Abstract
Reactive oxygen species act as signaling molecules but can also directly provoke cellular damage by rapidly oxidizing cellular components, including lipids. We developed a high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry-based quantitative method that allowed us to discriminate between free radical (type I)- and singlet oxygen (1O2; type II)-mediated lipid peroxidation (LPO) signatures by using hydroxy fatty acids as specific reporters. Using this method, we observed that in nonphotosynthesizing Arabidopsis (Arabidopsis thaliana) tissues, nonenzymatic LPO was almost exclusively catalyzed by free radicals both under normal and oxidative stress conditions. However, in leaf tissues under optimal growth conditions, 1O2 was responsible for more than 80% of the nonenzymatic LPO. In Arabidopsis mutants favoring 1O2 production, photooxidative stress led to a dramatic increase of 1O2 (type II) LPO that preceded cell death. Furthermore, under all conditions and in mutants that favor the production of superoxide and hydrogen peroxide (two sources for type I LPO reactions), plant cell death was nevertheless always preceded by an increase in 1O2-dependent (type II) LPO. Thus, besides triggering a genetic cell death program, as demonstrated previously with the Arabidopsis fluorescent mutant, 1O2 plays a major destructive role during the execution of reactive oxygen species-induced cell death in leaf tissues.
Plant leaves capture sun-derived light energy to drive CO2 fixation during photosynthesis. During this process, leaves need to cope with photooxidative stress when the balance between energy absorption and consumption is disturbed. Excess excitation energy in the photosystems (PSI and PSII) leads to the inhibition of photosynthesis via the production of various reactive oxygen species (ROS) at different spatial levels of the cell (Apel and Hirt, 2004; Asada, 2006; Van Breusegem and Dat, 2006). Both exposure to high light intensities and decreased CO2 availability direct linear electron transfer toward the reduction of molecular oxygen, generating superoxide radicals (O2−.) at PSI (the Mehler reaction). Superoxide dismutation generates hydrogen peroxide (H2O2), which is detoxified in the chloroplast by ascorbate peroxidases. As such, this so-called water-water cycle participates in the dissipation of excess energy (Asada, 2006). Decreased CO2 availability affects the first step in CO2 fixation by shifting the carboxylation of Rubisco by the Rubisco carboxylase-oxygenase enzyme toward oxygenation, a process called photorespiration. This leads, through the action of glycolate oxidase, to peroxisomal H2O2 production that is counteracted by catalases. Finally, when the intersystem electron carriers are overreduced, triplet excited P680 in the PSII reaction center as well as triplet chlorophylls in the light-harvesting antennae are produced, with the production of singlet oxygen (1O2) as a consequence (Krieger-Liszkay, 2005). In photosynthetic membranes, 1O2 is quenched by carotenoids and tocopherols. When antioxidant mechanisms are overwhelmed, increased cellular ROS levels trigger signal transduction events related to stress signaling and programmed cell death (Mittler et al., 2004; Van Breusegem and Dat, 2006). On the other hand, excessive ROS accumulation damages pigments, proteins, nucleic acids, and lipids (Halliwell and Gutteridge, 2007), thereby contributing to or executing cell death.
Since under environmental stress conditions different ROS are produced simultaneously, a causal link between the accumulation of a specific ROS and its signaling or damaging effects has always been difficult to establish. In recent years, the production of various transgenic Arabidopsis (Arabidopsis thaliana) plants with compromised levels of specific antioxidant enzymes and the identification of the conditional fluorescent (flu) mutant provided important tools to assess the specific effects of O2−., H2O2, and 1O2 within a particular subcellular compartment (Dat et al., 2003; op den Camp et al., 2003; Pnueli et al., 2003; Rizhsky et al. 2003; Vandenabeele et al., 2004; Wagner et al., 2004; Queval et al., 2007). For example, with catalase-deficient [Cat(−)] plants, the signaling effects of increased photorespiratory H2O2 levels could be identified (Dat et al., 2003; Vandenabeele et al., 2004; Queval et al., 2007). Similarly, in the conditional flu mutant increased plastid 1O2 levels were shown to induce a genetic program leading to cell death (op den Camp et al., 2003; Wagner et al., 2004). Nevertheless, whereas careful monitoring of gene expression on the whole-genome level enables to pinpoint specific signaling capacities for diverse ROS (Mittler et al., 2004; Gadjev et al., 2006), it remained impossible to discriminate between the oxidative damaging effects on cellular components of different ROS.
One consequence of ROS formation is lipid peroxidation (LPO; Halliwell and Gutteridge, 2007). Two nonenzymatic reaction types lead to specific patterns of oxidized membrane polyunsaturated fatty acids (PUFAs; Stratton and Liebler, 1997; Montillet et al., 2004; Mueller et al., 2006). Type I reactions are initiated by free radicals (FRs) having high redox potential, such as hydroxyl radicals (.OH) or organic oxyl and peroxyl radicals, and type II reactions are the result of 1O2 action. Notably, O2−. and H2O2 are not sufficiently reactive to oxidize any PUFA. However, both ROS can be nonenzymatically converted to .OH through Fenton-type reactions in the presence of transition metal ions such as Fe2+ (Halliwell and Gutteridge, 2007). Both type I and type II reactions lead to the formation of respective oxygenated fatty acids. Here, we propose a novel and quantitative approach to distinguish between FR- and 1O2-mediated LPO in plants by quantifying type II oxidation-specific hydroxy fatty acids with HPLC-tandem mass spectrometry (MS/MS), allowing us to monitor the relative contribution of LPO caused by PSI-dependent O2−./H2O2, photorespiratory H2O2, and photosynthetic 1O2 during photooxidative stress and cell death. We demonstrate that nonenzymatic LPO in leaves is almost exclusively mediated by 1O2 and that photooxidative stress-dependent cell death involves 1O2 production in its final stage.
RESULTS
An LPO Signature That Discriminates between 1O2 and FR-Dependent LPO in Plant Tissues
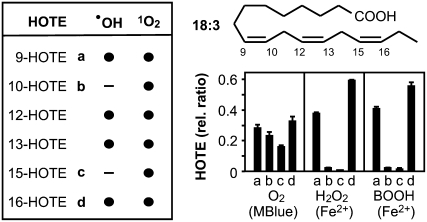
1O2 and FRs (as defined above) readily oxidize free and esterified PUFAs with a rate constant almost only limited by diffusion (Elstner, 1982). In both cases, the products are hydroperoxy PUFAs that are reduced in vivo to stable hydroxy fatty acids. We first assessed the differential oxidation of both linoleic (18:2) and linolenic acid (18:3) in vitro. 1O2 production was triggered by O2 photoactivation with methylene blue (Stratton and Liebler, 1997), whereas FR-mediated oxidation was initiated by H2O2 or tert-butyl hydroperoxide (BOOH) in the presence of Fe2+ (Halliwell and Gutteridge, 2007). After reduction of hydroperoxy PUFAs, hydroxy fatty acids were analyzed by HPLC-negative electrospray ionization-MS/MS. In contrast to gas chromatography-MS based methods (Mueller et al., 2006; Przybyla et al., 2008), HPLC-electrospray ionization-MS/MS does not require derivatization of hydroxy fatty acids and hence allows the direct and quantitative evaluation of all hydroxy fatty acid isomers. 1O2- and FR-mediated peroxidation of both 18:2 and 18:3 can be discriminated and quantified by the hydroxy fatty acid isomer distribution (Supplemental Fig. S1). Since it is the most abundant PUFA in leaves, we opted to analyze further only the 18:3 oxidation products (Fig. 1). Two out of the six possible hydroxy fatty acid isomers, 10-hydroxy-8,12,15(E,Z,Z)octadecatrienoic acid (HOTE) and 15-HOTE, were specific products exclusively formed by 1O2, whereas 9-HOTE, 12-HOTE, 13-HOTE, and 16-HOTE could be both generated through 1O2 or FR catalysis. The comparison of the relative abundance of 10-HOTE and 15-HOTE to that of 9-HOTE and 16-HOTE allowed for a clear distinction between 1O2 and FR-mediated 18:3 oxidation (Fig. 1). We estimated that it was possible to evaluate a combination of both LPO processes in the proportion between 80%/20% and 20%/80%.
Figure 1.
Peroxidation of linolenic acid (18:3). Peroxidation of 18:3 yields 9-, 10-, 12-, 13-, 15-, and 16-(hydro-)peroxy fatty acids, which can be reduced in vitro and in vivo to the corresponding HOTEs. All six theoretical possible isomers are formed by 1O2-mediated oxidation, while FRs such as .OH catalyze only the formation of four isomers. The HOTE signature, described as relative normalized levels of 9-HOTE (a), 10-HOTE (b), 15-HOTE (c), and 16-HOTE (d), characterizes: 1O2-mediated LPO, 1O2 generated by light activation of O2 in the presence of methylene blue (MBlue); FR-mediated LPO, FR generated from H2O2 or tert-BOOH through Fe2+ catalysis; see “Materials and Methods.” Results are expressed as mean ± sd (n = 3).
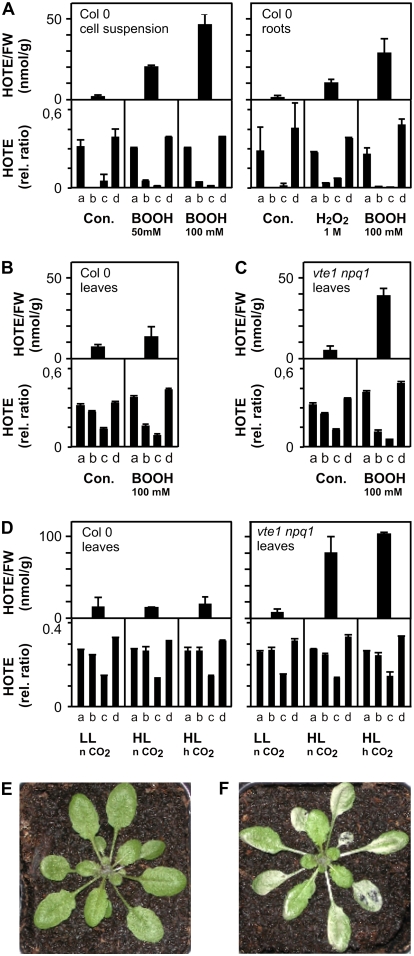
Next, we assessed both LPO processes in planta. Typically, lipid extracts from Arabidopsis tissues were reduced and hydrolyzed before total (free and esterified) hydroxy fatty acids were analyzed. We evaluated ROS-mediated LPO and lipoxygenase (LOX)-dependent LPO by HPLC-UV as previously described (Montillet et al., 2004), and then the 1O2- and FR-mediated LPO using the method established above. To obtain unambiguous observations for FR-mediated LPO, we investigated LPO processes in Arabidopsis cell suspensions and roots. Both tissues are devoid of 1O2-producing photosystems and displayed low basal levels of ROS-mediated LPO (approximately 1–2 nmol g−1 fresh weight [FW]) with an almost exclusively FR-mediated HOTE signature (Fig. 2A). Four hours after the addition of BOOH to cell suspensions and H2O2 or BOOH to roots, ROS-mediated LPO increased and the HOTE signature was indicative of FR-mediated LPO. Next, we compared HOTE isomer distribution in leaves from Arabidopsis wild-type and vte1 npq1 plants. The vte1 npq1 double mutant is sensitive to photooxidative stress due to a deficiency in two lipophilic 1O2 quenchers in the chloroplasts, α-tocopherol and the carotenoid zeaxanthin (Havaux et al., 2005). In both wild-type and vte1 npq1 leaves, basal levels of HOTE (approximately 5–10 nmol/g FW; Fig. 2, B and C) were approximately 5-fold higher than in roots. The HOTE signature in both wild-type and vte1 npq1 leaves displayed an almost exclusively 1O2-mediated LPO (Fig. 2, B and C). We then infiltrated the leaves of both plants with a mixture of BOOH and Fe3+ to favor the Fenton reaction. After 4 h incubation in the dark, the HOTE signature indicated a shift from a 1O2 LPO mechanism (Fig. 2, B and C, controls) to a mixed 1O2/FR LPO mechanism in treated samples (Fig. 2, B [50% FR-dependent LPO] and C [70% FR-dependent LPO]). Exposure of wild-type plants to photooxidative stress conditions (7°C and 1,300 μmol m−2 s−1 light) for 26 h at normal CO2 (380 ppm) and at high CO2 (2,000 ppm, to inhibit photorespiratory production of H2O2) did not increase 1O2-mediated LPO (Fig. 2D) and did not provoke tissue damage (Fig. 2E). However, in vte1 npq1 a strong 1O2-mediated LPO and tissue damage were observed (Fig. 2, D and F). Notably, total HOTE levels and the isomer distribution patterns were similar under normal and high CO2 conditions, suggesting that similar levels of 1O2 were produced in the presence or absence of photorespiration. Thus, in vte1 npq1, LPO and cell death are strictly correlated with 1O2 production. By comparison, in wild-type plants we observed both delayed 1O2-mediated LPO increase and light-induced damage that became only apparent after 54 h (data not shown).
Figure 2.
FR- and 1O2-mediated peroxidation of 18:3 in Arabidopsis. A, ROS-mediated LPO, expressed as HOTE levels, and HOTE signature (for details, see Fig. 1) of wild-type (Col-0) cell suspensions and roots under control conditions (Con.) and after treatment with tert-BOOH or H2O2 for 4 h. B to D, ROS-mediated LPO and HOTE signature (for details, see Fig. 1) in wild-type (Col-0) and vte1 npq1 leaves of plants grown under low light (LL = 100 μmol m−2 s−1). B and C, Plants were kept in the dark and infiltrated with a mixture of 100 mm BOOH/5 mm Fe2+ for 4 h. D, Plants were stressed at 7°C, high light (HL = 1,300 μmol m−2 s−1), and different CO2 (n CO2 = 380 ppm, h CO2 = 2,000 ppm) conditions for 26 h. Results are expressed as mean ± sd (n = 3). E and F, Images of wild-type (E) and vte1 npq1 (F) plants stressed with high light (1,300 μmol m−2 s−1; 7°C) for 26 h at 2,000 ppm CO2 showing leaf damage to the vte1 npq1 plant.
Taken together, these results demonstrate that the HOTE signature determined by HPLC-electrospray ionization-MS/MS allows us to discriminate between FR- and 1O2-mediated LPO processes in plant tissues.
1O2 Generation Induces Cell Death in Arabidopsis Leaf Tissues
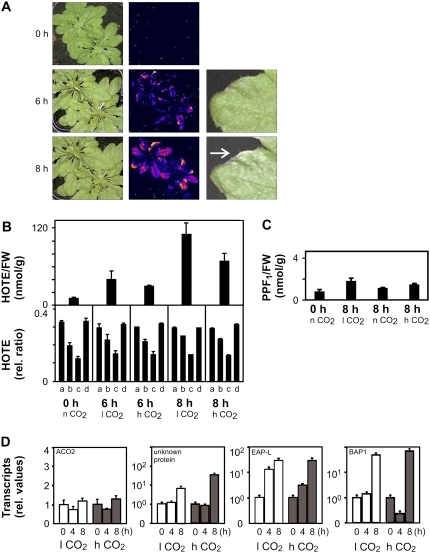
In leaves of the flu mutant, a genetic cell death program can be triggered by protochlorophyllide-dependent 1O2, involving 13-LOX LPO, and apparently occurs without any increase of nonenzymatic LPO early in the signaling phase, whereas in etiolated seedlings transferred to the light, 1O2 production appeared to be cytotoxic and led to nonenzymatic LPO and cell death (Kim et al., 2008; Przybyla et al., 2008). We observed in our experiments that the first photooxidative leave damage symptoms, which appeared in the light 4 h after the dark/light shift, were accompanied by a nonenzymatic LPO caused almost exclusively by 1O2 (about 15% of the 13-LOX LPO; Supplemental Fig. S2), suggesting that 1O2 cytotoxic effects may occur in photosynthetic tissues. Because we observed high levels of 1O2-mediated LPO in the vte1 npq1 mutant that directly preceded or occurred concurrently with cell death we hypothesized that 1O2 produced by the photosystems is also cytotoxic, damaging the photosynthetic tissues. To validate this hypothesis, we assessed LPO events in ch1 mutant plants that are deficient in chlorophyll b and PSII chlorophyll-protein antenna complexes and are characterized by a low nonphotochemical quenching (NPQ) capacity (Havaux et al., 2007). Because NPQ deactivates excited chlorophylls, thereby avoiding 1O2 production, its inhibition in ch1 leads to a highly sensitive photooxidation phenotype. Transfer of ch1 plants pregrown at low light (20°C, 100 μmol m−2 s−1) to photooxidative stress conditions (7°C, 1,300 μmol m−2 s−1) at high CO2 levels induced leaf damage within 8 h (Fig. 3A). Tissue damage was preceded by an increase of LPO after 6 h, as visualized by autoluminescence imaging (Havaux et al., 2006; Fig. 3A) and quantified by HOTE levels (Fig. 3B). Again, the HOTE signature had a typical 1O2-mediated LPO signature (Fig. 3B). Both in vte1 npq1 and ch1 plants, cell death symptoms were observed when LPO level exceeded a threshold level of 40 to 50 nmol HOTE g−1 FW.
Figure 3.
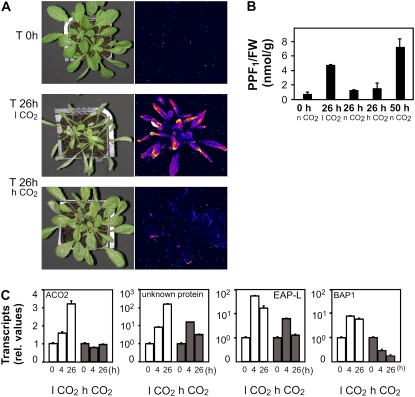
Photooxidation of the ch1 Arabidopsis mutant. ch1 plants, grown at low light (100 μmol m−2 s−1; 20°C) under normal CO2 (n CO2 = 380 ppm) conditions, were stressed with high light (1,300 μmol m−2 s−1; 7°C) at different CO2 levels (l CO2 = 70 ppm; n CO2; h CO2 = 2,000 ppm) for the time indicated. A, Images of control and stressed plants at high CO2 after 0, 6, and 8 h; left sections, photography of the plants; middle sections, autoluminescence measurement (Havaux et al., 2006) reflecting LPO; right sections, example of damage development on a leaf. B, ROS-mediated LPO, expressed as total HOTE levels (top), and HOTE signature (bottom; for details, see Fig. 1) of leaves from ch1 plants stressed for 0, 6, and 8 h. The HOTE signatures indicate the occurrence of 1O2-mediated LPO under all conditions. C, PPF1 levels of ch1 plants. D, Expression of H2O2 (ACO2 and unknown protein) and 1O2 (EAP-like and BAP1) marker genes in ch1 plants (for details, see “Materials and Methods”) for the time (h) indicated. Results are expressed as mean ± sd (n = 3) for B and C, and mean ± se (n = 6) for D.
The above results indicated that 1O2-mediated LPO directly precedes the onset of cell death. A similar sequence of events was seen at high and low (70 ppm) CO2 levels, suggesting that the observed LPO and cell death were independent of photorespiratory H2O2. The absence of FR-mediated LPO under these conditions was confirmed by monitoring the levels of F1-phytoprostanes (PPF1) that are sensitive and specific markers for FR-mediated LPO (Imbusch and Mueller, 2000; Mueller, 2004). PPF1 levels in wild-type and ch1 leaves were below 2 nmol/g FW under low or high light conditions both at low and high CO2 concentrations (Fig. 3C). Additional evidence of a close correlation between 1O2 accumulation and cell death in ch1 was obtained by monitoring H2O2 and 1O2-specific marker genes (Gadjev et al., 2006; Laloi et al., 2007). We analyzed the relative expression levels of two H2O2-responsive genes and two 1O2-responsive genes (Fig. 3D). In high light-stressed ch1 plants, H2O2 marker gene expression is maintained or slightly induced at both low and high CO2. In agreement with the HOTE signature, 1O2-specific transcripts were induced. Hence, PPF1 and gene expression analysis confirmed that ch1 cell death was not associated with H2O2 and FR-mediated LPO.
Taken together, these results indicate that the ch1 mutant is an appropriate model system to study 1O2-specific oxidative stress. Using the vte1 npq1, flu, and ch1 genetic models, we show that 1O2-mediated LPO directly precedes and/or correlates with cell death and that plastid 1O2 accumulation not only is able to trigger a genetic death program, as demonstrated with the flu mutant (op den Camp et al., 2003; Wagner et al., 2004; Przybyla et al., 2008), but is also directly cytoxic to photosynthetic cells by oxidizing cellular components, such as lipids.
Overproduction of O2−. or H2O2 Triggers Photooxidative 1O2 Formation, LPO, and Cell Death
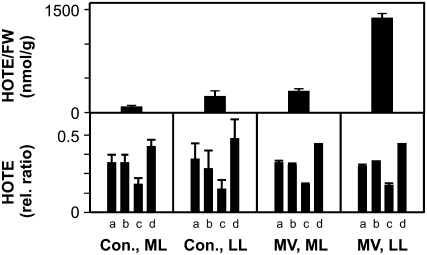
To assess LPO signatures in plant cell death events, we monitored the LPO signature triggered by increased O2−./H2O2 levels at the PSI site by applying 1 μm methyl viologen (MV) to Arabidopsis leaf discs from plants grown either under a low or moderate light regime (100 or 250 μmol m−2 s−1). MV is an herbicide that mimics the Mehler reaction by collecting electrons from the reduced ferredoxins at PSI and transferring them to O2 to produce O2−. that is rapidly converted into H2O2 (Asada, 2006). Leaf discs from low light-grown plants were more sensitive than those from plants grown at moderate light, as first bleaching appeared within 26 and 50 h, respectively (data not shown). In addition, MV-induced HOTE levels were significantly higher in leaf discs from low light-grown plants (Fig. 4). Surprisingly, in both cases a strong 1O2-mediated LPO was detected (Fig. 4). The mechanism of 1O2 formation is not clear but is likely the result of O2−./H2O2-propagated oxidative damage of PSII and/or release of chlorophyll from damaged PS.
Figure 4.
Photooxidation of wild-type Arabidopsis leaves treated with MV. ROS-mediated LPO, expressed as HOTE levels and HOTE signature (for details, see Fig. 1) of leaf discs from control plants (Con.) and MV-treated plants. Leaf discs of plants grown in low light (LL = 100 μmol m−2 s−1) and moderate light (ML = 250 μmol m−2 s−1) were incubated with or without MV (1 μm) for 30 and 50 h, respectively. The HOTE signature (for details, see Fig. 1) indicates an almost exclusive 1O2 LPO mechanism under all conditions. Results are expressed as mean ± sd (n = 3).
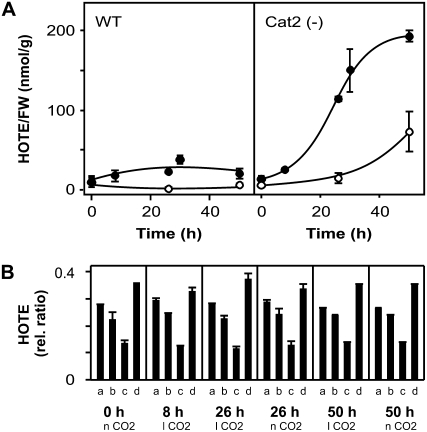
To determine the LPO signature provoked by increased photorespiratory H2O2 production, we used Cat(−) Arabidopsis plants (Vandenabeele et al., 2004) in which the activity of the major peroxisomal H2O2 scavenger is reduced below 10%. Cat(−) plants were grown under nonphotorespiratory conditions (low light, 100 μmol m−2 s−1; 2,000 ppm CO2) before transfer to photorespiration-stimulating conditions by applying 70 ppm (low level, near the compensation point), 380 ppm (normal level), or 2,000 ppm (high level) CO2 combined with high light (700 μmol m−2 s−1). In contrast to wild type, Cat(−) plants developed photorespiratory H2O2-dependent cell death within 26 h at low CO2 (Fig. 5A) and within 50 h at normal CO2 (data not shown). Cell death was completely inhibited at high CO2 (Fig. 5A). H2O2 accumulation was reflected by an increase in phytoprostane levels and H2O2-responsive gene expression (Fig. 5, B and C). LPO gradually increased in Cat(−) plants, but not in wild-type plants (Fig. 6A). Like for the ch1 mutant, tissue damage was correlated with LPO levels higher than 40 to 50 nmol HOTE g−1 FW. Strikingly, the HOTE isomer distribution again showed a strict 1O2-mediated LPO signature (Fig. 6B), suggesting that, also in Cat(−) plants, FR-mediated LPO is a minor process. The accumulation of 1O2 production was also reflected by the up-regulation of 1O2 specific transcripts. As expected, transcript levels of the H2O2-specific marker genes had also increased (Fig. 5B). In contrast, in Cat(−) plants transferred to high light and kept at high CO2, H2O2 as well as 1O2 marker gene expression was maintained or only slightly and transiently induced (Fig. 5B), and 1O2-mediated LPO increase (data not shown) and cell death (Fig. 5A) were completely suppressed. Hence, peroxisomal H2O2 overproduction ultimately leads to plastidic 1O2 formation and 1O2-dependent LPO preceding cell death. Taken together, our results demonstrate that, regardless of the chemical identity or subcellular production site of the initially produced ROS, plant cell death is correlated with 1O2-mediated LPO.
Figure 5.
Photooxidation in Cat(−) Arabidopsis plants. Wild-type and Cat(−) plants were grown at low light (100 μmol m−2 s−1) and high CO2 levels (2,000 ppm). Stress experiments were conducted at 21°C, medium light conditions (700 μmol m−2 s−1), and at different CO2 levels (l CO2 = 70 ppm; n CO2 = 380 ppm; h CO2 = 2,000 ppm) to modulate photorespiratory H2O2 production. A, Images of Cat(−) plants transferred to high light under l CO2 and h CO2 for 26 h. Leaf damage is observed only under l CO2 photorespiratory conditions (left) and is accompanied by increased autoluminescence (right; Havaux et al., 2006), reflecting LPO. B, PPF1 accumulation in Cat(−) plants under photorespiratory conditions for the time indicated. C, Expression of H2O2 (ACO2 and unknown protein) and 1O2 (EAP-like and BAP1) marker genes in Cat(−) plants (see “Materials and Methods” for details), under l CO2 and h CO2 stress conditions, for the time (h) indicated. Results are expressed as mean ± sd (n = 3) for B and as mean ± se (n = 6) for C.
Figure 6.
LPO in Cat(−) Arabidopsis plants. A, Changes in ROS-mediated LPO, expressed as HOTE levels, for wild-type and Cat(−) plants under photorespiratory stress conditions at 70 (•) or 380 ppm CO2 (○); see Figure 5 for conditions for B. The HOTE signatures in Cat(−) plants indicate the occurrence of 1O2-mediated LPO under all conditions (see Fig. 1 for details). Results are expressed as mean ± sd (n = 3).
DISCUSSION
In photosynthetic tissues, photooxidative stress involves the action of several ROS that are generated in different subcellular compartments. We established a novel method that allows us to discriminate between FR- and 1O2-mediated LPO by using 10-HOTE and 15-HOTE as specific reporters of 1O2-dependent LPO. Our results show that, whereas in Arabidopsis roots or cell suspensions nonenzymatic LPO depends on FRs, in leaves it is almost exclusively mediated by 1O2. Notably, levels of oxidized lipids were typically an order of magnitude higher in leaf tissues than in roots. Photooxidative stress conditions in the 1O2-sensitive vte1 npq1 double-mutant leaves provoked strongly increased 1O2-mediated LPO in combination with extensive tissue damage. In addition, using the chlorophyll b-deficient mutant ch1, it was shown that accelerating the accumulation of 1O2 was associated with more rapidly occurring tissue damage. Surprisingly, massive 1O2-mediated LPO was also detected in leaves under conditions that favored the accumulation of O2−. and H2O2, resulting in the production of .OH through the Fenton or Haber-Weiss reactions (Halliwell and Gutteridge, 2007). Together, these data indicate that photooxidative stress in leaf tissue results in 1O2-mediated LPO, whatever the initial ROS production is, and suggest that 1O2 can be directly toxic to cells.
The chemical reactivity of .OH and 1O2 toward a variety of organic scavenger molecules is almost equal and, in both cases, the reaction rate is diffusion limited (Elstner, 1982; Foyer and Noctor, 2005). The half-life of .OH in living cells is estimated to be around 1 ns (Cheng et al., 2002). In contrast, the decay time of 1O2 in cells is about 4 orders of magnitude higher (Baier et al., 2005). The decay time and the calculated diffusion path length of both species are also dependent on the microenvironment. For instance, the lifetime of 1O2 in water is 3 μs and in membranes about 14 μs, corresponding to a calculated diffusion length of about 200 and 400 nm, respectively (Baier et al., 2005). To explain these differences, it is known that .OH is the oxidizing species with the highest redox potential level (Halliwell and Gutteridge, 2007), leading to an indiscriminate hydrogen abstraction from any organic compound, whereas 1O2 is acting more specifically with unsaturated compounds, according to pericyclic addition reactions. Thus, 1O2 must diffuse until it reaches a suitable quencher. In algae at very high light, when the plastid 1O2-scavenging capacities are overwhelmed, long-range 1O2 diffusion has been experimentally measured, with 1O2 being detectable outside of chloroplasts (Fischer et al., 2007). In addition to this, .OH initiates a self-propagating radical chain reaction while 1O2 reacts with a 1:1 stoichiometry. All these considerations suggest that the relative damaging effect of equimolar concentrations of both ROS is largely in favor of .OH, therefore excluding that the 1O2 signature systematically found in our leaf experiments is due to a differential reactivity of 1O2 and FRs. It is established that .OH triggers a FR-catalyzed LPO chain reaction to form phytoprostanes that are validated specific markers of FR-dependent LPO (Mueller, 2004; Thoma et al., 2004). Although we did observe that in Cat(−) leaves increased H2O2 levels led to FR-mediated phytoprostane accumulation, LPO still exhibited a typical 1O2 signature, suggesting that FR-mediated LPO was completely overwhelmed by 1O2-mediated LPO. Therefore, our results show that the most relevant ROS, which is produced in the chlorophyll-containing lipid membranes of leaf tissue exposed to light, is 1O2 both under basal and stress conditions.
1O2 oxidizes cellular components, such as nucleic bases (Ravanat et al., 2000), proteins (Davies, 2003), and membranes and this characteristic is exploited in photodynamic therapies to kill cancer cells (Girotti and Kriska, 2004), bacteria (Maisch et al., 2007), and also to conceive herbicides (Fufezan et al., 2002; Tripathy et al., 2007). We have observed that 1O2-mediated LPO precedes cell death in our 1O2-overproducing mutants vte1 npq1 and ch1. Our data suggest that 1O2 production prevails over FR production and hence 1O2 appears as the most relevant ROS with respect to the execution of cell death in leaf tissues. An example of ROS-dependent plant cell death is also the hypersensitive response (Torres et al., 2006) during which light seems to be required both to initiate a cell death program, early on in the infection, as well as at later stages of cell death execution (Zeier et al., 2004; Liu et al., 2007). Interestingly, 1O2 HOTE markers significantly increased in Arabidopsis leaves challenged with the avirulent strain DC3000 of Pseudomonas syringae (Grun et al., 2007). In tobacco (Nicotiana tabacum), a pathogen-induced mitogen-activated protein kinase cascade disrupts chloroplast metabolism and leads to a rapid shutdown of CO2 fixation, a phenomenon that is expected to favor light-dependent excess excitation energy conditions in thylakoids and ROS production (Liu et al., 2007). It is also worth mentioning that compromised linear electron transport in transgenic tobacco plants with reduced ferredoxin-NADP(H) reductase levels also led to increased generation of 1O2, eventually followed by cell death (Palatnik et al., 2003). Apparently, environmental stresses that initiate cell death programs converge at halted chloroplast metabolism. As a result, excess light energy induces massive 1O2 formation, LPO, and membrane damage that may efficiently accelerate cell death. In the dark, the actual appearance of cell death is often delayed or even abolished. Taken together, these observations suggest that, to some extent, the photoproduction of 1O2 is responsible for light-dependent plant cell death. Therefore, we speculate that in chlorophyll-containing tissues, whatever the conditions of cell death initiation are, a photodynamic production of 1O2 is involved in the cell death process.
Besides a putative role as a physiologically relevant cytotoxin, a signaling role for 1O2 has been reported. Low subtoxic levels of 1O2 that do not cause any significant LPO are apparently sufficient to trigger signaling events in bacteria (Agnez-Lima et al., 2001), cyanobacteria (Anthony et al., 2005), algae (Ledford et al., 2007), and mammalian cells (Klotz et al., 2003). In the Arabidopsis flu mutant, 1O2 is produced by protochlorophyllides that accumulate after a dark-to-light shift hereby leading to cytotoxic effects in etiolated seedlings and to 1O2-mediated stress signaling and programmed cell death in green plantlets (op den Camp et al., 2003; Wagner et al., 2004; Kim et al., 2008; Przybyla et al., 2008). In agreement with the 1O2 signaling function, we observed an early accumulation of 1O2-specific transcripts in light-stressed ch1 and Cat(−) plants. We also observed that 1O2-mediated LPO was always associated with enhanced autoluminescence signals and preceded cell death in our 1O2-overproducing mutants. Hence, 1O2 appears to be involved in two clearly separable processes, signaling and execution, with respect to cell death. Such a dual role has been previously proposed for H2O2 also (Apel and Hirt, 2004; Asada, 2006; Torres et al., 2006; Van Breusegem and Dat, 2006).
In summary, we show that in addition to its involvement in stress and programmed cell death-related signaling events, 1O2 may be an important determinant during the execution phase of plant cell death.
MATERIALS AND METHODS
Plant Materials, Growth, and Stress Conditions
Arabidopsis (Arabidopsis thaliana) Heyhn. lines were issued from the Columbia ecotype (Col-0). The vte1 npq1, ch1, and Cat(−) plants have been previously described (Vandenabeele et al., 2004; Havaux et al., 2005, 2007). They were cultivated on soil under low light (100 μmol m−2 s−1; day/night 8/16; 20°C/18°C; 70% relative humidity) and normal (380 ppm) or high CO2 (2,000 ppm) for 4 to 5 weeks. For photooxidative stress conditions, plants were transferred in controlled chambers at the beginning of the day period (day/night 14/10), either at low temperature (7°C) and high light (1,300 μmol m−2 s−1) or normal temperature (20°C) and medium light (700 μmol m−2 s−1), and under various CO2 conditions (70, 380, and 2,000 ppm). For root treatments, plants were grown on sand and then transferred in an hydroponic oxygenated device for 3 d before application of H2O2 or BOOH for 4 h. Arabidopsis cells of Col-0 (100 mL of cell suspension in a 250 mL Erlenmeyer flask; rotatory shaking at 125 rpm) were grown at 25°C under continuous low light (90 μmol m−2 s−1) in Chandler's medium (Chandler et al., 1972). Cells were collected at 24% packed cell volume (15 g/100 mL) and treated similarly in the dark. For hydroperoxide application to the leaves, the treatment was carried out by infiltration of a solution of BOOH (100 mm) and FeCl2 (5 mm) at the end of the night period, and 4 h incubation in the dark. For MV treatment, leaf discs (diameter 1.2 cm) of Col-0 plants were floated for 30 to 50 h on a Tween 20 (0.1%) solution with or without 1 μm MV.
PUFA Oxidation
PUFAs (3–5 mg of 18:2 or 18:3) as a slurry mixture in methanol/water (0.5/1.5 mL) were oxidized (1) by 0.1 mm methylene blue in the light (300–340 μmol m−2 s−1) to generate 1O2; or (2) by 50 mm H2O2 or BOOH (5–10 mm) in the presence of 0.5 mm FeCl2 to generate FRs. The reaction was incubated for 10 to 120 min and stopped by adding 1 mL of CHCl3 containing 1 mg/mL triphenyl phosphine and 0.05% (w/v) butylated hydroxytoluene. The organic phase was recovered and analyzed for hydroxy fatty acid content and distribution.
LPO Analyses
Imaged autoluminescence signal is attributed to the spontaneous decomposition of peroxides (Havaux et al., 2006). Spontaneous photon emission from whole Arabidopsis plants was measured after 2 h dark adaptation with a liquid N2 cooled CCD camera, as previously described (Havaux et al., 2005, 2007). Integration time was 20 min. For hydroxy fatty acid analyses, lipids were extracted from 0.5 g frozen plant material by grinding with 2 × 1 mL CHCl3 containing 1 mg/mL triphenyl phosphine and 0.05% (w/v) butylated hydroxytoluene, with 15-hydroxy-11,13(Z,E)-eicosadienoic acid as internal standard. The organic phase was evaporated under a stream of N2. The residue was recovered in 1.25 mL ethanol and 1.25 mL 3.5 m NaOH and hydrolyzed at 80°C for 15 min. After addition of 2.2 mL 1 m citric acid, hydroxy fatty acids were extracted with 2 × 1 mL hexane/ether (50/50). The organic phase was used for the straight phase HPLC-UV analysis, as previously described (Montillet et al., 2004). PPF1 extraction was carried out on 0.5 g frozen material, as previously described (Imbusch and Mueller, 2000). For the HPLC-electrospray ionization-MS/MS analyses, aliquots were evaporated and recovered in aqueous 1 mm ammonium acetate/acetonitrile (60:40, v/v) with [18O2]13-HOTE or [18O3]PPF1 used as internal standards. Hydroxy fatty acids were separated by HPLC on a Purospher Star RP-18e column (125 × 2 mm i.d., 5 μm particle size, Merck). HOTE were eluted from the column using a linear gradient (0.2 mL/min) starting with 1 mm ammonium acetate in water/acetonitrile (65:35, v/v) at 0 min to 40:60 (v/v) at 10 min. PPF1 were eluted with a linear gradient starting with 90:10 (v/v) at 0 min to 60:40 (v/v) at 15 min. Hydroxy fatty acids and PPF1 were analyzed using a Waters Micromass Quatro Premier triple quatrupole mass spectrometer in the negative electrospray ionization mode (for conditions, see Supplemental Table S1).
RNA Isolation and Quantitative Reverse Transcription-PCR
For each sample, RNA was isolated from leaves of four plants with the TRIzol reagent (Invitrogen). First-strand cDNA was prepared using 2.5 μg total RNA, Superscript II reverse transcriptase (Invitrogen), and an oligo(dT)15 primer. Five microliters of 1:6 or 1:12 diluted first-strand cDNA was used as a template in the subsequent PCR, performed on a Bio-Rad iCycler using 200 nm primers and Platinum Supermix-UDG (Invitrogen) supplemented with fluorescein dye in a final volume of 25 μL per reaction, according to the manufacturer's instructions. All PCR reactions were performed in triplicate on material from two biological repeat experiments. The H2O2-specific genes used were At1g49150 (unknown protein) and At1g62380 (ACO2), the 1O2-specific genes At5g10830 (embryo abundant protein-like) and At3g61190 (BAP1; Gadjev et al., 2006; Laloi et al., 2007). Universal ProbeLibrary probes (Roche Applied Science) and primers used can be found in Supplemental Table S2. All transcript levels were normalized using actin-related protein 7 (At3g60830) as a control gene.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Hydroxy fatty acid distribution for the oxidation of linoleic (18:2) and linolenic (18:3) acids.
Supplemental Figure S2. Photooxidation of the flu Arabidopsis mutant.
Supplemental Table S1. Mass transitions and conditions for negative electrospray ionization HPLC-MS/MS analysis of PPF1 and of the hydroxy fatty acids issued from the oxidation of linoleic (18:2) and linolenic (18:3) acids.
Supplemental Table S2. Primers and universal probe library probes (Roche Applied Science) that were used in the quantitative PCR reactions.
Supplementary Material
Acknowledgments
We thank Christophe Laloi (ETH, Zurich) for helpful discussions; Jean-Luc Montillet (IBEB, CEA Cadarache) for the root and cell experiments; Michaël Vandorpe (DPSB, Ghent) for excellent technical assistance; Martine De Cock (DPSB, Ghent) for help in preparing the manuscript; and the Groupe de Recherche Appliquée en Phytotechnie (CEA Cadarache) for the controlled growth facilities.
This work was supported by grants from the exchange programs Tournesol (grant nos. F/11498QB and B/T2006.14) and Procope (grant nos. F/14925QF and D/0628204), in part by the Sonderforschungsbereiche number 567 of the Deutsche Forschungsgemeinschaft (to M.J.M.), and by the Ghent University (Geconcerteerde Onderzoeksacties no. 12051403) and the Research Foundation-Flanders (grant no. G.0350.04).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christian Triantaphylidès (ctriantaphylid@cea.fr).
The online version of this article contains Web-only data.
References
- Agnez-Lima LF, Di Mascio P, Demple B, Menck CFM (2001) Singlet molecular oxygen triggers the soxRS regulon of Escherichia coli. Biol Chem 382 1071–1075 [DOI] [PubMed] [Google Scholar]
- Anthony JR, Warczak KL, Donohue TJ (2005) A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc Natl Acad Sci USA 102 6502–6507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier J, Maier M, Engl R, Landthaler M, Baumler W (2005) Time-resolved investigations of singlet oxygen luminescence in water, in phosphatidylcholine, and in aqueous suspensions of phosphatidylcholine or HT29 cells. J Phys Chem B 109 3041–3046 [DOI] [PubMed] [Google Scholar]
- Chandler MT, Tandeau de Marsac N, Kouchkovsky Y (1972) Photosynthetic growth of tobacco cells in liquid suspension. Can J Bot 50 2265–2270 [Google Scholar]
- Cheng FC, Jen JF, Tsai TH (2002) Hydroxyl radical in living systems and its separation methods. J Chromatogr B Analyt Technol Biomed Life Sci 781 481–496 [DOI] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Beeckman T, Van De Cotte B, Langebartels C, Kangasjärvi J, Inzé D, Van Breusegem F (2003) Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J 33 621–632 [DOI] [PubMed] [Google Scholar]
- Davies MJ (2003) Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci 3 17–25 [DOI] [PubMed] [Google Scholar]
- Elstner EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33 73–96 [Google Scholar]
- Fischer BB, Krieger-Liszkay A, Hideg E, Snyrychová I, Wiesendanger M, Eggen RI (2007) Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett 581 5555–5560 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufezan C, Rutherford AW, Krieger-Liszkay A (2002) Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett 532 407–410 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti AW, Kriska T (2004) Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid Redox Signal 6 301–310 [DOI] [PubMed] [Google Scholar]
- Grun C, Berger S, Matthes D, Mueller MJ (2007) Early accumulation of non-enzymatically synthesised oxylipins in Arabidopsis thaliana after infection with Pseudomonas syringae. Funct Plant Biol 34 65–71 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J (2007) Free Radicals in Biology and Medicine, Ed 4. Oxford University Press, Oxford
- Havaux M, Dall'Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P (2005) Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17 3451–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Triantaphylidès C, Genty B (2006) Autoluminescence imaging: a non-invasive tool for mapping oxidative stress. Trends Plant Sci 11 480–484 [DOI] [PubMed] [Google Scholar]
- Imbusch R, Mueller MJ (2000) Analysis of oxidative stress and wound-inducible dinor isoprostanes F1 (phytoprostanes F1) in plants. Plant Physiol 124 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Apel K, Laloi C (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz LO, Kröncke KD, Sies H (2003) Singlet oxygen-induced signaling effects in mammalian cells. Photochem Photobiol Sci 2 88–94 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56 337–346 [DOI] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford HK, Chin BL, Niyogi KK (2007) Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot Cell 6 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S (2007) Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J 51 941–954 [DOI] [PubMed] [Google Scholar]
- Maisch T, Baier J, Franz B, Maier M, Landthaler M, Szeimies RM, Bäumler W (2007) The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc Natl Acad Sci USA 104 7223–7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network in plants. Trends Plant Sci 9 490–498 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Cacas JL, Garnier L, Montané MH, Douki T, Bessoule JJ, Polkowska-Kowalczyk L, Maciejewska U, Agnel JP, Vial A, et al (2004) The upstream oxylipin profile of Arabidopsis thaliana: a tool to scan for oxidative stresses. Plant J 40 439–451 [DOI] [PubMed] [Google Scholar]
- Mueller MJ (2004) Archetype signals in plants: the phytoprostanes. Curr Opin Plant Biol 7 441–448 [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Mène-Saffrané L, Grun C, Karg K, Farmer EE (2006) Oxylipins analysis methods. Plant J 45 472–489 [DOI] [PubMed] [Google Scholar]
- op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, et al (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Tognetti VB, Poli HO, Rodríguez RE, Blanco N, Gattuso M, Hajirezaei M-R, Sonnewald U, Valle EM, Carrillo N (2003) Transgenic tobacco plants expressing antisense ferredoxin-NADP(H) reductase transcripts display increased susceptibility to photo-oxidative damage. Plant J 35 332–341 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34 187–203 [DOI] [PubMed] [Google Scholar]
- Przybyla D, Göbel C, Imboden A, Hamberg M, Feussner I, Apel K (2008) Enzymatic, but not non-enzymatic 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J 54 236–248 [DOI] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52 640–657 [DOI] [PubMed] [Google Scholar]
- Ravanat JL, Di Mascio P, Martinez GR, Medeiros MHG, Cadet J (2000) Singlet oxygen induces oxidation of cellular DNA. J Biol Chem 275 40601–40604 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278 38921–38925 [DOI] [PubMed] [Google Scholar]
- Stratton SP, Liebler DC (1997) Determination of singlet oxygen-specific versus radical-mediated lipid peroxidation in photosensitized oxidation of lipid bilayers: effect of β-carotene and α-tocopherol. Biochemistry 36 12911–12920 [DOI] [PubMed] [Google Scholar]
- Thoma I, Krischke M, Loeffler C, Mueller MJ (2004) The isoprostanoid pathway in plants. Chem Phys Lipids 128 135–148 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Mohapatra A, Gupta I (2007) Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX. Biochim Biophys Acta 1767 860–868 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39 45–58 [DOI] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306 1183–1185 [DOI] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219 673–683 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.