Vol. 128: 714–725, 2002
Zhang D.-P., Wu Z.-Y., Li X.-Y., and Zhao Z.-X. Purification and Identification of a 42-Kilodalton Abscisic Acid-Specific-Binding Protein from Epidermis of Broad Bean Leaves.
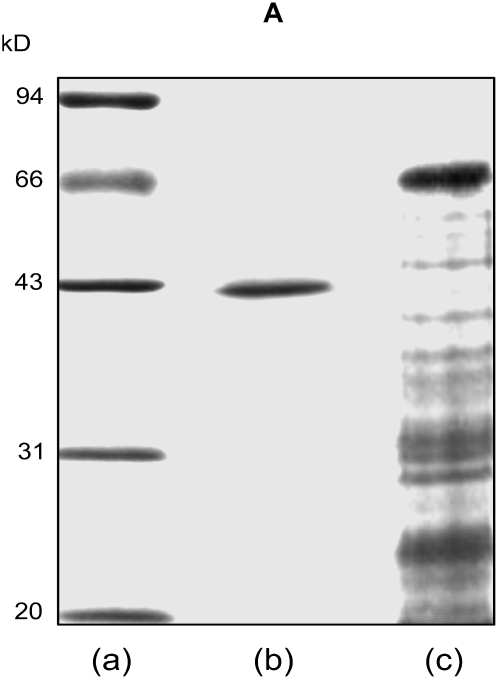
The authors regret that the SDS-PAGE figure of the purified abscisic acid (ABA)-binding protein in this paper (Fig. 4A; see http://www.plantphysiol.org/cgi/content/full/128/2/714/F4) had been used in a previously published paper in Acta Botanica Sinica (Wu Z-Y, Zhang D-P, Jia W-S [1999] Isolation and purification of ABA binding protein from abaxial epiderm of Vicia faba leaf. Acta Bot Sin 41: 842–845; figure 3B), in which the preliminary results of the ABA-binding proteins from our laboratory were published. In the preliminary assays, it was observed that the purified ABA-binding proteins included two bands, one at about 42 kD and another much less prominent band at about 70 kD. With the improved purification procedures published in the Plant Physiology paper, the 70-kD protein was no longer apparent, but the ABA-binding activity did not substantially change, which indicated that the 42-kD protein was an ABA-binding protein. Furthermore, immunoblotting experiments demonstrated that the antiserum raised against the 42-kD protein did not react with the purified 70-kD protein. We therefore concluded that the 70-kD protein was a contaminating peptide. The error of using the same SDS-PAGE gel in both papers was the result of an oversight when choosing among many pictures of the SDS-PAGE gels that we used to assay the ABA-binding proteins. The misused picture in the Plant Physiology paper (Figure 4A) was an overexposed photograph of the same gel that we presented in the earlier Acta Botanica Sinica paper, such that the 70-kD band could hardly be seen, and thus it was reused in error. A new version of the SDS-PAGE gel for the 42-kD ABA-binding protein has been provided below. It was derived from the protocols that were described in the Plant Physiology paper. The legend of Figure 4 needs no modification with this substitution of the figure; however, it is reprinted here for convenience.
Figure 4.
Coomassie blue-stained SDS-PAGE (A), silver-stained native IEF (B), and silver-stained IEF/SDS-PAGE (C) of the purified ABA-binding protein. In A: a, molecular mass standards; b, purified ABA-binding proteins (3 μg), of which the calculated molecular mass was 42 kD; c, proteins in the crude extract (15 μg). In B: a, the purified ABA-binding protein, of which the measured pI was 4.86; b, the protein standards. C, The purified ABA-binding protein (2 μg) was resolved by IEF in the first dimension followed by SDS-PAGE; a, molecular mass markers; b, the purified ABA-binding protein. The measured molecular mass and pI were, respectively, 42 kD and 4.86.