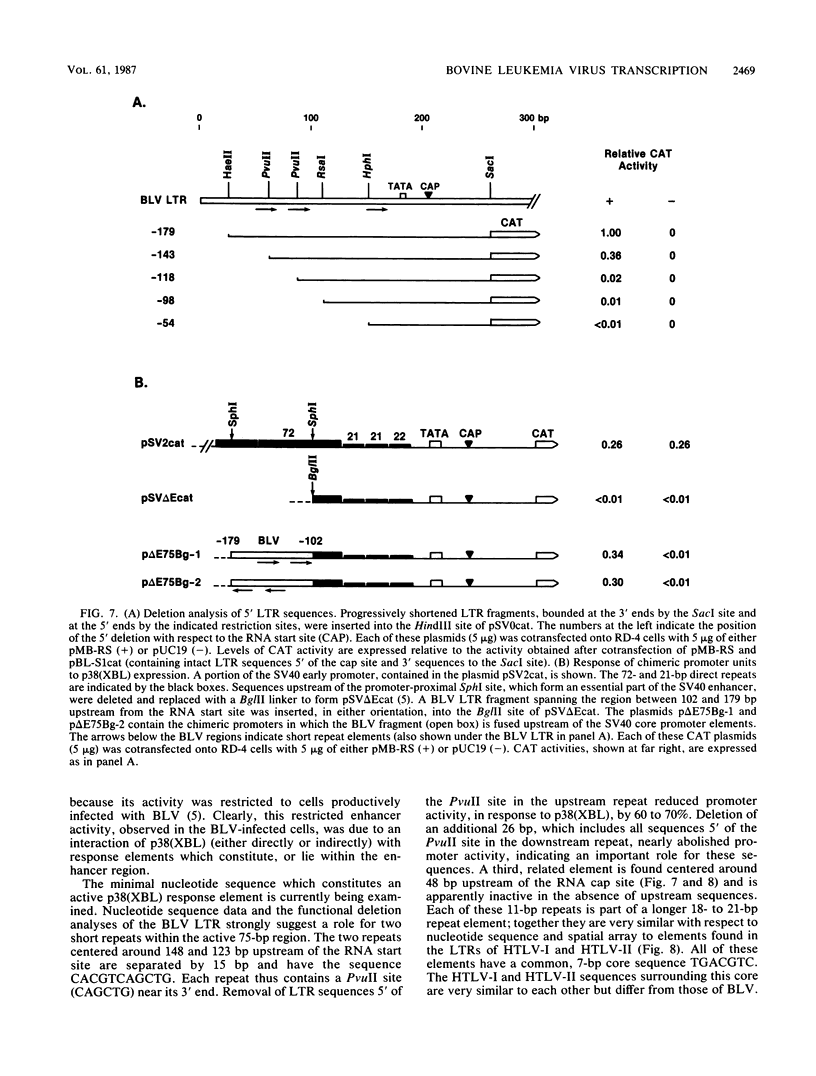
Bovine leukemia virus (BLV) gene expression is exquisitely regulated at multiple levels, including a transcriptional control effected by virus-encoded trans-acting factors and cis-acting target sequences. Like the human T-cell leukemia viruses type I and type II, but unlike other RNA tumor viruses, BLV contains several open reading frames at the 3' end of its genome. A subgenomic mRNA which encodes two overlapping reading frames from this region could produce proteins of 38 and 18 kilodaltons (kDa). A series of cis-trans experiments using transfected virus gene constructs in different combinations revealed that expression of the 38-kDa protein was both necessary and sufficient to activate, in trans, the BLV promoter. This activation was specific for the BLV long terminal repeat, as a variety of related retroviral promoters were not responsive to the expression of the 38-kDa protein p38(XBL). Deletion analysis and construction of chimeric promoters identified a 75-base-pair long terminal repeat region which functions like a p38(XBL)-dependent enhancer element.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Chen I. S., McLaughlin J., Golde D. W. Long terminal repeats of human T-cell leukaemia virus II genome determine target cell specificity. Nature. 1984 May 17;309(5965):276–279. doi: 10.1038/309276a0. [DOI] [PubMed] [Google Scholar]
- Derse D., Caradonna S. J., Casey J. W. Bovine leukemia virus long terminal repeat: a cell type-specific promoter. Science. 1985 Jan 18;227(4684):317–320. doi: 10.1126/science.2981431. [DOI] [PubMed] [Google Scholar]
- Derse D., Casey J. W. Two elements in the bovine leukemia virus long terminal repeat that regulate gene expression. Science. 1986 Mar 21;231(4744):1437–1440. doi: 10.1126/science.3006241. [DOI] [PubMed] [Google Scholar]
- Derse D., Diniak A. J., Casey J. W., Deininger P. L. Nucleotide sequence and structure of integrated bovine leukemia virus long terminal repeats. Virology. 1985 Feb;141(1):162–166. doi: 10.1016/0042-6822(85)90193-x. [DOI] [PubMed] [Google Scholar]
- Derse D., Dorn P. L., Levy L., Stephens R. M., Rice N. R., Casey J. W. Characterization of equine infectious anemia virus long terminal repeat. J Virol. 1987 Mar;61(3):743–747. doi: 10.1128/jvi.61.3.743-747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Kiyokawa T., Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa J., Seiki M., Sato M., Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40 chi HTLV-I. EMBO J. 1986 Apr;5(4):713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Bruck C., Kettmann R., Burny A. Bovine leukemia virus. Curr Top Microbiol Immunol. 1984;112:1–19. doi: 10.1007/978-3-642-69677-0_1. [DOI] [PubMed] [Google Scholar]
- Goh W. C., Sodroski J., Rosen C., Essex M., Haseltine W. A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985 Mar 8;227(4691):1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Wong-Staal F., Manzari V., Gallo R. C., Sodroski J. G., Trus M. D., Perkins D., Patarca R., Haseltine W. A. Long terminal repeat structure of an American isolate of type I human T-cell leukemia virus. Virology. 1984 Dec;139(2):340–345. doi: 10.1016/0042-6822(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Sodroski J. G., Haseltine W. A., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Essex M. Antigens encoded by the 3'-terminal region of human T-cell leukemia virus: evidence for a functional gene. Science. 1984 Oct 5;226(4670):57–61. doi: 10.1126/science.6089350. [DOI] [PubMed] [Google Scholar]
- Mamoun R. Z., Astier-Gin T., Kettmann R., Deschamps J., Rebeyrotte N., Guillemain B. J. The pX region of the bovine leukemia virus is transcribed as a 2.1-kilobase mRNA. J Virol. 1985 May;54(2):625–629. doi: 10.1128/jvi.54.2.625-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Portetelle D., Bruck C., Burny A., Dekegel D., Mammerickx M., Urbain J. Detection of complement-dependent lytic antibodies in sera from bovine leukemia virus-infected animals. Ann Rech Vet. 1978;9(4):667–674. [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Sagata N., Tsuzuku-Kawamura J., Nagayoshi-Aida M., Shimizu F., Imagawa K., Ikawa Y. Identification and some biochemical properties of the major XBL gene product of bovine leukemia virus. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7879–7883. doi: 10.1073/pnas.82.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ikawa Y. Two distinct polypeptides may be translated from a single spliced mRNA of the X genes of human T-cell leukemia and bovine leukemia viruses. FEBS Lett. 1985 Nov 11;192(1):37–42. doi: 10.1016/0014-5793(85)80038-7. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Yoshida M. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6899–6902. doi: 10.1073/pnas.79.22.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Golde D. W., Miwa M., Sugimura T., Chen I. S. Nucleotide sequence analysis of the long terminal repeat of human T-cell leukemia virus type II. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1079–1083. doi: 10.1073/pnas.81.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Miwa M., Slamon D. J., Chen I. S., Hoshino H., Takano M., Fujino M., Sugimura T. Identification of new gene products coded from X regions of human T-cell leukemia viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(2):302–306. doi: 10.1073/pnas.82.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Wachsman W., Takahashi Y., Golde D. W., Miwa M., Sugimura T., Chen I. S. Nucleotide sequence of the 3' region of an infectious human T-cell leukemia virus type II genome. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6657–6661. doi: 10.1073/pnas.81.21.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Press M. F., Souza L. M., Murdock D. C., Cline M. J., Golde D. W., Gasson J. C., Chen I. S. Studies of the putative transforming protein of the type I human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1427–1430. doi: 10.1126/science.2990027. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Shimotohno K., Cline M. J., Golde D. W., Chen I. S. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science. 1984 Oct 5;226(4670):61–65. doi: 10.1126/science.6089351. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Rosen C., Goh W. C., Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985 Jun 21;228(4706):1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Temin H. M. Function of the retrovirus long terminal repeat. Cell. 1982 Jan;28(1):3–5. doi: 10.1016/0092-8674(82)90367-1. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 1981 Nov;27(1 Pt 2):1–3. doi: 10.1016/0092-8674(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Wachsman W., Golde D. W., Temple P. A., Orr E. C., Clark S. C., Chen I. S. HTLV x-gene product: requirement for the env methionine initiation codon. Science. 1985 Jun 28;228(4707):1534–1537. doi: 10.1126/science.2990032. [DOI] [PubMed] [Google Scholar]







