Abstract
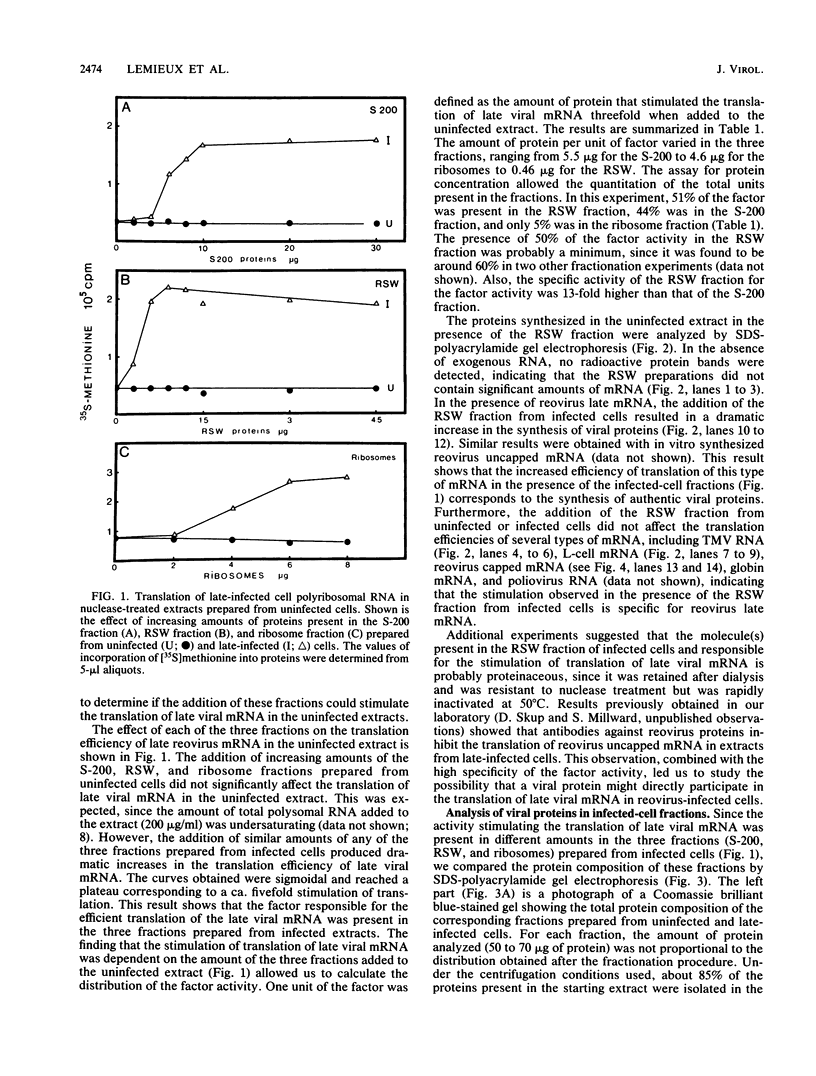
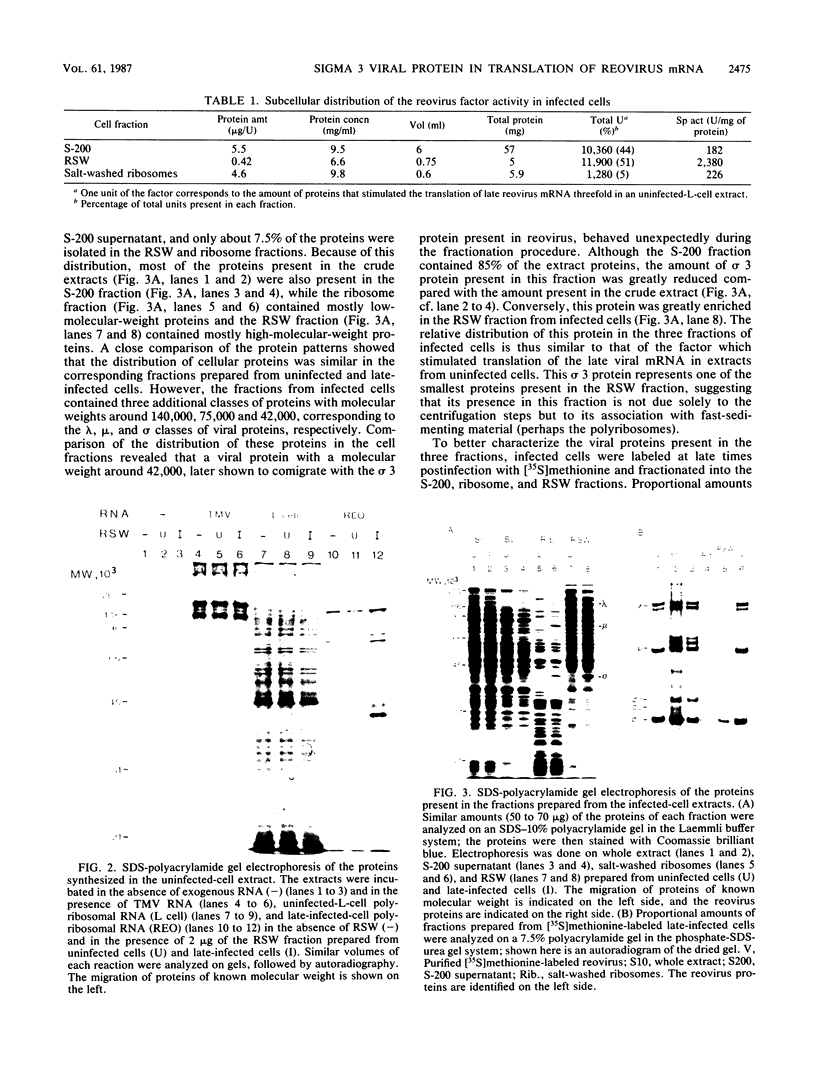
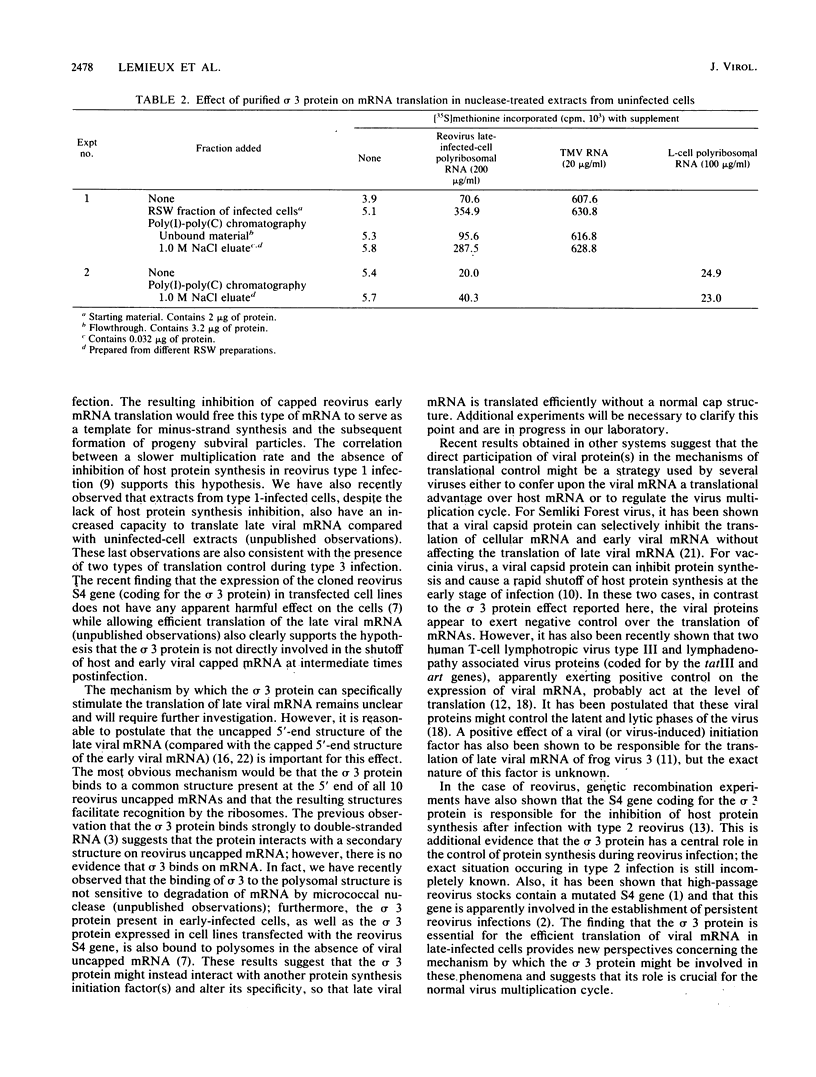
Reovirus late (uncapped) mRNA was previously shown to be efficiently translated in vitro extracts prepared from infected cells but not from uninfected cells. We demonstrated that different fractions from infected cells can stimulate translation of late viral mRNA when added to uninfected extracts. The activity of the different fractions correlated with their relative content of the sigma 3 capsid protein; the fraction prepared by high-salt wash of the ribosomes had the highest specific activity. The activity present in this fraction was abolished by preincubation with an anti-sigma 3 serum. Purified sigma 3 protein also stimulated the translation of late viral mRNA, confirming that it was the factor involved. Altogether, these results suggest that this protein plays the role of a late-viral-mRNA-specific initiation factor. The absence of an inhibitory effect of sigma 3 on the translation of other mRNAs indicates that this protein is not directly involved in the inhibition of host and early viral mRNA translation that occurs in infected cells but that a second mechanism is probably operative.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Chakraborty P. R., Fields B. N. Genetic variation during lytic reovirus infection: high-passage stocks of wild-type reovirus contain temperature-sensitive mutants. J Virol. 1980 Apr;34(1):285–287. doi: 10.1128/jvi.34.1.285-287.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Fields B. N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982 Mar;28(3):605–612. doi: 10.1016/0092-8674(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Huismans H., Joklik W. K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976 Apr;70(2):411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Lemay G., Millward S. Expression of the cloned S4 gene of reovirus serotype 3 in transformed eucaryotic cells: enrichment of the viral protein in the crude initiation factor fraction. Virus Res. 1986 Nov;6(2):133–140. doi: 10.1016/0168-1702(86)90045-6. [DOI] [PubMed] [Google Scholar]
- Lemieux R., Zarbl H., Millward S. mRNA discrimination in extracts from uninfected and reovirus-infected L-cells. J Virol. 1984 Jul;51(1):215–222. doi: 10.1128/jvi.51.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S. M., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Multiplication rate but not yield of reovirus serotypes 1 and 3 correlates with the level of virus-mediated inhibition of cellular protein synthesis. Virology. 1984 Jul 15;136(1):133–143. doi: 10.1016/0042-6822(84)90254-x. [DOI] [PubMed] [Google Scholar]
- Person-Fernandez A., Beaud G. Purification and characterization of a protein synthesis inhibitor associated with vaccinia virus. J Biol Chem. 1986 Jun 25;261(18):8283–8289. [PubMed] [Google Scholar]
- Raghow R., Granoff A. Cell-free translation of frog virus 3 messenger RNAs. Initiation factors from infected cells discriminate between early and late viral mRNAs. J Biol Chem. 1983 Jan 10;258(1):571–578. [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology. 1982 Oct 30;122(2):381–391. doi: 10.1016/0042-6822(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Skup D., Millward S. Highly efficient translation of messenger RNA in cell-free extracts prepared from L-cells. Nucleic Acids Res. 1977 Oct;4(10):3581–3587. doi: 10.1093/nar/4.10.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Millward S. Reovirus-induced modification of cap-dependent translation in infected L cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):152–156. doi: 10.1073/pnas.77.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Zarbl H., Millward S. Regulation of translation in L-cells infected with reovirus. J Mol Biol. 1981 Sep 5;151(1):35–55. doi: 10.1016/0022-2836(81)90220-5. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Skup D., Trachsel H., Millward S. In vitro translation in reovirus- and poliovirus-infected cell extracts. Effects of anti-cap binding protein monoclonal antibody. J Biol Chem. 1981 May 10;256(9):4138–4141. [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbl H., Skup D., Millward S. Reovirus progeny subviral particles synthesize uncapped mRNA. J Virol. 1980 May;34(2):497–505. doi: 10.1128/jvi.34.2.497-505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Kasperaitis M., Voorma H. O., Benne R. Infection of neuroblastoma cells by Semliki Forest virus. The interference of viral capsid protein with the binding of host messenger RNAs into initiation complexes is the cause of the shut-off of host protein synthesis. Eur J Biochem. 1984 Feb 1;138(3):473–478. doi: 10.1111/j.1432-1033.1984.tb07940.x. [DOI] [PubMed] [Google Scholar]