Abstract
B-cell lymphomas induced in chickens infected with avian leukosis viruses are characterized by integration of the virus within the cellular myc locus and alteration of c-myc expression. Although avian leukosis viruses are intact, replication-competent retroviruses, the structures of many myc-associated proviruses are altered by deletions, raising the possibility that proviral defectiveness plays an essential role in oncogenesis. We found that all myc-associated proviruses in 21 independent tumors had deletions, which were confined to the viral genome and did not extend into adjacent cellular sequences. Deletions were not random but, in at least 85% of the myc-associated proviruses, involved a region near the 5' end of the proviral genome where elements implicated in control of viral gene expression have been localized. A second class of deletions involved sequences in the 3' half of the viral genome and included the splice acceptor site used in generating viral env mRNA. Both the 5' and 3' long terminal repeats of myc-associated proviruses appeared to be structurally intact in most tumors, although the 5' long terminal repeats were not involved in expression of either U5-myc transcripts or detectable steady-state viral RNAs. A complex array of repeated sequence elements surrounded the junctions of the internal deletions in two myc-associated proviruses. The organization of the deleted proviruses was similar to that of deleted unintegrated viral molecules, consistent with a model in which deletions occurred prior to integration.
Full text
PDF
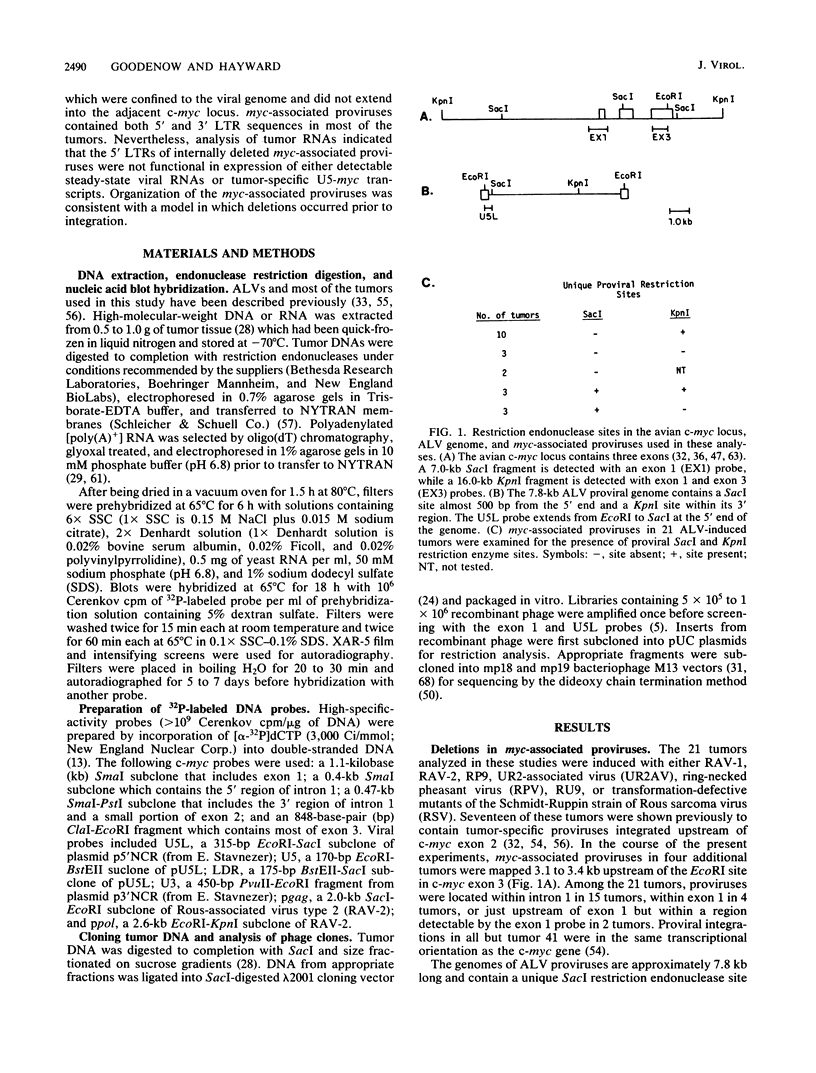

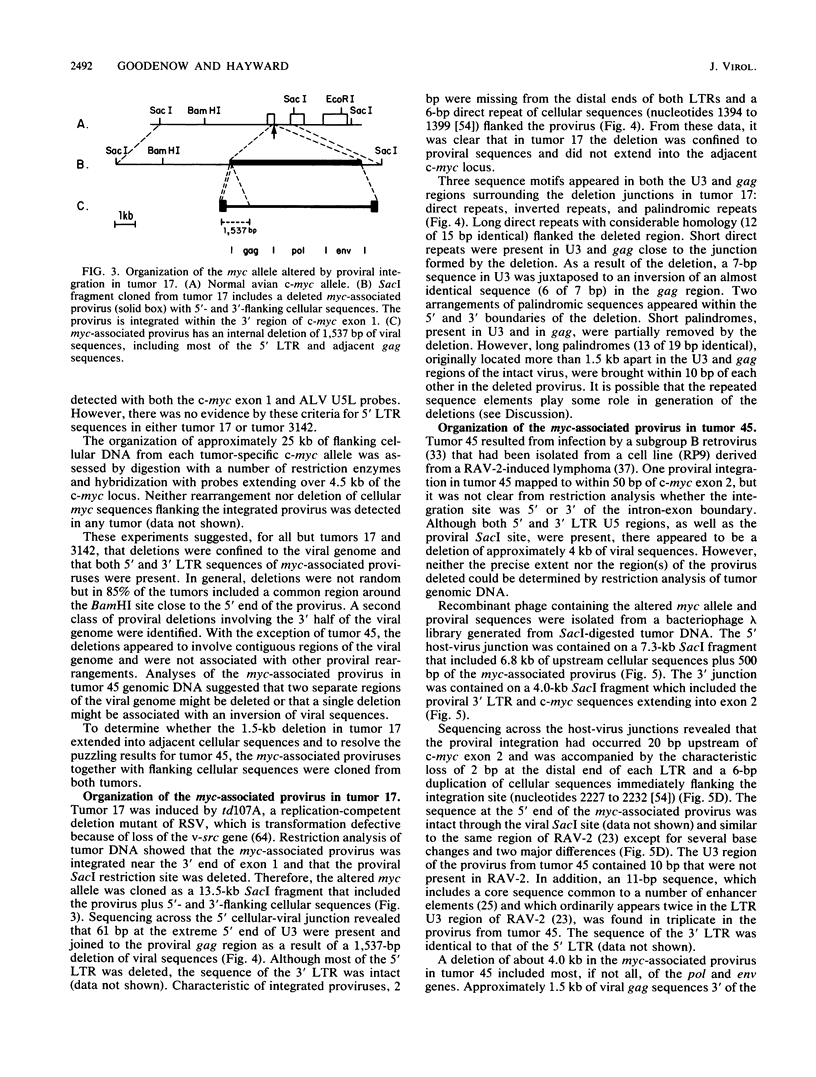
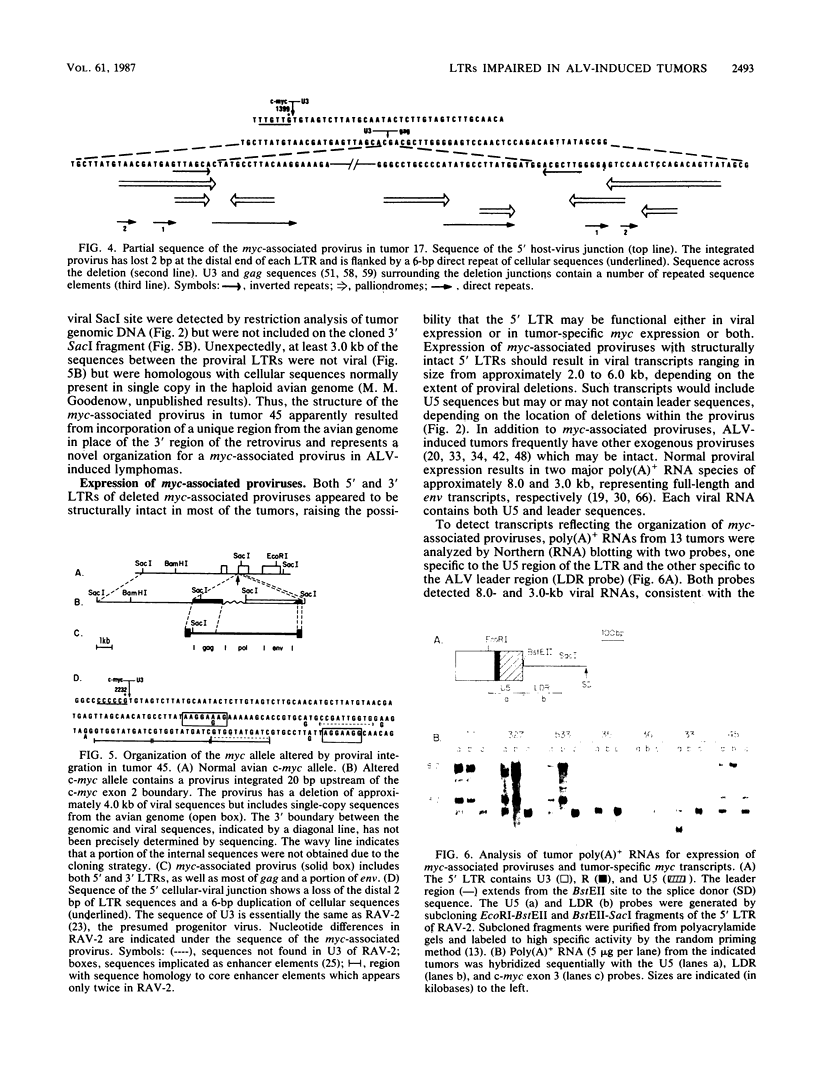





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Avian leukosis virus infection: analysis of viremia and DNA integration in susceptible and resistant chicken lines. J Virol. 1984 Jul;51(1):123–130. doi: 10.1128/jvi.51.1.123-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A. 1985 Jan;82(1):213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Selective integration of avian leukosis virus in different hematopoietic tissues. Virology. 1986 Dec;155(2):557–566. doi: 10.1016/0042-6822(86)90216-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Rous sarcoma virus encodes a transcriptional activator. Cell. 1985 Mar;40(3):537–546. doi: 10.1016/0092-8674(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Colicelli J., Goff S. P. Mutants and pseudorevertants of Moloney murine leukemia virus with alterations at the integration site. Cell. 1985 Sep;42(2):573–580. doi: 10.1016/0092-8674(85)90114-x. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Duyk G., Leis J., Longiaru M., Skalka A. M. Selective cleavage in the avian retroviral long terminal repeat sequence by the endonuclease associated with the alpha beta form of avian reverse transcriptase. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6745–6749. doi: 10.1073/pnas.80.22.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyk G., Longiaru M., Cobrinik D., Kowal R., deHaseth P., Skalka A. M., Leis J. Circles with two tandem long terminal repeats are specifically cleaved by pol gene-associated endonuclease from avian sarcoma and leukosis viruses: nucleotide sequences required for site-specific cleavage. J Virol. 1985 Nov;56(2):589–599. doi: 10.1128/jvi.56.2.589-599.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Crittenden L. B., Kung H. J. Orientation and position of avian leukosis virus DNA relative to the cellular oncogene c-myc in B-lymphoma tumors of highly susceptible 15I5 X 7(2) chickens. J Virol. 1982 Nov;44(2):742–746. doi: 10.1128/jvi.44.2.742-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Vora A. C., Swanstrom R., Olsen J. C. Nuclease mechanism of the avian retrovirus pp32 endonuclease. J Virol. 1986 Jun;58(3):970–974. doi: 10.1128/jvi.58.3.970-974.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Differential transcription from the long terminal repeats of integrated avian leukosis virus DNA. J Virol. 1986 Nov;60(2):497–505. doi: 10.1128/jvi.60.2.497-505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Groudine M. Transcription of three c-myc exons is enhanced in chicken bursal lymphoma cell lines. Proc Natl Acad Sci U S A. 1985 Jan;82(1):53–57. doi: 10.1073/pnas.82.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Gasic G. P., Rogler C. E., Skalka A. M., Ju G., Hishinuma F., Papas T., Astrin S. M., Hayward W. S. Molecular analysis of the c-myc locus in normal tissue and in avian leukosis virus-induced lymphomas. J Virol. 1982 Oct;44(1):158–166. doi: 10.1128/jvi.44.1.158-166.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Nottenburg C., Varmus H. E. Features of the chicken c-myc gene that influence the structure of c-myc RNA in normal cells and bursal lymphomas. Mol Cell Biol. 1986 Aug;6(8):2800–2806. doi: 10.1128/mcb.6.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. C., Swanstrom R. A new pathway in the generation of defective retrovirus DNA. J Virol. 1985 Dec;56(3):779–789. doi: 10.1128/jvi.56.3.779-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachl C., Schubach W., Eisenman R., Linial M. Expression of c-myc RNA in bursal lymphoma cell lines: identification of c-myc-encoded proteins by hybrid-selected translation. Cell. 1983 Jun;33(2):335–344. doi: 10.1016/0092-8674(83)90415-4. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. Circles with two tandem LTRs are precursors to integrated retrovirus DNA. Cell. 1984 Mar;36(3):673–679. doi: 10.1016/0092-8674(84)90347-7. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. A., Lewis W. G., Crittenden L. B., Kung H. J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway A. A., Swift R. A., Kung H. J., Fujita D. J. In vitro transcription analysis of the viral promoter involved in c-myc activation in chicken B lymphomas: detection and mapping of two RNA initiation sites within the reticuloendotheliosis virus long terminal repeat. J Virol. 1985 Apr;54(1):161–170. doi: 10.1128/jvi.54.1.161-170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins T., Bister K., Garon C., Papas T., Duesberg P. Structural relationship between a normal chicken DNA locus and the transforming gene of the avian acute leukemia virus MC29. J Virol. 1982 Feb;41(2):635–642. doi: 10.1128/jvi.41.2.635-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Gagnon G. C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986 Jan;57(1):28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Potter M., Mushinski J. F., Lavu S., Reddy E. P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984 Nov 30;226(4678):1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- Shih C. K., Linial M., Goodenow M. M., Hayward W. S. Nucleotide sequence 5' of the chicken c-myc coding region: localization of a noncoding exon that is absent from myc transcripts in most avian leukosis virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4697–4701. doi: 10.1073/pnas.81.15.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Neckameyer W. S., Hayward W. S., Smith R. E. Genetic determinants of neoplastic diseases induced by a subgroup F avian leukosis virus. J Virol. 1987 Apr;61(4):1203–1212. doi: 10.1128/jvi.61.4.1203-1212.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Smith R. E., Hayward W. S. Mechanisms of oncogenesis by subgroup F avian leukosis viruses. J Virol. 1984 Oct;52(1):1–8. doi: 10.1128/jvi.52.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., DeLorbe W. J., Bishop J. M., Varmus H. E. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc Natl Acad Sci U S A. 1981 Jan;78(1):124–128. doi: 10.1073/pnas.78.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. Nucleotide sequence of the 5' noncoding region and part of the gag gene of Rous sarcoma virus. J Virol. 1982 Feb;41(2):535–541. doi: 10.1128/jvi.41.2.535-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Snyder P., Hanafusa T., Hanafusa H. Evidence for the common origin of viral and cellular sequences involved in sarcomagenic transformation. J Virol. 1980 Jul;35(1):52–64. doi: 10.1128/jvi.35.1.52-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Westaway D., Payne G., Varmus H. E. Proviral deletions and oncogene base-substitutions in insertionally mutagenized c-myc alleles may contribute to the progression of avian bursal tumors. Proc Natl Acad Sci U S A. 1984 Feb;81(3):843–847. doi: 10.1073/pnas.81.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



