Abstract
During barley (Hordeum vulgare) seed development, phosphoenolpyruvate carboxylase (PEPC) activity increased and PEPC-specific antibodies revealed housekeeping (103-kD) and inducible (108-kD) subunits. Bacterial-type PEPC fragments were immunologically detected in denatured protein extracts from dry and imbibed conditions; however, on nondenaturing gels, the activity of the recently reported octameric PEPC (in castor [Ricinus communis] oil seeds) was not detected. The phosphorylation state of the PEPC, as judged by l-malate 50% inhibition of initial activity values, phosphoprotein chromatography, and immunodetection of the phosphorylated N terminus, was found to be high between 8 and 18 d postanthesis (DPA) and during imbibition. In contrast, the enzyme appeared to be in a low phosphorylation state from 20 DPA up to dry seed. The time course of 32/36-kD, Ca2+-independent PEPC kinase activity exhibited a substantial increase after 30 DPA that did not coincide with the PEPC phosphorylation profile. This kinase was found to be inhibited by l-malate and not by putative protein inhibitors, and the PEPC phosphorylation status correlated with high glucose-6-phosphate to malate ratios, thereby suggesting an in vivo metabolic control of the kinase. PEPC phosphorylation was also regulated by photosynthate supply at 11 DPA. In addition, when fed exogenously to imbibing seeds, abscisic acid significantly increased PEPC kinase activity. This was further enhanced by the cytosolic protein synthesis inhibitor cycloheximide but blocked by protease inhibitors, thereby suggesting that the phytohormone acts on the stability of the kinase. We propose that a similar abscisic acid-dependent effect may contribute to produce the increase in PEPC kinase activity during desiccation stages.
Phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31) catalyzes the β-carboxylation of PEP to yield oxaloacetate (OAA) and inorganic phosphate, a reaction that is involved in several metabolic contexts in plants (Chollet et al., 1996). Subsequently, OAA can be reduced to malate and/or transaminated to generate Asp. In seeds, the enzyme recycles respired CO2, thus providing the Krebs cycle with OAA whenever the demand for amino acid and protein synthesis is high (Huppe and Turpin, 1994; Golombek et al., 1999). In addition, the enzyme has been proposed to contribute to fatty acid synthesis (Sangwan et al., 1992; Tripodi et al., 2005) and to starchy endosperm acidification during seed maturation (Macnicol and Jacobsen, 1992). Gel-blot experiments and specific antibodies have constantly detected several PEPC subunits (of about 100–110 kD) thought to act in vivo as a homotetrameric enzyme. All plant PEPCs sequenced so far, including C4, Crassulacean acid metabolism (CAM), and C3 species, contain the N-terminal phosphorylation domain (Chollet et al., 1996; Nimmo, 2003). Recently, a bacterial-related PEPC (BTPC) lacking the N-terminal phosphorylation domain was identified in Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa; Sánchez and Cejudo, 2003); BTPC gene and transcripts have also been identified in soybean (Glycine max) and castor bean (Ricinus communis) plants (Sullivan et al., 2004; Gennidakis et al., 2007). In addition, two PEPC isoforms (p107 homotetrameric, class 1 PEPC; and p107/p64 heterooctameric, class 2 PEPC) have been described in developing castor oil seed, where the p64 polypeptide is structurally and immunologically unrelated to p107 (Blonde and Plaxton, 2003; Tripodi et al., 2005). More recent data have established that this peptide is a fragment of a phosphorylated bacterial-like PEPC subunit (118 kD) that is rapidly proteolyzed in protein extracts by a Cys endopeptidase and that the molecular mass of the heterooctameric complex is close to 910 kD (Gennidakis et al., 2007; Uhrig et al., 2008). These results were reminiscent of the class 2 PEPC formerly characterized in green microalgae (Chlamydomonas reinhardtii and Selenastrum minutum; Rivoal et al., 1996, 1998) and studied further by others (Mamedov et al., 2005; Moellering et al., 2007).
All phosphorylatable plant-type PEPCs show allosteric properties and are typically activated by Glc-6-P and inhibited by l-malate (Echevarría et al., 1994; Chollet et al., 1996). In plant tissues active in nitrogen assimilation and/or transamination reactions, other allosteric inhibitors, such as Asp and Glu, are also operative (Law and Plaxton, 1997; Golombek et al., 1999; Blonde and Plaxton, 2003). The phosphorylation of a highly conserved Ser at the N-terminal end of the PEPC affects the kinetic properties of the enzyme (Nimmo et al., 1987; Echevarría et al., 1994; Chollet et al., 1996). It is now a well-established fact that PEPC kinase is a low molecular mass Ser/Thr protein kinase (32 kD) without N- and C-terminal extensions that does not possess calcium-binding domains, although it is structurally related to the calcium-dependent family of protein kinases. It is regulated at the transcriptional level in most physiological situations investigated so far (for review, see Echevarría and Vidal, 2003; Nimmo, 2003; Izui et al., 2004). PEPC-related metabolites (Echevarría et al., 1994, Bakrim et al., 1998, Murmu and Plaxton, 2007), a redox mechanism involving thioredoxins (Tsuchida et al., 2004; Murmu and Plaxton, 2007), and a protein inhibitor (Nimmo et al., 2001) have been suggested also to regulate PEPC kinase activity. Notably, metabolites have been shown to operate in vivo (e.g. malate inhibits while Glc-6-P antagonizes this effect) in protoplasts of sorghum (Sorghum bicolor) leaves (Bakrim et al., 1998). In addition, it has been proposed, in CAM plants, that a high concentration of l-malate reduces both mRNA and the accumulation of the kinase itself (Borland et al., 1999). In vitro, PEPC phosphorylation modulates the metabolite control of the enzyme (increasing the 50% inhibition of initial activity [IC50] for the feedback inhibitor l-malate and decreasing the activation constant for the activator Glc-6-P; Echevarría et al., 1994; Chollet et al., 1996; Vidal and Chollet, 1997). This allows a powerful protection against the l-malate that the enzyme activity contributes to produce, keeping the catalytic rate at levels required for the physiological context. However, dephosphorylation of typical p107 subunits of the novel, high molecular mass PEPC isoform (PEPC2) of castor oil seeds led to an opposite behavior (e.g. decreasing the sensitivity to negative effectors, including l-malate) and to the suggestion that this PEPC form is specifically engaged in fatty acid synthesis during seed development (Tripodi et al., 2005, Gennidakis et al., 2007).
Pharmacologically based experiments with mesophyll cell protoplasts from C4 (Giglioli-Guivarc'h et al., 1996) and CAM (Bakrim et al., 2001) leaves led to the identification of components of the signaling cascade controlling PEPC kinase activity via rapid synthesis of the kinase (Jiao et al., 1991; Hartwell et al., 1999) and the phosphorylation of photosynthetic PEPC isoforms. The phosphoinositide cycle and calcium are central elements of this cascade (Coursol et al., 2000). Very recently, the degradation of the PEPC kinase by the ubiquitin-proteasome pathway was demonstrated to occur in transformed mesophyll cell protoplasts from maize (Zea mays; Agetsuma et al., 2005) and was suggested to operate in sorghum leaves (Monreal et al., 2007).
PEPC phosphorylation has been studied extensively in leaves (especially from C4 and CAM plants), and only a few studies have dealt with seeds. This has been investigated in developing seeds of the leguminous species Vicia faba (Golombek et al., 1999) and during castor oil seed development, as mentioned above (Tripodi et al., 2005; Gennidakis et al., 2007; Murmu and Plaxton, 2007: Uhrig et al., 2008). In barley (Hordeum vulgare), wheat (Triticum aestivum), and sorghum seeds, our results established that PEPC phosphorylation occurred in situ during early germination, and the dedicated Ca2+-independent PEPC kinase appeared to be present (Osuna et al., 1996, 1999; Nhiri et al., 2000). However, in contrast to the leaf context, the PEPC kinase was constitutive during barley seed imbibition, and the cascade controlling its activity by protein synthesis was not operative (Osuna et al., 1999). This led us to study when and how the kinase was accumulated during seed development.
In this work, we first biochemically and immunologically characterized the barley seed PEPC, after which the time course of PEPC phosphorylation, PEPC kinase activity, and possible interactions with metabolite effectors were determined during seed development and imbibition. In addition, we report the positive effect of the phytohormone abscisic acid (ABA) on the PEPC phosphorylation process during seed germination, and it is proposed that such a mechanism is involved in the increase in PEPC kinase activity during the late stages of seed development.
RESULTS AND DISCUSSION
Characterization of PEPC during Seed Development
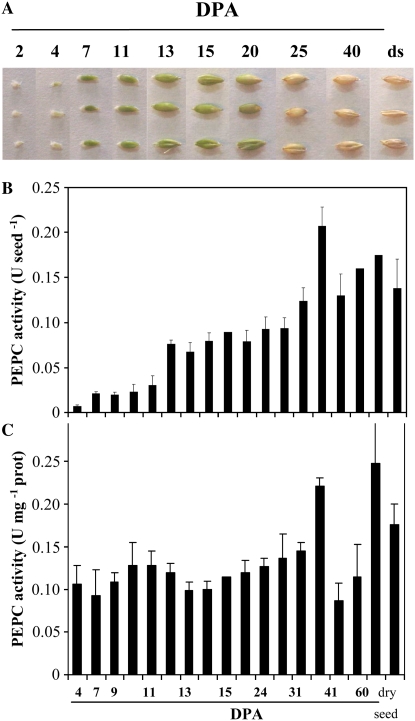
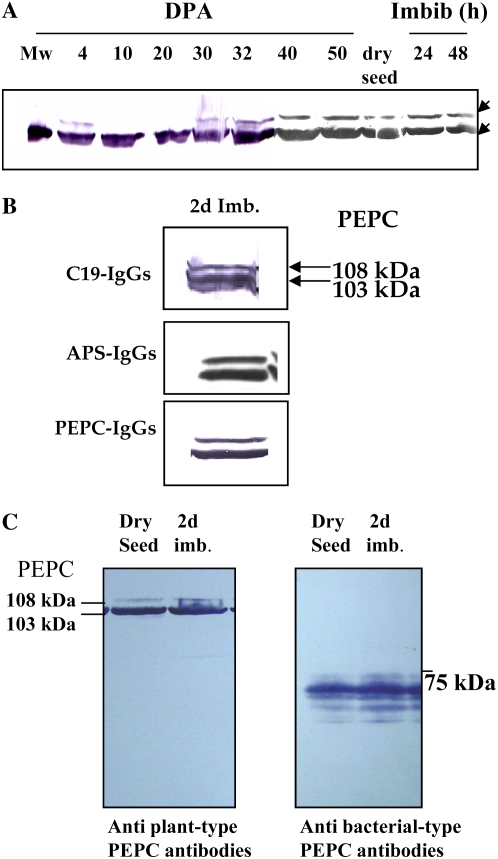
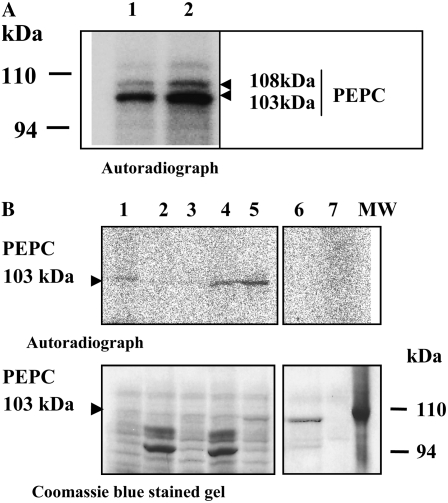
PEPC protein was detected at different stages of barley seed development, as shown in Figure 1. On a per seed basis, the enzyme activity showed an approximately 15-fold increase (Fig. 1B), whereas on a protein basis, it remained almost unchanged (Fig. 1C). Western-blot experiments showed the presence of a 103-kD PEPC subunit during the time course of seed development and subsequent imbibition; however, at early stages of development (4 DPA) and at 30 DPA, a second protein band with a molecular mass of 108 kD was also detected, and it remained until dry seed formation and during imbibition (Fig. 2A). These polypeptides were demonstrated to be intact and classical plant-type PEPC subunits (i.e. containing the N-terminal phosphorylation domain at variance with the bacterial-type PEPC; Sánchez and Cejudo 2003) using specific N- and C-terminal antibodies (Fig. 2B). Two immunologically related subunits have also been identified in developing and germinating wheat seeds (Osuna et al., 1996; González et al., 1998), castor oil seed (Sangwan et al., 1992; Uhrig et al., 2008), and during the germination of barley and sorghum seeds (Osuna et al., 1999; Nhiri et al., 2000). However, whether these different subunits (108 and 103 kD in barley, 110 and 107 kD in castor oil seed) are combined to form a heterotetrameric structure in vivo (as in banana [Musa spp.] fruit; Law and Plaxton 1997) is not known. Recently, the accumulation of a 110-kD plant-type PEPC subunit was found in depodded developing castor oil seed, which appeared to correspond to a larger form of the 107-kD subunit (differing by five supplemental amino acids in the N terminus; Uhrig et al., 2008). It is interesting that the barley seed 108-kD subunit was found to be absent during the green stages of seed development (Fig. 1; 7–20 DPA) and present in early stages of development (4 DPA) and during the desiccation period (Fig. 1; 4 and 25 DPA up to dry seed). Whether this subunit is regulated by photosynthate supply in barley seeds and is a larger version of the 103-kD form (or coded by a different gene) needs to be investigated further.
Figure 1.
A, Barley seeds. Developmental stages considered in this work are as follows: 4 to 7 DPA, early development; 10 to 25 DPA, maturation; and 25 DPA up to dry seed (ds), desiccation (González et al., 1998). B and C, Time course of PEPC activity in crude extracts from seeds at different development stages and dry seeds. PEPC activity was assayed in nondesalted crude extracts from whole seeds at optimal pH (8.0), 2.5 mm PEP, and 30°C. PEPC activity was expressed on a per seed basis (B) or on a protein basis (C). Results are means of three independent experiments.
Figure 2.
Immunocharacterization of PEPC. A, At the indicated times, soluble proteins (200 μg) of nondesalted extracts from whole seed were separated by SDS-PAGE (10% acrylamide), transferred onto nitrocellulose, and probed with polyclonal antibodies raised against sorghum leaf PEPC. The arrows indicate 108- and 103-kD PEPC subunits. B, The 103- and 108-kD polypeptides are intact and classical plant-type PEPC (PTPC) subunits. The experiment was performed as in A and probed with specific C19-IgGs, APS-IgGs, or anti-sorghum leaf PEPC IgGs (30 μg per 20 mL of incubation medium). C, The experiment was performed as in A. The transferred protein from dry and 2-d-imbibed seeds was incubated with polyclonal anti-sorghum leaf PEPC IgGs (30 μg per 20 mL of incubation medium) or antibacterial-type PEPC4 IgGs from Arabidopsis (20 μg per 20 mL of incubation medium). Immunolabeled proteins were detected by a horseradish peroxidase assay. [See online article for color version of this figure.]
In castor oil seeds, the 118-kD BTPC subunit was shown to form a heterooctameric complex (associated with the classical 107-kD subunit) with a molecular mass of 910 kD (Gennidakis et al., 2007). In vitro, the BTPC was rapidly proteolyzed to generate a 64-kD fragment (Blonde and Plaxton, 2003; Tripodi et al., 2005). Using specific antibodies, we detected the presence of low molecular mass BTPC polypeptides (60–70 kD), but not the corresponding full-size subunit, in dry and imbibed barley seeds (Fig. 2C). Similar fragments were obtained when crude extracts were prepared using denaturing conditions (TCA and acetone) in the extraction protocol (Fig. 3B; antibacterial-type PEPC antibodies), suggesting that the truncated BTPC and the heterooctameric PEPC (as described by Blonde and Plaxton, 2003) may exist in vivo. However, neither the fragments nor the full-size subunit were detected in crude extracts from 15 DPA prepared using denaturing conditions (Supplemental Fig. S1A). In addition, nondenaturing PAGE performed on 15-DPA dry and imbibing seed extracts containing phenylmethylsulfonyl fluoride (PMSF) but not thiol-reducing agents (Gennidakis et al., 2007) only revealed the activity of tetrameric and dimeric PEPC (Supplemental Fig. S1B, lanes 2–4). These tetrameric and dimeric PEPCs were also obtained in the presence of dithiothreitol (Supplemental Fig. S1B, lanes 6 and 7). The activity detected as a high molecular mass in dry seeds (Supplemental Fig. S1B, asterisk) was not revealed by the bacterial-type PEPC-IgGs after transfer of the proteins onto a nitrocellulose membrane (data not shown), so it could be considered a precipitated classical plant-type PEPC due to the method used to obtain concentrated samples (precipitation of proteins in crude extract with polyethylene glycol; see “Materials and Methods”) needed to measure PEPC activity on nondenaturing gels. Taken together, these results indicated that PEPC activity in barley seeds was mostly due to classical tetrameric/dimeric plant-type isoforms. On the basis of distinct kinetic and regulatory properties, it has been suggested that the class 2 enzyme (the octameric isoenzyme in castor oil seed) may be linked to fatty acid synthesis, while the class 1 PEPC (classical tretrameric isoenzyme) is involved in the synthesis of reserve proteins (Blonde and Plaxton, 2003). In the microalgae C. reinhardtii and S. minutum, the genes encoding PEPC subunits forming class 1 and 2 enzyme oligomers are responsive to the availability of inorganic nitrogen and carbon supplied to the culture medium (Moellering et al., 2007). The presence of a class 2 PEPC and its physiological role in the context of cereal seeds also need to be investigated further.
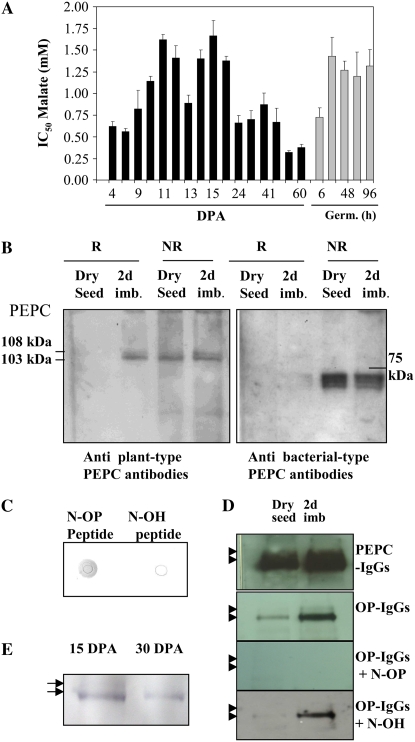
Figure 3.
A, Time course of PEPC phosphorylation as performed by measuring the malate IC50 for PEPC of crude nondesalted extracts from whole seeds as described by Nimmo et al. (1987) and Echevarría et al. (1994). This corresponds to the PEPC activity at pH 7.3 in the presence or absence of malate. Results are means of three experiments with seeds obtained from three different plant cultures. B, Analysis of phosphorylated PEPC by phosphoprotein affinity chromatography. Dry and imbibed seeds (200 mg) were extracted by the protocol described by Meimoun et al. (2007) based on protein extraction using denaturing conditions (TCA and acetone) and phosphoprotein chromatography (Qiagen). Proteins in the flow-through and retained fractions were analyzed by SDS-PAGE and revealed using polyclonal anti-sorghum leaf PEPC IgGs (30 μg per 20 mL of incubation medium) or antibacterial-type PEPC4 (from Arabidopsis) antibodies (20 μg per 20 mL of incubation medium). Immunolabeled proteins were detected by a chemiluminescence detection system (Super Signal West Dura Signal; Pierce) according to the manufacturer's instructions. R and NR indicate retained and not retained proteins in the column, respectively. C, Specificity of the anti-phosphorylated N-terminal antibodies (OP-IgGs). This was tested on dots (5 μg of protein on a nitrocellulose membrane) of the N-terminal phosphorylated peptide (N-OP) or nonphosphorylated peptide (N-OH). The membrane was incubated with the OP-IgGs (30 μg per 15 mL of incubation medium). D, Detection of the phosphorylated PEPC using the OP-IgGs. Eight dry or 2-d-imbibed seeds were extracted using the nondenaturing protocol (see “Materials and Methods”), and protein was analyzed by SDS-PAGE and western blot. The membrane was incubated with the specific OP-IgGs (30 μg per 15 mL of incubation medium) in the absence (OP-IgGs) or presence of 30 μg per 15 mL of the corresponding phosphopeptide (OP-IgGs + N-OP) or dephosphopeptide (OP-IgGs + N-OH). Immunolabeled proteins were detected by a chemiluminescence detection system (Super Signal West Dura Signal; Pierce) according to the manufacturer's instructions. E, Seeds (15 and 30 DPA) were extracted using the nondenaturing protocol (see “Materials and Methods”). Proteins from 15 and 30 DPA (0.042 units of PEPC) were immunoprecipitated for 3 h on ice by specific OP-IgGs using the immunoprecipitation protocol described by Osuna et al. (1996). The immunoprecipitated proteins were analyzed by SDS-PAGE and western blot. The membrane was incubated with polyclonal anti-sorghum leaf PEPC IgGs (30 μg per 20 mL of incubation medium). Experiments described in C and E used the horseradish peroxidase detection method. The arrows in D and E indicate the 103- and 108-kD PEPC subunits. [See online article for color version of this figure.]
PEPC Phosphorylation during Seed Development
The l-malate test (concentration of l-malate that inhibits the PEPC activity by 50% [IC50]), when performed at suboptimal pH (7.3), has been established to reflect the phosphorylation state of PEPC (e.g. IC50 was high, around 1.5 mm l-malate, and low, around 0.3 mm, for the phosphorylated and nonphosphorylated forms of the C4 sorghum enzyme, respectively; Echevarría et al., 1994; Chollet et al., 1996). When applied to barley seed extracts, this test revealed two main periods when the enzyme was highly phosphorylated: between 8 and 20 DPA (Fig. 3A) and during germination (Fig. 3A; Osuna et al., 1996). In contrast, the enzyme was in a low phosphorylation state from 20 DPA until dry seed (Fig. 3A). A similar pattern of PEPC (p107) phosphorylation has been established in castor oil seed (Tripodi et al., 2005), with a high phosphorylated PEPC during development (coincident with a 5-fold increase in the IC50 malate value for total PEPC activity in desalted extracts) and a low phosphorylated PEPC during seed desiccation. As mentioned above, the barley seed may contain the atypical heterooctameric PEPC; this form has been demonstrated to possess unexpected regulatory properties in vitro, in particular l-malate sensitivity, which is decreased following dephosphorylation of the 107-kD plant-type subunits of the class 2 complex (Tripodi et al., 2005). This was in marked contrast to typical tetrameric 107-kD PEPCs; therefore, the validity of the l-malate test could be questioned. This was checked by the use of affinity chromatography with a commercial kit (Qiagen P-phosphokit) that has been shown previously to distinguish phosphorylated PEPC from its nonphosphorylated counterpart in crude extract from sorghum and Arabidopsis leaves (Meimoun et al., 2007) and using antiphosphorylation site-specific antibodies raised against the corresponding phosphorylated synthetic peptide. Protein extracts prepared using denaturing conditions (Meimoun et al., 2007) from dry and imbibed seeds were subjected to affinity chromatography, and the eluted fractions (retained phosphoproteins) were subsequently analyzed by SDS-PAGE and western blotting. Figure 3B shows that the 103- and 108-kD PEPC polypeptides were partially but significantly retained (around 50%) on the column in the case of imbibed seed crude protein extracts (Fig. 3B, R, 2 d imb., anti-plant-type PEPC antibodies). In contrast, no binding was observed in the case of dry seeds (Fig. 3B, R, dry seed, anti-plant-type PEPC antibodies). This result was in good agreement with the l-malate IC50 values described above, thereby indicating that there was little or no phosphorylated PEPC in dry seeds and a substantial part phosphorylated in the imbibed seeds. It has to be noted that the BTPC fragments were detected in the pass-through fractions (Fig. 3B, NR, antibacterial-type PEPC antibodies). The immunological approach was based on the use of a specific antiphosphorylated site PEPC antiserum (OP-IgGs) raised in rabbits against the phosphorylated synthetic peptide from C3-type PEPC [Cys-ERLSS(P)IDAQLR]. This antiserum specifically recognized the phosphorylated peptide, as shown in Figure 3C. In western-blot experiments, it detected the presence of phosphorylated PEPC in imbibed seeds but much less in dry seed protein extracts, as expected (Fig. 3D, OP-IgGs). The specificity of antibodies for its epitope was ascertained following preincubation with either the phosphorylated peptide, which blocked the detection response (Fig. 3D, OP-IgGs + N-OP), or the nonphosphorylated peptide, which did not (Fig. 3D, OP-IgGs + N-OH). These antibodies made it possible to analyze further the seed PEPC at 15 and 30 DPA. Due to the low amount (units per seed) of the enzyme in 15-DPA seeds and the scarcity of this material, it was necessary to immunoprecipitate PEPC from the crude extract. Samples (0.02 PEPC units in 200 μL of extraction medium) were incubated with OP-IgGs and finally with protein A-Sepharose. Figure 3E shows that the phosphorylated PEPC was present at both stages; however, the amount of phosphorylated PEPC was higher at 15 DPA. Collectively, these results validated the l-malate test as reflecting the phosphorylation state of the classical tetrameric seed PEPC and showed that the phosphorylated PEPC content varied during seed development, maturation, and germination. It was high at 15 DPA, decreased at 30 DPA, and was undetectable in dry seeds, before it increased again during germination.
PEPC Kinase during Seed Development
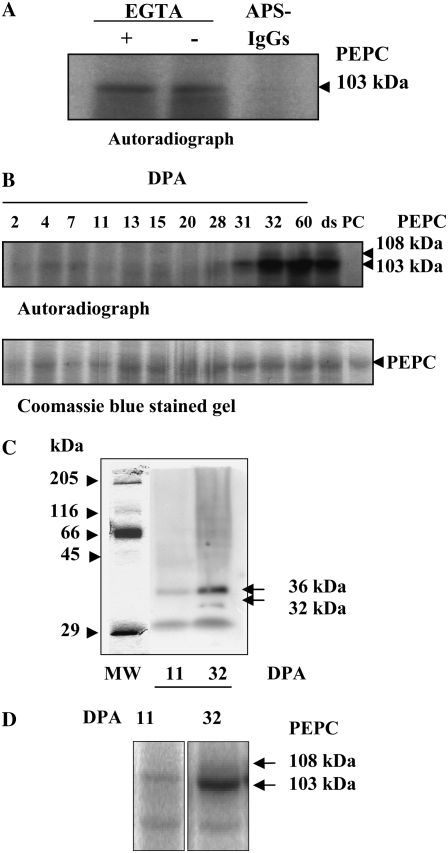
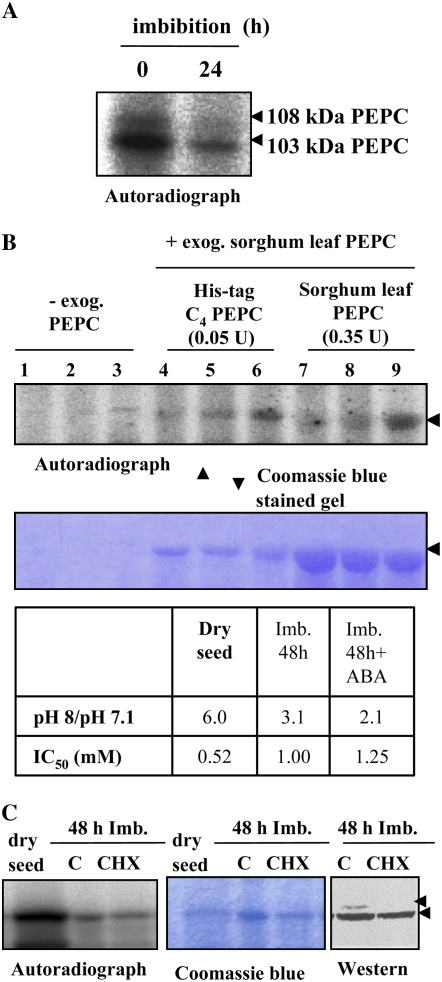
The next step was to characterize the PEPC kinase activity in seed protein extracts. Several PEPC kinase genes and cDNAs were cloned recently (for review, see Nimmo, 2003), and this calcium-independent protein kinase is now definitely recognized as the genuine, physiological PEPC kinase. In reconstituted assays, the PEPC kinase activity from dry barley seeds was found to be independent of calcium and to phosphorylate its endogenous PEPC target at the N-terminal domain, as expected (Fig. 4A). This kinase also phosphorylated exogenous sorghum C4 PEPC (Figs. 5, A and C, and 8B below). It is to be noted that this exogenous substrate accurately reflected the radioactive signal obtained with the endogenous PEPC (Figs. 5, A and C, and 8B below). Using this assay, the PEPC kinase activity was barely detected at early and mid developmental stages (Fig. 4B, lanes 2–20 DPA), but it showed a marked increase during desiccation, including dry seeds (Fig. 4B, lanes 31 DPA to dry seed). At these stages, the endogenous 108-kD PEPC was also found to be phosphorylated (Fig. 4B, lanes 32, 60, and ds [for dry seed], and Fig. 5C, lanes 1 and 3 for dry seed). In gel experiments conducted in the presence of EGTA detected the “classical” 32/36-kD double band (Chollet et al., 1996; Nimmo, 2003) at 32 DPA and a much weaker single 36-kD band at 11 DPA (Fig. 4C). This 36-kD protein has been proposed to be the ubiquitinated form of the PEPC kinase (Agetsuma et al., 2005; Monreal et al., 2007). Thus, our data suggested that the 32-kD kinase was present in seed tissues, although at early developmental stages the low level of this kinase made it undetectable by the native gel method (Fig. 4B). At 11 DPA, PEPC kinase activity was clearly detected in a reconstituted assay containing higher amounts of protein extract (0.03 units of endogenous PEPC and about 100 μg of total protein; Fig. 4D). However, it was surprising how such low levels of the PEPC kinase detected at 7 to 18 DPA may be responsible for such high levels of in vivo PEPC phosphorylation. It could be that another seed kinase, like a Ca2+-dependent protein kinase (which has been shown to phosphorylate PEPC in vitro in previous studies; Echevarría et al., 1988; Duff et al., 1996; Ogawa et al., 1998), phosphorylates PEPC at this stage of development. However, this scenario is not probable, since only phosphorylation by the Ca2+-independent PEPC kinase alters PEPC sensitivity to malate (Duff et al., 1996; Nhiri et al., 1998; Ogawa et al., 1998). Furthermore, during imbibition of barley seeds, PEPC phosphorylation was not affected by the Ca2+-dependent protein kinase inhibitor W7 (Osuna et al., 1999). The comparison of the in vitro PEPC kinase activity (Fig. 4, B and C) and the in vivo PEPC phosphorylation profiles (Fig. 3, A, B, D, and E) revealed the apparent lack of correlation between these two processes. This discrepancy suggested that a regulatory mechanism controlling PEPC kinase/PEPC phosphorylation occurred in vivo. It should be noted that this is at variance with the castor oil seed PEPC, whose activity, polypeptide, and transcripts showed a pronounced decrease in dry seeds, then reappeared during germination (Gennidakis et al., 2007), and PEPC kinase activity, which was detected only during seed development and correlated with the in vivo PEPC phosphorylation state (Murmu and Plaxton, 2007).
Figure 4.
Characterization of the PEPC kinase. A, Effects of EGTA and APS-IgGs on in vitro PEPC kinase activity. Aliquots of desalted extracts from whole dry seeds (150 μg of total protein, containing 0.02 units of endogenous PEPC) were incubated at 30°C for 1 h in the presence of components of the phosphorylation assay lacking sorghum PEPC and 1 mm EGTA or 40 μg of APS-IgGs (APS-IgGs). B, PEPC kinase activity profile during the development of barley seeds. Aliquots of desalted extracts from seeds harvested at different stages of development (0.02 units of endogenous PEPC) were incubated at 30°C for 1 h in the presence of 0.08 units of purified, nonphosphorylated sorghum leaf PEPC as an exogenous substrate and the components of the phosphorylation assay. The in vitro phosphorylation reaction was stopped by the addition of dissociation buffer, pH 6.8, and heat denaturation. Proteins were separated by SDS-PAGE (10% acrylamide) and analyzed by autoradiography. ds, Dry seed; PC, 0.08 units of purified, nonphosphorylated sorghum PEPC. C, In gel assay of PEPC kinase activity from crude extracts at two stages (11 and 32 DPA) of seed development. Soluble proteins from crude extracts (500 μg) were separated by SDS-PAGE (15% acrylamide). After elimination of SDS, proteins were renatured as described in “Materials and Methods,” then the gel was incubated in the presence of [γ-32P]ATP (50 μCi per 15 mL) and 10 mm MgCl2 and analyzed by autoradiography. D, The experiment was performed as described in B, in the absence of exogenous PEPC and using concentrated aliquots containing 250 μg of protein.
Figure 5.
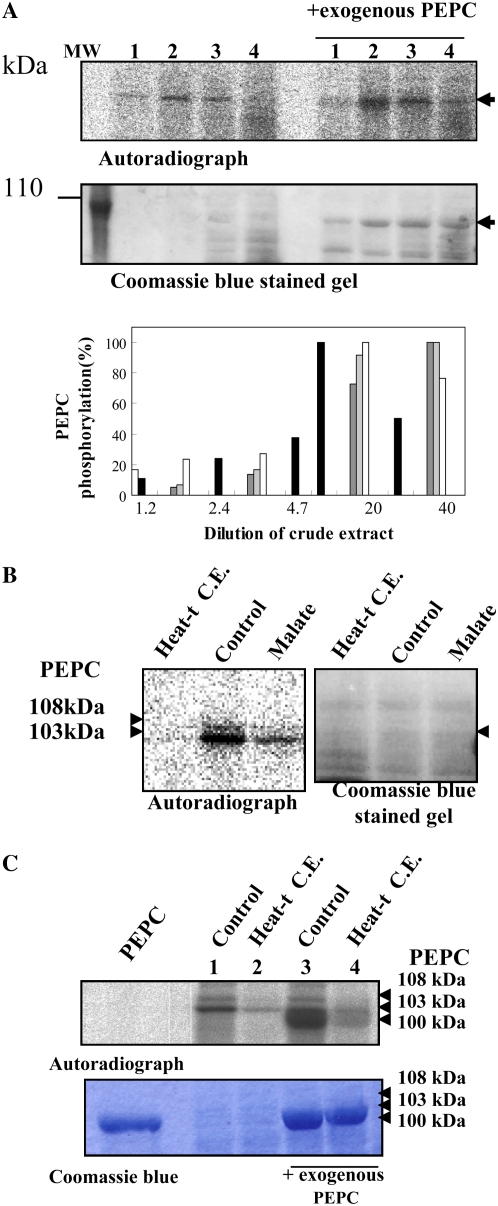
A, Effect of nondesalted extract concentration on in vitro PEPC kinase activity. The phosphorylation assays were performed at different crude extract concentrations from whole dry seeds, at 30°C during 45 min, in the presence of 1 mm EGTA, the presence or absence of a purified, nonphosphorylated sorghum leaf PEPC as an exogenous substrate (0.2 units), and the remaining components of the phosphorylation assay. Lanes 1 to 4 correspond to 24, 48, 242, and 485 μg of protein that contained 0.005, 0.01, 0.05, and 0.1 units of endogenous PEPC in a final volume of 120 μL. MW, Molecular mass marker. The histogram shows the results of different experiments (different gray intensities), and phosphorylation was quantified by the program Image J.130. B, Effect of a heat-treated nondesalted seed extract and malate on PEPC phosphorylation. The phosphorylation assays were performed in the standard conditions described above (control) using nondesalted crude extracts from dry seeds (40 μg of total protein containing 0.01 units of endogenous PEPC in 120 μL) and in the presence of an aliquot (200 μg of total protein) of the supernatant of denatured (2 min at 90°C) and centrifuged crude extracts (removing most proteins including PEPC) prepared from dry seeds (Heat-t C.E.) or l-malate (malate) at a concentration of 0.6 mm. After the phosphorylation assay, proteins were separated by SDS-PAGE (10% acrylamide) and analyzed by autoradiography. C, The experiment was performed as indicated in B, with denatured and centrifuged crude extracts (Heat-t C.E.) from 11-DPA seeds. The sorghum leaf PEPC was added as a marker (Coomassie Brilliant Blue; PEPC). Phosphorylated seed PEPC bands (108 and 103 kD) and the sorghum leaf PEPC band (100 kD) are clearly detected on the autoradiograph. [See online article for color version of this figure.]
Figure 8.
A, Degradation of PEPC kinase during seed imbibition. The phosphorylation assay was performed using desalted crude extracts from deembryonated barley seeds imbibed in water for 24 h at room temperature. B, ABA and CHX affect PEPC kinase activity during imbibition and germination. Seeds were allowed to imbibe in water (lanes 1, 4, and 7), 40 μm ABA (lanes 2, 5, and 8), or 40 μm ABA and 25 μm CHX (lanes 3, 6, and 9) for 78 h at room temperature. In addition to the endogenous PEPC, this experiment was performed using two different preparations of exogenous PEPC in the phosphorylation assays: purified nonphosphorylated or recombinant (His-tag C4 PEPC) sorghum leaf PEPC. Proteins were separated by SDS-PAGE (11% acrylamide) and analyzed by autoradiography. The table shows the in vivo phosphorylation state of PEPC as determined by the ratio between PEPC activity at pH 8 and pH 7.1 and by the malate test (IC50). The arrow indicates 103-kD PEPC. C, Seeds were imbibed for 72 h in the absence (C) or presence (CHX) of 25 μm CHX. Phosphorylation assays were carried out as above in the absence of exogenous PEPC. Transferred proteins on nitrocellulose membranes (western) were incubated with polyclonal anti-PEPC IgGs from sorghum leaves (30 μg per 20 mL of incubation medium), and immunolabeled proteins were detected by a horseradish peroxidase assay. The arrows indicate 103- and 108-kD PEPC subunits. [See online article for color version of this figure.]
The PEPC Kinase Is Subject to Metabolite Control in Seeds
PEPC kinase activity is mainly controlled at the transcriptional level in most plant organs and tissues (for review, see Nimmo, 2003), with the notable exception of germinating seeds (Osuna et al., 1999). Other mechanisms acting at the posttranslational level have been reported to affect PEPC kinase activity: in vitro thioredoxin-mediated changes of PEPC kinase activity, but this has still to be shown in planta (Tsuchida et al., 2004; Murmu and Plaxton, 2007); a metabolic control, both in vitro (Echevarría et al., 1994; Murmu and Plaxton, 2007) and in situ by feeding mesophyll protoplasts with metabolite effectors (Bakrim et al., 1998); and, finally, a heat-sensitive 100-kD protein inhibitor interacting with the kinase in extracts from maize and Kalanchoe fedtschenkoi leaves (Nimmo et al., 2001). In fact, Nimmo et al. (2001) had shown that PEPC kinase activity in crude leaf extracts increased markedly upon dilution. We tested this latter possibility using nondesalted extracts from dry seeds. Increasing the concentration of the extract in reconstituted assays led to a marked inhibition of both endogenous and exogenous (nonphosphorylated PEPC from sorghum leaves) PEPC phosphorylation (Fig. 5A, lanes 3 and 4). Conversely, diluting the crude extract with a 20-fold dilution led to a 5-fold increase in the initial PEPC kinase activity (Fig. 5A, histogram). However, inhibition of PEPC phosphorylation was maintained after the addition of an aliquot of a heat-denatured (90°C for 10 min) and centrifuged crude seed extract to the phosphorylation assay (Fig. 5B, Heat-t C.E.). A similar inhibition of PEPC kinase activity was observed when heat-treated protein extracts from 11-DPA seeds were added to the dry seed reconstituted assay (Fig. 5C, Heat-t C.E.). Alternatively, the kinase inhibitor could be a low molecular mass, heat-stable compound such as l-malate, which decreased the kinase activity when added to the reconstituted assay (Fig. 5B, malate). Indeed, both Sephadex G-25 gel filtration (Fig. 6A) and NAD-malate dehydrogenase (MDH) treatment (removing malate) of the crude extract (Fig. 6B) restored the PEPC kinase activity.
Figure 6.
The inhibitory effect related to crude extract concentration was abolished by desalting the crude extract or by a preincubation of the denatured crude extracts with MDH. A, Phosphorylation assays were performed using the standard conditions and desalted crude extracts (filtration through Sephadex G-25) from whole dry seeds. Lane 1, 0.05 PEPC units per 242 μg of proteins; lane 2, 0.1 PEPC units per 484 μg of proteins. B, The phosphorylation assays were performed using the conditions described for Figure 5A, with nondesalted crude extracts of whole dry seeds (48 μg of protein containing 0.01 units of PEPC in 120 μL). Lane 1, crude extract; lane 2, crude extract + an aliquot of the supernatant of heat-treated nondesalted crude extract (262 μg of total protein); lane 3, crude extract + 4 mm l-malate; lane 4, crude extract + an aliquot of the supernatant of heat-treated nondesalted crude extract (262 μg of protein) treated with 50 units of MDH and 4 mm NAD+; lane 5, crude extract + a purified nonphosphorylated sorghum leaf PEPC as exogenous substrate (0.05 units); lane 6, nonphosphorylated sorghum leaf PEPC (0.15 units); lane 7, components of the ADP scavenging system (adenylate kinase inhibitor, phosphocreatine, and creatine phosphokinase). The phosphorylation assays were performed in the presence of 1 mm EGTA at 30°C during 45 min. After denaturation, the proteins were separated by SDS-PAGE (11% acrylamide) and analyzed by autoradiograph. MW, Molecular mass markers.
Collectively, these results showed that the protein inhibitor reported by Nimmo et al. (2001) was not operational in crude extracts from 11-DPA or dry barley seeds; rather, l-malate was responsible for the inhibition of PEPC kinase in crude extracts under our experimental conditions. In addition, these data indicated that l-malate accumulated to high levels in dry seeds, thereby supporting the hypothesis that PEPC kinase activity was modulated in vivo by this metabolite during seed maturation.
l-Malate and Glc-6-P Content in Relation to PEPC Phosphorylation Status during Seed Development and Germination
l-Malate content was shown to increase sharply between 10 and 15 DPA, reaching values as high as 400 nmol seed−1 (around 40 mm, considering a barley seed volume of 10 μL) and remaining stable, thereafter, up to the dry seed stage (Fig. 7A). High content of malate has also been found in the starchy endosperm of dry barley seeds (800 nmol endosperm−1) by Drozdowicz and Jones (1995). Since the kinase IC50 for l-malate was found to be around 0.5 mm (Fig. 5B, malate), such high l-malate concentrations may explain why PEPC phosphorylation was low or absent (Fig. 3A), although the PEPC kinase reached maximal levels during late maturation and in dry seeds (Fig. 4, B and C). However, it could not account for the high levels of PEPC phosphorylation detected in both the mid part of seed development (Fig. 3A, 10–18 DPA) and during imbibition and germination (Fig. 3A; Osuna et al., 1996). The l-malate effect on PEPC kinase has been shown to be indirect, acting on the PEPC rather than the protein kinase (Wang and Chollet, 1993). In agreement, Glc-6-P, which decreases malate inhibition of PEPC activity, also antagonized malate inhibition of PEPC phosphorylation by the kinase both in vitro and in situ (Echevarría et al., 1994; Bakrim et al., 1998). We reasoned that this metabolite may be present in seeds at levels capable of counteracting the l-malate effect. Indeed, Figure 7B shows that Glc-6-P levels peaked at around 20 DPA and early imbibition, with a consistent value of approximately 200 and 400 μmol (20 and 40 mm) per seed, when PEPC phosphorylation was observed, albeit l-malate had already attained its maximum level (Fig. 7A). These results support the hypothesis that PEPC kinase activity/PEPC phosphorylation status is controlled mainly by the Glc-6-P to malate ratio during the development and germination of barley seeds (Fig. 7C). The in vivo regulation of PEPC phosphorylation by Glc-6-P and l-malate has also been reported in sorghum mesophyll protoplasts and during imbibition of sorghum seeds (Bakrim et al., 1998; Nhiri et al., 2000). The mechanistic basis for this metabolite (l-malate and Glc-6-P) effect has been demonstrated to be indirect, by promoting changes in the phosphorylability of the PEPC (Wang and Chollet, 1993; Kai et al., 1999; Matsumura et al., 2002).
Figure 7.
Determination of l-malate and Glc-6-P levels in barley seeds. A and B, Malate (A) and Glc-6-P (B) were quantified in crude extracts from seeds at different stages of development, imbibition, and germination, as described in “Materials and Methods.” The germinated seeds were soaked, whole, at 25°C. C, Glc-6-P to l-malate ratio. The in vivo phosphorylation state of PEPC was determined by the l-malate test, and the IC50 l-malate is given in parentheses.
We also checked the role of photosynthate supply in the regulation of PEPC phosphorylation during seed development. The IC50 (l-malate) for PEPC decreased from 1.2 to 0.62 mm in crude extracts from 11 DPA at 20 h following pod excision. This result confirmed the high phosphorylation state of the enzyme at this developmental stage and pointed to the important role of photosynthate supply in sustaining high levels of phosphorylated PEPC. The elimination of photosynthate supply has been shown to be responsible for the down-regulation of PEPC kinase activity in castor oil seed following pod excision or prolonged darkness (Tripodi et al., 2005; Murmu and Plaxton, 2007) and in soybean root nodules after shoot decapitation, stem girdling, or prolonged darkness conditions (Zhang et al., 1995; Wadham et al., 1996; Zhang and Chollet 1997; Sullivan et al., 2004).
Collectively, ours results showed that the PEPC kinase activity was intrinsically weak at 11 DPA and in the imbibing seeds and increased at 30 DPA and in the dry seeds. During imbibition, the Glc-6-P to malate ratio may account for the up-regulation of PEPC kinase and the high level of phosphorylated PEPC. In addition, photosynthate supply seemed to be essential to maintain high levels of phosphorylated PEPC during seed development (11–18 DPA).
Effect of the Phytohormone ABA on the Regulation of PEPC Kinase and PEPC Phosphorylation in Germinating Barley Seeds
Despite the fact that this study provides evidence for a metabolite regulation of PEPC kinase activity during the development and germination of barley seeds, so far there is no available information concerning the mechanism(s) underlying the large increase in kinase activity during late maturation. In the C4 leaf, it has been proposed that PEPC kinase gene expression and protein synthesis are increased in the light following activation of a complex cascade that involves the phosphoinositide cycle and calcium (Giglioli-Guivarc'h et al., 1996; Coursol et al., 2000). This change in the amount of the kinase implies that synthesis dominates over degradation. The reverse occurs when plants are returned to darkness, leading to degradation of the kinase and its disappearance in the mesophyll tissues (Echevarría et al., 1990; Jiao et al., 1991). In germinating seeds, since the cascade is not operative during imbibition and germination (Osuna et al., 1999), the PEPC kinase accumulated during the preceding phase should be constitutively present.
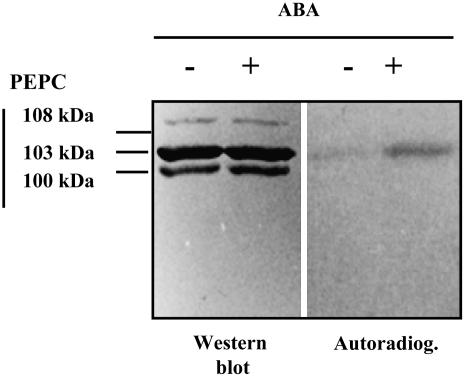
The phytohormone ABA is well known to control several aspects of seed development and dormancy by a complex signaling machinery, implying calcium, kinases, and phosphatases, which ultimately modulates the transcription of several related target genes (Leung and Giraudat, 1998; Finkelstein et al., 2002). The hypothesis that ABA contributes to the regulation of PEPC kinase levels in seeds was assessed. During imbibition (24 h), PEPC kinase activity levels decreased significantly in whole seeds (Fig. 8A), while PEPC phosphorylation (as judged by the l-malate test and confirmed by phosphoprotein affinity chromatography and an immunological test) was seen to rapidly increase, reaching a maximum after 24 h and then staying stable for up to 96 h (Fig. 3A; Osuna et al., 1996). The decrease in PEPC kinase activity was reduced (together with an increase in the PEPC phosphorylation state, as judged by the pH 8 to pH 7.1 activity ratio and the l-malate test) when seeds were germinated in the presence of ABA (Fig. 8B, lanes 2, 5, and 8), thus indicating that the hormone may play some role in positively controlling the amount of PEPC kinase. The cytosolic protein synthesis inhibitor cycloheximide (CHX) alone, which was taken up by the seed (Fig. 8C, western; see inhibition of the 108-kD PEPC that was reported previously by Osuna et al., 1999), did not affect the phosphorylation process (Fig. 8C, autoradiograph), in good agreement with previous results from Osuna et al. (1999), but it appeared to markedly amplify the effect of ABA on PEPC kinase activity during seed imbibition (Fig. 8B, lanes 3, 6, and 9). These ABA and ABA/CHX effects were observed for the phosphorylation of both the endogenous PEPC and the exogenous PEPC from sorghum leaves (which accurately reflects the radioactive signal obtained with the endogenous PEPC). The use of exogenous PEPC ensured that the ABA and CHX effects were not due to changes in the amount and/or phosphorylability of the endogenous PEPC. On the other hand, it has been established that CHX enhanced the light-dependent increase in PEPC kinase-translatable mRNA in maize, Arabidopsis, and barley leaves, yet it blocked the kinase synthesis and PEPC phosphorylation, as expected (Hartwell et al., 1999; Fontaine et al., 2002; Fig. 8C). Thus, if PEPC kinase synthesis was involved in the ABA response of barley seeds, CHX should have inhibited this process; therefore, the observed enhancing effect of the phytohormone was probably on the degradation of the kinase.
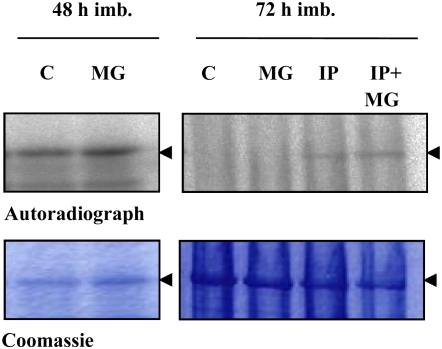
A recent report on maize mesophyll protoplasts has suggested that the degradation process of the PEPC kinase involves the ubiquitin-proteasome pathway (Agetsuma et al., 2005). In sorghum leaves, ABA was found to decrease the disappearance of PEPC kinase activity in the dark, an effect that was mimicked by either the 26S proteasome inhibitor MG132 or CHX (Monreal et al., 2007). It was concluded that the phytohormone stabilizes the kinase by decreasing its degradation rate and that a rapid protein synthesis event is involved in this process. We have verified whether a similar mechanism may account for the ABA-mediated increase in the stability of the barley seed PEPC kinase. To this end, seeds were imbibed in the presence of the proteasome inhibitor. In contrast to sorghum leaves, MG132 did not prevent proteolysis of PEPC kinase at either 48 or 72 h of imbibition time (Fig. 9, MG), although its possibly ubiquitinated form (36 kD, according to Agetsuma et al. [2005]) was detected in the in gel experiment (Fig. 4C). However, incubation of barley seed with a cocktail of protease inhibitors (betstatin, chymostatin, and PMSF) did prevent the decrease in PEPC kinase activity (Fig. 9, IP). The effect of the protease inhibitors was not modified by the presence of MG132 (Fig. 9, IP + MG). We propose that the increased stability of the kinase was due to the modulation of seed proteases. As we have no evidence that MG132 enters the seed, whether the ubiquitin-proteosome pathway plays some role in this seed context needs further investigation. Thus, ABA and ABA combined with CHX modulated the degradation rate of the kinase. Since the CHX effect is observed only when ABA is present, it is suggested that the inhibitor acts on steps triggered by the phytohormone. How and at which step CHX acts to further enhance this effect are open questions.
Figure 9.
Effects of the 26S proteasome inhibitor MG132 and protease inhibitors on PEPC kinase activity during seed imbibition. Eight deembryonated seeds were imbibed during 48 or 72 h in the absence or presence of 200 μm MG132 in 1% dimethyl sulfoxide (MG) or during 72 h in the absence or presence of protease inhibitors (IP; 10 μg mL−1 leupeptin, 10 μg mL−1 chymostatin, and 1 mm PMSF) or MG132 and IP (IP + MG). After the phosphorylation assay, proteins were separated by SDS-PAGE (11% acrylamide) and analyzed by autoradiography. The arrows indicate the 103-kD PEPC subunit. [See online article for color version of this figure.]
The effect of ABA was also investigated in wheat seeds. Seeds were imbibed in 200 μCi of [32P]phosphate in the absence or presence of ABA (40 μm). SDS-PAGE and autoradiography showed that PEPC phosphorylation was considerably higher in seeds treated with the hormone (Fig. 10). This shows that a similar mechanism may occur in wheat seeds as well as barley seeds.
Figure 10.
Effect of ABA on the in situ phosphorylation of PEPC during imbibition of wheat seeds. Eight deembryonated seeds were allowed to imbibe (72 h) in a buffer (100 mm Tris-Cl, pH 7.4) containing 200 μCi of [32P]phosphate and 10 mm CaCl2 in the absence (−) or presence (+) of 40 μm ABA at room temperature and in the dark. Proteins from the aleurone-endosperm tissue were extracted in a buffer (as described in “Materials and Methods”) containing 1 mm nonradioactive ATP, and PEPC was immunoprecipitated (immunoprecipitation as described by Osuna et al. [1996]) with polyclonal sorghum-leaf PEPC IgG (134 μg of protein) and analyzed by SDS-PAGE (8% acrylamide). The proteins were electrotransferred to nitrocellulose, autoradiographed, and probed with 300 μg of polyclonal sorghum leaf PEPC antibodies. An immunoblot from an aleurone-endosperm immunoprecipitated sample and the corresponding autoradiograph are shown.
Our results establish that ABA promotes PEPC kinase activity in cereal seeds during germination through a mechanism that alters its degradation rate. Thus, given that ABA concentration increases in seed tissues just before the maturation phase (Rock and Quatrano, 1995; Finkelstein et al., 2002), a consistent hypothesis is that it is an endogenous signal triggering PEPC kinase accumulation during this phase (Fig. 3B) via a decrease in its degradation rate. This constitutes a new mechanism controlling PEPC kinase levels, but it does not rule out the occurrence of a transduction cascade similar to that described in the C4 leaf during early stages of barley seed development.
Finally, PEP metabolism via PEPC and cytosolic pyruvate kinase appears to play a pivotal role in partitioning developing seed carbohydrates toward plastidic fatty acid biosynthesis as well as the mitochondrial production of ATP and the organic acids required for amino acid synthesis and interconversion (Sangwan et al., 1992; Ruuska et al., 2002; Schwender et al., 2004; Turner et al., 2005). It has been firmly established that the carbon flux through PEPC by the PEPC branch is three to five times greater than that through the pyruvate kinase branch in the aleurone cell layer of maturing barley seeds (Macnicol and Raymond, 1998). In addition, acidification of the endosperm following malate accumulation is thought to have an important role during the development of barley seeds (Macnicol and Jacobsen, 1992). Our data showed that PEPC activity gradually increased during barley seed development, with two main phosphorylation periods. The first one, between 11 and 15 DPA, may be correlated with the beginning of the synthesis of reserve compounds and malate accumulation for acidification of the endosperm. The second phase started sharply during early imbibition. This may be to continue malate synthesis, while this metabolite progressively decreases following its use in fueling the glycolytic pathway and the tricarboxylic acid cycle during germination. The octameric PEPC described by Gennidakis et al. (2007) has been proposed to contribute specifically to fatty acid biosynthesis in castor oil seeds (Blonde and Plaxton, 2003). In barley seeds, the activity of the octameric PEPC form was not detected. This may be related to the low accumulation of lipids in these seeds.
MATERIALS AND METHODS
Plant Material and Antibodies
Barley (Hordeum vulgare ‘Beka’; Rhône-Poulenc) and wheat (Triticum aestivum ‘Chinese Spring’; Pioneer) seeds were sterilized in 2% (v/v) NaOCl for 20 to 30 min and washed with sterile water, 0.01 m HCl, and, finally, sterile water. Seeds were placed on filter paper soaked with sterile water in a glass petri dish. Seeds were allowed to imbibe for 6 to 92 h at room temperature. Plants were cultivated under controlled conditions in a greenhouse under a 16-h-day/8-h-night cycle at 22°C to 25°C. Seed were harvested at different postanthesis stages, frozen in liquid nitrogen, and kept at −20°C until use. For this study, seeds harvested at three different times and at different periods of the year were used.
The APS-IgGs and C19-IgGs were raised against the N-terminal synthetic peptide (4-ERHHSIDAQLRALAPGKVSEE-24) containing the phosphorylation motif and the C-terminal synthetic peptide ([Y]942-EDTLILTMKGIAAGMQNTG-960) containing the last 19 amino acids of sorghum (Sorghum bicolor) leaf C4 PEPC. The OP-IgGs used in the experiments shown in Figure 3, C to E, were raised against the N-terminal synthetic peptide (phosphorylated on the regulatory Ser: Cys-ERLSS[PO3H2]IDAQLR) derived from a wheat PEPC sequence (González et al., 2002). Polyclonal antibodies against native sorghum leaf PEPC were prepared as described by Pacquit et al. (1995) and Vidal et al. (1981). Polyclonal antibodies against the bacterial-type PEPC4 from Arabidopsis (Arabidopsis thaliana) were kindly supplied by Prof. F.J. Cejudo.
Seed Imbibition, in Situ 32P Labeling, and Immunoprecipitation
Seed imbibition was carried out on plastic dishes (Nunc Multidish 6) for 6 to 96 h (depending on the experiment) in water. Deembryonated seeds were imbibed in 0.1 m Tris-HCl buffer, pH 7.4, and 10 mm CaCl2 (Hamabata et al., 1988; Heimovaara-Dijkstra et al., 1994) in the absence or presence of ABA and/or CHX (see Fig. 8 legend), MG132, and/or proteases inhibitors (see Fig. 9 legend).
Wheat seeds (eight deembryonated half seeds) were allowed to imbibe in 200 μL of buffer containing 100 mm Tris-HCl, pH 7.5, 10 mm CaCl2, and 200 μCi of 32P in the absence or presence of 40 μm ABA (Fig. 10). Incubation with 32P, seed extraction, and inmunoprecipitation were as described by Osuna et al. (1996).
Protein Extraction
Eight whole or deembryonated seeds were ground in a chilled mortar with washed sand and 1 mL of 0.1 m Tris-HCl buffer, pH 7.5, 10 mm MgCl2, 1 mm EDTA, 20% glycerol, 14 mm β-mercaptoethanol, 1 mm PMSF, 10 μg mL−1 chymostatin, 10 μg mL−1 leupeptin, 50 mm KF, and 1 μm microcystin-LR. The homogenate was centrifuged at 15,000g for 7 min at 4°C, and the supernatant was used as the nondesalted crude extract. When desalted crude extracts were used, proteins were first precipitated by the addition of (NH4)2SO4 to 60% saturation, sedimented by centrifugation at 15,000g, resuspended in 200 μL of extraction buffer, and filtered through Sephadex G-25 equilibrated with the extraction buffer without mercaptoethanol. All experiments were performed at 4°C. Denatured, nondesalted crude extracts (Figs. 5 and 6, Heat-t C.E.) were prepared from an aliquot containing 200 μg of protein, heated for 2 min at 90°C, and centrifuged at 15,000g for 5 min. This treatment removed the PEPC (103- and 108-kD PEPC) from the supernatant, which was used directly, or preincubated with 4 mm NAD+ and 50 units of NAD-MDH, to eliminate l-malate. In the case of denaturing extraction, proteins were precipitated in 1.8 mL of acetone containing 10% (w/v) TCA and 0.07% (v/v) 2-mercaptoethanol for 1 h at −20°C. After centrifugation (20,000g, 10 min, 4°C), the precipitate was washed three times with 2 mL of acetone and 0.07% (v/v) 2-mercaptoethanol for 1 h at –20°C and then vacuum dried. The resulting pellet was solubilized in 3 × 600 μL of 20 mm MOPS/NaOH, pH 7, 0.5% SDS (heated to 95°C for 5 min) for phosphoprotein chromatography using the Qiagen column (Meimoun et al., 2007). Protein in extracts was determined by the method of Bradford (1976), using BSA as a standard.
PEPC Immunocharacterization and Electrophoresis
Samples were subjected to SDS-PAGE (10%–13% [w/v] acrylamide) according to Laemmli (1970) for 12 h at 85 V and at room temperature using an electrophoresis cell (Bio-Rad). Alternatively, a miniprotean electrophoresis cell from Bio-Rad was used, and samples were subjected to SDS-PAGE for 3 to 5 h at 100 V. After electrophoresis, proteins were stained with Coomassie Brilliant Blue R-250 or electroblotted onto a nitrocellulose membrane (N-8017; Sigma) at 10 V (3 mA cm−2) for 2 h in a semidry transfer-blot apparatus (Bio-Rad). Membranes were blocked in Tris-buffered saline (0.02 m Tris-HCl and 0.15 m NaCl, pH 7.5) containing 5% (w/v) powdered milk, and PEPC bands were immunochemically labeled by overnight incubation of the membrane at 6°C in 20 mL of Tris-buffered saline containing antisera (protein A-purified polyclonal PEPC IgGs [30 μg of protein], APS-IgGs [40 μg of protein], OP-IgGs [40 μg of protein], or C19-IgGs [30 μg of protein]). Subsequent detection was with a peroxidase assay using affinity-purified goat anti-rabbit IgG horseradish peroxidase conjugate (Bio-Rad). Alternatively, immunolabeled proteins were detected by a chemiluminescence detection system (Super Signal West Dura Signal; Pierce) according to the manufacturer's instructions.
Determination of PEPC Activity and l-Malate Test
PEPC activity was measured spectrophotometrically at optimal pH (8.0) using the NAD-MDH-coupled assay at 2.5 mm PEP as described by Echevarría et al. (1994). Assays were initiated by addition of an aliquot of the extract. An enzyme unit was defined as the amount of PEPC that catalyzes the carboxylation of 1 μmol PEP min−1 at pH 8, 30°C. The phosphorylation state of PEPC was determined by the malate test (malate inhibition at suboptimal pH of 7.3 and expressed as the IC50) and/or by the velocity test (ratio of PEPC activity determined at pH 8 and pH 7.1, which decreases when the enzyme becomes phosphorylated due to an increase in velocity at pH 7.1). Both tests are described by Echevarría et al. (1994). A high IC50 and a low pH 8/pH 7.1 PEPC activity are correlated to a high degree of PEPC phosphorylation.
In Gel PEPC Activity
Proteins from seed (16 whole seeds) were extracted in 0.1 m HEPES-NaOH, pH 7.9 (4 mL), containing 5% (v/v) glycerol, 1 mm EGTA, 10 mm MgCl2, 25 mm KF, 1 mm PMSF, 10 μg mL−1 chymostatin, 10 mm leupeptin, and a protease inhibitor cocktail from Sigma (P9599) in the absence or presence of thiol-reducing agents. The extract was centrifuged at 15,000g, 15 min, 4°C, and the supernatant was concentrated by addition of 20% (w/v) PEG8000 (P4463-250G; Sigma). The pellet was collected by centrifugation (15,000g, 15 min, 4°C) and solubilized in 200 μL of extraction medium. Finally, the samples were submitted to nondenaturing polyacrylamide gels (5% acrylamide without staking gel) at 8°C. After protein migration, the gels were treated and revealed as described by Rivoal et al. (2002).
Affinity Chromatography of Phosphorylated PEPC
This was essentially performed as described by Meimoun et al. (2007) using the denaturing extraction protocol (200 mg of dry and imbibed seeds) and the phosphoprotein purification kit from Qiagen. Proteins in the flow-through and retained fractions were analyzed by SDS-PAGE and western blotting using anti-sorghum PEPC antibodies and revealed by a chemiluminescence detection system (Super Signal West Dura Signal; Pierce) according to the manufacturer's instructions (Fig. 3B).
In Vitro Phosphorylation and In Gel Phosphorylation Assay
For most experiments, in vitro phosphorylation using seed extracts (aliquots containing 0.005–0.2 units of endogenous PEPC) was performed in a reaction medium containing 100 mm Tris-HCl, pH 7.5, 20% (v/v) glycerol, 5 mm MgCl2, 1 μm microcystin-LR, 0.25 mm P1P5-di(adenosine-5′)-pentaphosphate (adenylate kinase inhibitor), 4 mm phosphocreatine, and 10 mm creatine phosphokinase (components of the ADP scavenging system; Echevarría et al., 1990) and in the presence or absence of 0.2 units of nonphosphorylated sorghum PEPC, EGTA, or APS-IgGs as specified in the figure legends. The phosphorylation reaction was initiated by the addition of 1 μCi of [γ-32P]ATP (10 Ci mmol−1) and incubated at 30°C for 45 min. The reaction was stopped by boiling the samples for 3 min at 90°C in the presence of dissociation buffer (100 mm Tris-HCl, pH 6.8, 25% [v/v] glycerol, 1% [w/v] SDS, 10% [v/v] 2-mercaptoethanol, and 0.05% [w/v] bromphenol blue). The denatured proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue R-250. The gel was analyzed with a phosphor imager (Fujix BAS 1000; Fuji).
The polypeptides with PEPC kinase activity were detected according to the method described by Wang and Chollet (1993). Aliquots of crude extracts (100 μL per 500 μg of proteins) were mixed with dissociation buffer and subjected to 15% SDS-PAGE (polymerized in the presence of 0.4 mg mL−1 purified, nonphosphorylated PEPC from sorghum leaves). After electrophoresis, SDS was eliminated by incubation of the gel twice for 1 h in 100 mL of a medium containing 50 mm Tris-HCl, pH 8.0, 20% [v/v] 2-propanol, then in buffer A (50 mm Tris-HCl, pH 8.0, 5 mm 2-mercaptoethanol, and 0.1 mm EDTA) for 1 h. Proteins were denatured by a 2-h incubation in buffer A containing 100 mL of 6 m guanidine-HCl and renatured by a 5-h incubation with 250 mL of 0.04% Tween 40 (P-1504; Sigma) in buffer A. The gel was finally preincubated for 30 min in the assay solution containing 40 mm HEPES-NaOH, pH 8.0, 2 mm dithiothreitol, 5 mm Mg(CH3COO)2, and 1 mm EGTA and then incubated during 2 h in the presence of [γ-32P]ATP (50 μCi 15 mL−1). Unbound phosphate was removed by washing the gel overnight with 5% [w/v] TCA and 1% [w/v] sodium pyrophosphate. Then, the gel was dried and analyzed by autoradiography.
Determination of l-Malate and Glc-6-P Levels
Malate and Glc-6-P were determined in crude extracts prepared at different stages of development and imbibition by grinding whole seeds (0.2 g fresh weight) with 1 mL of 7% (v/v) perchloric acid. The acid suspension was neutralized with 15% (v/v) 1 m tetramethylammonium chloride/5 n KOH, and the residue was removed by centrifugation at 15,000g for 2 min. The supernatant was used for l-malate and Glc-6-P quantification or stored at −35°C. l-Malate concentration was determined by measuring the increase in absorbance at 340 nm due to the enzymatic reduction of NAD+ according to Lowry and Passoneau (1972). The reaction was carried out in 1 mL of a reaction mixture containing an aliquot of the supernatant, 50 mm 2-amino-2-methyl-1 propanol, pH 9.9, 4 mm NAD+, 4 mm Glu, 3.5 units of NAD-MDH, and 0.9 units of Asp transaminase. Glc-6-P levels were determined by measuring the increase in A340 due to the enzymatic reduction of NADP+. The reaction was carried out in 1 mL of a reaction mixture containing an aliquot of the supernatant in 100 mm Tris-HCl, pH 7.5, 5 mm NADP+, and 2 units of Glc-6-P-dehydrogenase.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Immunodetection of bacterial-type PEPC at 15 DPA and in gel PEPC activity from barley seed.
Supplementary Material
Acknowledgments
The authors thank Dr. Michael Hodges for valuable comments and careful reading of the manuscript. Thanks are also due to Prof. F.J. Cejudo for providing the antiserum against the bacterial-type PEPC4 from Arabidopsis.
This work was supported by the Ministerio de Educación y Ciencia (grant no. BCM2001–2366–CO3–02) and by the Grupo de Investigación de Fosforilación de Proteínas en Plantas y Metabolismo del Carbono BIO–298 from La Junta de Andalucía.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Cristina Echevarría (echeva@us.es).
Some figures in this article are displayed in color online but in black and white in print.
The online version of this article contains Web-only data.
References
- Agetsuma M, Furumoto T, Yanagisawa S, Izui K (2005) The ubiquitin-proteosome pathway is involved in rapid degradation of phosphoenolpyruvate carboxylase kinase for C4 photosynthesis. Plant Cell Physiol 46 389–398 [DOI] [PubMed] [Google Scholar]
- Bakrim N, Brulfert J, Vidal J, Chollet R (2001) Phosphoenolpyruvate carboxylase kinase is controlled by a similar signaling cascade in CAM and C4 plants. Biochem Biophys Res Commun 286 1158–1162 [DOI] [PubMed] [Google Scholar]
- Bakrim N, Nhiri M, Pierre JN, Vidal J (1998) Metabolite control of Sorghum C4 phosphoenolpyruvate carboxylase catalytic activity and phosphorylation state. Photosynth Res 58 153–162 [Google Scholar]
- Blonde JD, Plaxton WC (2003) Structural and kinetic properties of high and low molecular mass phosphoenolpyruvate carboxylase isoforms from the endosperm of developing castor oil seeds. J Biol Chem 278 11867–11873 [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins BW, Wilkins MB, Nimmo HG (1999) Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in Crassulacean acid metabolism. Plant Physiol 121 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47 273–298 [DOI] [PubMed] [Google Scholar]
- Coursol S, Giglioli-Guivarc'h N, Vidal J, Pierre JN (2000) An increase in phosphoinositide-specific phospholipase C activity precedes induction of C4 phosphoenolpyruvate carboxylase phosphorylation in illuminated and NH4Cl-treated protoplasts from Digitaria sanguinalis. Plant J 23 497–506 [DOI] [PubMed] [Google Scholar]
- Drozdowicz YM, Jones RL (1995) Hormonal regulation of organic and phosphoric acid release by barley aleurone layers and scutela. Plant Physiol 108 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SMG, Giglioli-Guivarc'h N, Pierre JN, Vidal J, Condon SA, Chollet R (1996) In-situ evidence for the involvement of calcium and bundle-sheath-derived photosynthetic metabolites in the C4 phosphoenolpyruvate carboxylase kinase signal transduction chain. Planta 199 467–474 [Google Scholar]
- Echevarría C, Pacquit V, Bakrim N, Osuna L, Delgado B, Arrio-Dupont M, Vidal J (1994) The effect of pH on the covalent and metabolic control of C4 phosphoenolpryruvate carboxylase from Sorghum leaf. Arch Biochem Biophys 315 425–430 [DOI] [PubMed] [Google Scholar]
- Echevarría C, Vidal J (2003) The unique phosphoenolpyruvate carboxylase kinase. Plant Physiol Biochem 41 541–547 [Google Scholar]
- Echevarría C, Vidal J, Jiao JA, Chollet R (1990) Reversible light activation of the phosphoenolpyruvate carboxylase protein-serine kinase in maize leaves. FEBS Lett 275 25–28 [DOI] [PubMed] [Google Scholar]
- Echevarría C, Vidal J, Le Marechal P, Brulfert J, Ranjeva R, Gadal P (1988) The phosphorylation of Sorghum leaf phosphoenolpyruvate carboxylase is a Ca2+-calmodulin dependent process. Biochem Biophys Res Commun 155 835–840 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedling. Plant Cell (Suppl) 14 S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine V, Hartwel G, Jenkins HG, Nimmo HG (2002) Arabidopsis thaliana contains two phosphoenolpyruvate carboxylase kinase genes with different expression pattern. Plant Cell Environ 25 115–122 [Google Scholar]
- Gennidakis S, Rao S, Greenham K, Glen Uhrig R, O'Leary B, Snedden WA, Lu C, Plaxton WC (2007) Bacterial- and plant-type phosphoenolpyruvate carboxylase polypeptides interact in the hetero-oligomeric class-2 PEPC complex of developing castor oil seeds. Plant J 52 839–849 [DOI] [PubMed] [Google Scholar]
- Giglioli-Guivarc'h N, Pierre JN, Brown S, Chollet R, Vidal J, Gadal P (1996) The light-dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell 8 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek S, Heim U, Horstmann C, Wobus U, Weber H (1999) Phosphoenolpyruvate carboxylase in developing seeds of Vicia faba L.: gene expression and metabolic regulation. Planta 208 66–72 [DOI] [PubMed] [Google Scholar]
- González MC, Echevarría C, Vidal J, Cejudo FJ (2002) Isolation and characterisation of a wheat phosphoenolpyruvate carboxylase gene: modelling of the encoded protein. Plant Sci 162 233–238 [Google Scholar]
- González MC, Osuna L, Echevarría C, Vidal J, Cejudo FJ (1998) Expression and localization of phosphoenolpyruvate carboxylase in developing and germinating wheat grains. Plant Physiol 116 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamabata A, García-Maya M, Romero T, Bernal-Lugo I (1988) Kinetics of the acidification capacity of aleurone layer and its effect upon solubilization of reserve substances from starchy endosperm of wheat. Plant Physiol 86 643–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J, Gill A, Nimmo GA, Wilkins MB, Jenkins GL, Nimmo HG (1999) Phosphoenolpyruvate carboxylase kinase is a novel protein kinase regulated at the level of expression. Plant J 20 333–342 [DOI] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Heistek JC, Wang M (1994) Counteractive effects of ABA and GA3 on extracellular and intracellular pH and malate in barley aleurone. Plant Physiol 106 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45 577–607 [Google Scholar]
- Izui K, Matsumura H, Furumoto T, Kai Y (2004) Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol 55 69–84 [DOI] [PubMed] [Google Scholar]
- Jiao JA, Echevarría C, Chollet R (1991) Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci USA 88 2712–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai Y, Matsumura H, Inoue T, Terada K, Nagara Y, Yoshinaga T, Kihara A, Tsumura A, Izui K (1999) Three-dimensional structure of phosphoenolpyruvate carboxylase: a proposed mechanism for allosteric inhibition. Proc Natl Acad Sci USA 96 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277 680–685 [DOI] [PubMed] [Google Scholar]
- Law RD, Plaxton WC (1997) Regulatory phosphorylation of banana fruit phosphoenolpyruvate carboxylase by a copurifying phosphoenolpyruvate carboxylase-kinase. Eur J Biochem 247 642–651 [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 19 199–222 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passoneau JV (1972) A Flexible System of Enzymatic Analysis. Academic Press, New York
- Macnicol PK, Jacobsen JV (1992) Endosperm acidification and related metabolic changes in the developing barley grain. Plant Physiol 98 1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol PK, Raymond P (1998) Role of phosphoenolpyruvate carboxylase in malate production by the developing barley aleurone layer. Physiol Plant 103 132–138 [Google Scholar]
- Mamedov TG, Moellering ER, Chollet R (2005) Identification and expression analysis of two inorganic C- and N-responsive genes encoding novel and distinct molecular forms of eukaryotic phosphoenolpyruvate carboxylase in the green microalga Chlamydomonas reinhardtii. Plant J 42 832–843 [DOI] [PubMed] [Google Scholar]
- Matsumura H, Xie Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y (2002) Crystal structures of C4 from maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases. Structure 10 1721–1730 [DOI] [PubMed] [Google Scholar]
- Meimoun P, Ambard-Bretteville F, Colas des Francs-Small C, Valot B, Vidal J (2007) Analysis of plant phosphoproteins. Anal Biochem 371 238–246 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Ouyang Y, Mamedov TG, Chollet R (2007) The two divergent PEP-carboxylase catalytic subunits in the green microalga Chlamydomonas reinhardtii respond reversibly to inorganic-N-supply and co-exist in the high-molecular-mass, hetero-oligomeric class-2-PEPC complex. FEBS Lett 581 4871–4876 [DOI] [PubMed] [Google Scholar]
- Monreal JA, Feria AB, Vinardell JM, Vidal J, Echevarría C, García-Mauriño S (2007) ABA modulates the degradation of phosphoenolpyruvate carboxylase kinase in sorghum leaves. FEBS Lett 581 3468–3472 [DOI] [PubMed] [Google Scholar]
- Murmu J, Plaxton WC (2007) Phosphoenolpyruvate carboxylase protein kinase from developing castor oil seeds: partial purification, characterization, and reversible control by photosynthate supply. Planta 226 1299–1310 [DOI] [PubMed] [Google Scholar]
- Nhiri M, Bakrim N, Bakrim N, El Hachimi-Messouak Z, Echevarría C, Vidal J (2000) Posttranslational regulation of phosphoenolpyruvate carboxylase during germination of Sorghum seeds: influence of NaCl and L-malate. Plant Sci 151 29–37 [Google Scholar]
- Nhiri M, Bakrim N, Pacquit V, El Hachimi-Messouak Z, Osuna L, Vidal J (1998) Calcium-dependent and -independent phosphoenolpyruvate carboxylase kinases in sorghum leaves: further evidence for the involvement of the calcium-independent protein kinase in the in situ regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase. Plant Cell Physiol 39 241–246 [Google Scholar]
- Nimmo GA, McNaughton GA, Fewson CA, Wilkins MB, Nimmo HG (1987) Changes in the kinetic properties and phosphorylation state of phosphoenolpyruvate carboxylase in Zea mays leaves in response to light and dark. FEBS Lett 213 18–22 [Google Scholar]
- Nimmo GA, Wilkins MB, Nimmo HG (2001) Partial purification and characterization of a protein inhibitor of phosphoenolpyruvate carboxylase kinase. Planta 213 250–257 [DOI] [PubMed] [Google Scholar]
- Nimmo HG (2003) Control of the phosphorylation of phosphoenolpyruvate carboxylase in higher plants. Arch Biochem Biophys 414 189–196 [DOI] [PubMed] [Google Scholar]
- Ogawa N, Yabuta N, Ueno Y, Izui K (1998) Characterization of a maize Ca(2+)-dependent protein kinase phosphorylating phosphoenolpyruvate carboxylase. Plant Cell Physiol 39 1010–1019 [DOI] [PubMed] [Google Scholar]
- Osuna L, González MC, Cejudo FJ, Vidal J, Echevarría C (1996) In vivo and in vitro phosphorylation of the phosphoenolpyruvate carboxylase from wheat seed during germination. Plant Physiol 111 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna L, Pierre JN, González MC, Alvarez R, Cejudo FJ, Echevarría C, Vidal J (1999) Evidence for a slow-turnover form of the Ca2+-independent phosphoenolpyruvate carboxylase kinase in the aleurone endosperm tissue of germinating barley seeds. Plant Physiol 119 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquit V, Giglioli N, Crétin C, Pierre JN, Vidal J, Echevarría C (1995) Regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase from sorghum: an immunological study using specific anti-phosphorylation site-antibodies. Photosynth Res 43 283–288 [DOI] [PubMed] [Google Scholar]
- Rivoal J, Dunford R, Plaxton WC, Turpin DH (1996) Purification and properties of four phosphoenolpyruvate carboxylase isoforms from the green alga Selenastrum minutum: evidence that association of the 102-kDa catalytic subunit with unrelated polypeptides may modify the physical and kinetic properties of the enzyme. Arch Biochem Biophys 332 47–57 [DOI] [PubMed] [Google Scholar]
- Rivoal J, Plaxton WC, Turpin DH (1998) Purification and characterization of high- and low-molecular-mass isoforms of phosphoenolpyruvate carboxylase from Chlamydomonas reinhardtii: kinetic, structural and immunological evidence that the green algal enzyme is distinct from the prokaryotic and higher plant enzyme. Biochem J 331 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoal J, Smith CR, Moraes TF, Turpin DH, Plaxton WC (2002) A method for activity attaining after native polyacrylamide gel electrophoresis using a coupled enzyme assay and fluorescence detection: application to the analysis of several glycolytic enzymes. Anal Biochem 300 94–99 [DOI] [PubMed] [Google Scholar]
- Rock CD, Quatrano RS (1995) The role of hormones during seed development. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 671–697
- Ruuska SA, Girke T, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during the Arabidopsis seed filling. Plant Cell 14 1191–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez R, Cejudo FJ (2003) Identification and expression analysis of a gene encoding a bacterial-type phosphoenolpyruvate carboxylase from Arabidopsis and rice. Plant Physiol 132 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan RS, Singh N, Plaxton WC (1992) Phosphoenolpyruvate carboxylase activity and concentration in the endosperm of developing and germinating castor oil seeds. Plant Physiol 99 445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB, Shachar-Hill Y (2004) Understanding flux in plant metabolic networks. Curr Opin Plant Biol 7 309–317 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Jenkins GI, Nimmo HG (2004) Roots, cycles and leaves: expression of the phosphoenolpyruvate carboxylase gene family in soybean. Plant Physiol 135 2078–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi KE, Turner WL, Gennidakis S, Plaxton WC (2005) In vivo regulatory phosphorylation of novel phosphoenolpyruvate carboxylase isoforms in endosperm of developing castor oil seed. Plant Physiol 139 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y, Furumoto T, Hisabori T, Izui K (2004) Mechanism of redox regulation of a C4-phosphoenolpyruvate carboxylase (PEPC) via thioredoxin (Trx). Plant Cell Physiol 45 S154 [Google Scholar]
- Turner WL, Knowles VL, Plaxton WC (2005) Cytosolic pyruvate kinase: subunit composition, activity, and amount in developing castor and soybean seeds, and biochemical characterization of the purified castor seed enzyme. Planta 6 1051–1062 [DOI] [PubMed] [Google Scholar]
- Uhrig RG, O'Leary B, Spang HE, MacDonald JA, She YM, Plaxton WC (2008) Co-immunopurification of phosphorylated bacterial- and plant-type phosphoenolpyruvate carboxylase with the plastidial pyruvate dehydrogenase complex from developing castor oil seeds. Plant Physiol 146 1346–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Chollet R (1997) Regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase. Trends Plant Sci 2 230–237 [Google Scholar]
- Vidal J, Godbillon P, Gadal P (1981) Recovery of active highly purified phosphoenolpyruvate carboxylase from specific immunoabsorbent column. FEBS Lett 118 31–34 [Google Scholar]
- Wadham C, Winter H, Schuller KA (1996) Regulation of soybean nodule phosphoenolpyruvate carboxylase in vivo. Physiol Plant 97 531–535 [Google Scholar]
- Wang YH, Chollet R (1993) Partial purification and characterization of phosphoenolpyruvate carboxylase protein-serine kinase from illuminated maize leaves. Arch Biochem Biophys 304 496–502 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Chollet R (1997) Phosphoenolpyruvate carboxylase protein kinase from soybean root nodules: partial purification, characterization, and up/down regulation by photosynthate supply from the shoots. Arch Biochem Biophys 343 260–269 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li B, Chollet R (1995) In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol 108 1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.