Abstract
Plant innate immunity to pathogenic microorganisms is activated in response to recognition of extracellular or intracellular pathogen molecules by transmembrane receptors or resistance proteins, respectively. The defense signaling pathways share components with those involved in plant responses to UV radiation, which can induce expression of plant genes important for pathogen resistance. Such intriguing links suggest that UV treatment might activate resistance to pathogens in normally susceptible host plants. Here, we demonstrate that pre-inoculative UV (254 nm) irradiation of Arabidopsis (Arabidopsis thaliana) susceptible to infection by the biotrophic oomycete Hyaloperonospora parasitica, the causative agent of downy mildew, induces dose- and time-dependent resistance to the pathogen detectable up to 7 d after UV exposure. Limiting repair of UV photoproducts by postirradiation incubation in the dark, or mutational inactivation of cyclobutane pyrimidine dimer photolyase, (6-4) photoproduct photolyase, or nucleotide excision repair increased the magnitude of UV-induced pathogen resistance. In the absence of treatment with 254-nm UV, plant nucleotide excision repair mutants also defective for cyclobutane pyrimidine dimer or (6-4) photoproduct photolyase displayed resistance to H. parasitica, partially attributable to short wavelength UV-B (280–320 nm) radiation emitted by incubator lights. These results indicate UV irradiation can initiate the development of resistance to H. parasitica in plants normally susceptible to the pathogen and point to a key role for UV-induced DNA damage. They also suggest UV treatment can circumvent the requirement for recognition of H. parasitica molecules by Arabidopsis proteins to activate an immune response.
During their lifetime, plants are exposed to abiotic stressors, including cold, drought, heat, salinity, and UV radiation, and biotic stressors, such as fungi, oomycetes, bacteria, viruses, nematodes, or phytophagous insects, which perturb growth, development, and reproduction. To counter the effects of these agents, plants have evolved a range of responses such as stress neutralization, damage repair, shedding of affected tissues, and renewal of tissue growth. In particular, through innate immunity, plants perceive and limit microbial pathogens to small regions of tissue or individual cells where they may be killed by induced defense components. Recognition of pathogen molecules by host transmembrane receptors or resistance (R) proteins initiates signal transduction pathways that activate the defensive systems (Flor, 1971; Dangl and Jones, 2001; Allen et al., 2004; Chisholm et al., 2006; Dodds et al., 2006; Jones and Dangl, 2006). Plants unable to mobilize such defenses, or in which pathogens can suppress immunity, succumb to infection.
Plants face multiple stressors concurrently, and convergence of mechanisms that regulate stress responses likely underlies one stress causing cross tolerance to others (Xiong et al., 2002; Holley et al., 2003; Stratmann, 2003). Interestingly, UV-B (280–320 nm) radiation is known to act through signaling pathways, the components of which closely resemble those for pathogen resistance (Frohnmeyer et al., 1999; A-H-Mackerness et al., 2001; Ulm et al., 2001; Nawrath et al., 2002; Brosché and Strid, 2003). Indeed, UV stimulates transcription of genes important for defense, including those encoding chalcone synthase, pathogenesis-related proteins such as chitinase and β-1,3-glucanase, Phe ammonia lyase, or stilbene synthase (El Ghaouth et al., 2003; Bonomelli et al., 2004; Borie et al., 2004; Sävenstrand et al., 2004; Maeda et al., 2005). UV-enhanced transcription of the β-1,3-glucanase gene was photoreactivable (Kucera et al., 2003), implicating cyclobutane pyrimidine dimers (CPDs) or (6-4) photoproducts (6-4PPs) in transcription induction (Jiang et al., 1997a; Landry et al., 1997; Nakajima et al., 1998). However, no correlation was found between UV photoproduct levels and increased expression of several other pathogen defense genes (Green and Fluhr, 1995; Kalbin et al., 2001). Although the nature of the inducing signal is not yet clear, overlap in signaling pathways for pathogen resistance and UV-B responses, as well as enhanced defense gene expression, suggests UV may promote immunity to plant disease.
Two very distinct responses to UV during pathogen attack can be envisaged depending on the treatment regimen (Paul, 2000). There might be a direct impact on the pathogen, if UV exposure is concurrent with or follows infection by the pathogen (Gunasekera et al., 1997), or an indirect effect through enhanced host resistance if irradiation precedes infection. There are reports consistent with UV influencing pathogenesis (Brederode et al., 1991; Yalpani et al., 1994; Stevens et al., 1998, 2004, 2005; Mercier et al., 2000, 2001; Paul, 2000; Brown et al., 2001; de Capdeville et al., 2002), but most must be interpreted cautiously, either because they likely involved UV directly attenuating the pathogen or investigated postharvest treatment of fruit rather than the intact plant. On the other hand, UV irradiation of tobacco (Nicotiana tabacum) leaves reduced the symptoms of infection by tobacco mosaic virus inoculated 24 h or 5 d postirradiation (Brederode et al., 1991; Yalpani et al., 1994). However, the UV treatment potentiated resistance in resistant cultivars, and UV-induced leaf pigmentation and surface layer changes may have contributed to this effect. Thus, some studies suggest UV treatment may indirectly increase plant pathogen resistance, but none has determined whether UV can activate pathogen resistance in a susceptible host.
Here, we demonstrate that pre-inoculation UV-C (254 nm) treatment of normally susceptible Arabidopsis (Arabidopsis thaliana) accessions induces prolonged, dose-dependent resistance to virulent isolates of the phytopathogenic oomycete Hyaloperonospora parasitica. Conditions known to interfere with repair of CPDs or 6-4PPs enhance UV-C-induced defense against H. parasitica. In the absence of UV-C, an Arabidopsis triple mutant defective in the production of UV-absorbing flavonoids, photoreactivation, and nucleotide excision repair (NER) is highly resistant to the pathogen. We present evidence linking resistance in such mutants partly to exposure of the plants to shorter, low-fluence UV-B (280–320 nm) wavelengths present in incubator light. Our results suggest that UV treatment can bypass the need for recognition of H. parasitica molecules by Arabidopsis proteins to trigger pathogen defense and point to the involvement of DNA damage in UV-induced activation of the immune response.
RESULTS
Arabidopsis Landsberg erecta and Columbia Are Susceptible to Different H. parasitica Isolates
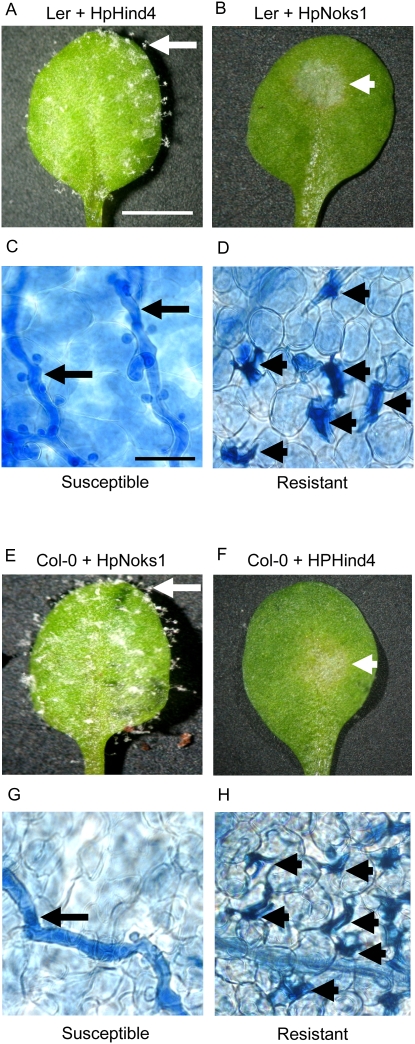
H. parasitica is an obligate biotroph that reproduces asexually by the production of conidiophores (Channon, 1981; Koch and Slusarenko, 1990; Donofrio and Delaney, 2001). Although a native pathogen of Arabidopsis, different H. parasitica isolates are virulent on different accessions (Koch and Slusarenko, 1990; Holub et al., 1994; Mohr and Cahill 2003; Allen et al., 2004). In particular, HpHind4 and HpNoks1 are virulent on wild-type Landsberg erecta (Ler) and Columbia (Col-0), respectively, whereas Ler is resistant to HpNoks1 and Col-0 to HpHind4. By 7 d postinoculation of 26-d-old plants, infection with HpHind4 or HpNoks1 resulted in the production of conidiophores and the spread of hyphae bearing haustoria within the infected tissue (Fig. 1, A, C, E, and G). In contrast, immunity to the pathogen was associated with a small area of tissue necrosis at the site of inoculation, internal lesions reflecting a hypersensitive cell death response (HR), and no hyphal spread (Fig. 1, B, D, F, and H).
Figure 1.
Interaction of Arabidopsis with H. parasitica. A to D, Representative samples of Ler leaves 7 d after inoculation with H. parasitica isolates HpHind4 (A and C) or HpNoks1 (B and D). Conidiophores or areas of necrotic cells on whole leaves (A and B) are indicated by white arrow or arrowhead, respectively. Hyphae bearing haustoria or HR lesions in lactophenol-trypan blue-stained leaves (C and D) are indicated by black arrows or arrowheads, respectively. Bar = 5 mm (A) or 50 μm (C).
UV Induces Resistance to H. parasitica
Biologically relevant doses of UV-B and sunlight induce CPDs in seedling leaves (Quaite et al., 1992b). Furthermore, Arabidopsis mutants defective in UV shielding and repair of CPDs and 6-4PPs die within 1 week of commencing daily sunlight exposure (Britt and Fiscus, 2003). These same effects can be induced by 254-nm radiation (UV-C; Jenkins et al., 1995; Jiang et al., 1997b; Gallego et al., 2000), but, unlike UV-B, this wavelength is close to the DNA absorption peak of 260 nm and is not absorbed well by proteins so UV-C damage is relatively specific for DNA. UV-C also more efficiently induces the same types of direct DNA damage as higher UV-B doses (Mitchell et al., 1991; Quaite et al., 1992a; Perdiz et al., 2000; Birrell et al., 2001). Thus, to test the effect of UV-induced CPDs and 6-4PPs on resistance to H. parasitica, we used a lamp emitting primarily 254-nm UV-C (hereafter designated UV) to conveniently produce these dipyrimidines.
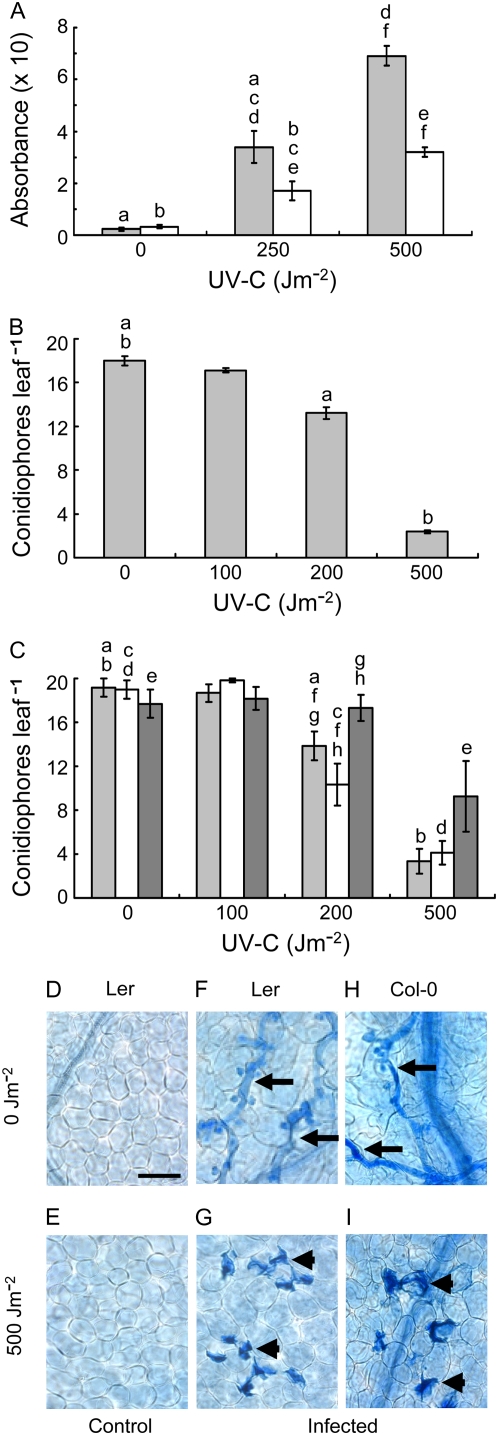
Twenty-six-day-old Ler and Col-0 plants were UV irradiated and incubated for 24 h prior to pathogen inoculation, thereby avoiding any direct effect of UV on H. parasitica. We used monoclonal antibodies to CPDs to confirm that UV treatment produced damage in plant DNA and repair took place during postirradiation incubation (Fig. 2A; P < 0.001 for all differences between doses for corresponding treatments and between treatments at the same dose, except 0 Jm−2). Because we lack a DNA standard bearing a defined number of CPDs, we could not relate absorbance to a specific number of CPDs. CPDs were examined because they are the most abundant UV photoproducts (Brash, 1988; Mitchell and Nairn, 1989), but it is reasonable to assume that 6-4PPs were also induced and repaired. Presumably, elimination of damage induced by the relatively low doses used here involved primarily photoreactivation during the 10-h photoperiod and NER during the remaining time in the dark (Pang and Hays, 1991; Chen et al., 1994; Quaite et al., 1994; Landry et al., 1997). Under these conditions, only the highest UV dose we used, 500 Jm−2, had any phenotypic effect on whole plants, a slight stunting of growth.
Figure 2.
UV-induced resistance to H. parasitica. A, Plant DNA was isolated 0 h (light gray bars) or 24 h (white bars) after UV irradiation or mock treatment, and CPDs were detected using an ELISA with anti-CPD monoclonal antibodies and spectrophotometric A492. Each column in A represents the mean ± se of three independent measurements. B and C, Ler (B) or Col-0 (C) leaves were inoculated with HpHind4 24 h after UV exposure or with HpNoks1 24 h (light gray bars), 72 h (white bars), or 168 h (dark gray bars) after UV exposure, respectively, and the number of conidiophores per leaf was determined 7 d after inoculation. Each column in B and C, and in all succeeding figures, represents the mean ± se of at least four independent experiments in each of which conidiophores on 48 leaves (four leaves per plant) were counted. D to I, The six bottom images show representative samples of Ler (D–G) or Col-0 (H and I) leaves that were mock inoculated (D and E) or inoculated with HpHind4 (F and G) or HpNoks1 (H and I) 24 h after mock UV treatment (D, F, and H) or UV irradiation (E, G, and I), incubated for 7 d, and then stained with lactophenol-trypan blue. Hyphae-bearing haustoria or HR lesions are indicated by black arrows or arrowheads, respectively. In this, and all succeeding figures, the same lowercase letters above different columns indicate pairs of values that differ significantly (P less than at least 0.05). Bar in D = 50 μm. [See online article for color version of this figure.]
Following pathogen inoculation, plants were incubated for 7 d and examined. Ler and Col-0 exhibited UV dose-dependent resistance to H. parasitica, as shown by reduced conidiophore formation (P < 0.01 or 0.001 for 0 Jm−2 compared to 200 Jm−2 or 500 Jm−2, respectively, for Ler and P < 0.05 for both doses for Col-0) and the presence of HR lesions within the inoculated tissue (Fig. 2, B, C, G, and I). We also observed a dose-dependent decrease in conidiophore production in irradiated Arabidopsis Wassilewskija inoculated with HpNoks-1 (data not shown). The absence of HR lesions in irradiated leaves that were not inoculated (Fig. 2E) demonstrated the lesions were not induced by UV alone. Instead, their appearance in irradiated and then inoculated leaves showed they only developed in irradiated tissue in response to the pathogen. Furthermore, because the occurrence of HR lesions requires tissue invasion by hyphae (Koch and Slusarenko, 1990), UV exposure did not cause resistance simply by preventing the pathogen penetrating the leaf surface. Thus, UV-induced resistance to H. parasitica appeared to mirror natural resistance wherein the initial stages of infection (spore germination, hyphal penetration between epidermal cells) occur in the same fashion as observed for susceptible plants (Koch and Slusarenko, 1990). In early experiments, we detected UV-induced resistance if plants were irradiated and then immediately inoculated with H. parasitica. On this basis, we infer resistance develops within several hours of UV treatment, given that in susceptible hosts the oomycete has passed the initial infection stages by 12 to 18 h after inoculation (Koch and Slusarenko, 1990; Donofrio and Delaney, 2001). We did not continue with this treatment regimen, which proved logistically difficult because of the numbers of plants required.
Resistance to H. parasitica was evident when plants were inoculated 72 h or 168 h postirradiation (Figure 2C; P < 0.05 for the same inoculation times at 0 Jm−2 compared to 200 Jm−2 [except 168 h postirradiation inoculation] or 500 Jm−2). But when plants were inoculated 7 d postirradiation with 200 Jm−2, resistance was not apparent compared to the resistance observed for inoculation 24 h or 72 h after UV exposure (P < 0.05 in each case). Although the resistance of plants treated with 500 Jm−2 and inoculated 7 d later appeared to be slightly reduced compared to inoculation 24 h or 72 h post-UV exposure, the difference was not significant (P > 0.05). Collectively, our observations indicate that the magnitude of UV-induced resistance to H. parasitica is dose dependent and perhaps may be time dependent (at least for treatment with 200 Jm−2).
Impairing UV Photoproduct Repair Increases UV-Induced Resistance to H. parasitica
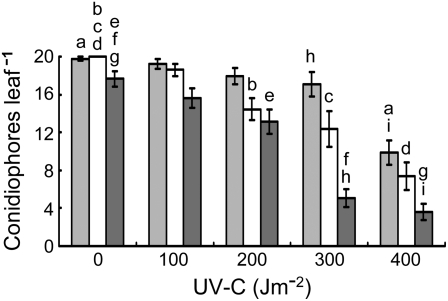
The dose dependency of UV-induced resistance to H. parasitica suggests DNA damage, in particular CPDs and 6-4PPs, was involved. Arabidopsis is able to photoreactivate both types of damage, and withholding photoreactivating wavelengths from irradiated wild-type plants hinders the light-mediated reversal of CPDs and 6-4PPs (Pang and Hays, 1991; Britt et al., 1993; Chen et al., 1994; Jiang et al., 1997a; Landry et al., 1997; Tanaka et al., 2002; Waterworth et al., 2002). Thus, we first asked whether these photoproducts might have a role in UV-induced resistance to the pathogen by incubating irradiated Col-0 plants for 72 h under light, 24 h in the dark followed by 2 d incubation under light, or 72 h in the dark, prior to inoculation with H. parasitica. This approach permitted irradiated plants to photoreactivate UV damage throughout the entire 3-d postirradiation pre-inoculation period, only during the final 2 d or not at all. Figure 3 shows that incubation in the light or dark alone had no effect on pathogen resistance. For UV doses >200 Jm−2, dark incubation for 72 h following UV treatment significantly increased the magnitude of resistance compared to incubation under light for 72 h (P < 0.01). The results must be interpreted cautiously, because the absence of light conceivably could have intensified the UV effects via a mechanism not involving photoreactivation.
Figure 3.
Effect of photoreactivation on UV-induced resistance to H. parasitica. Mock-treated or UV-irradiated Col-0 leaves were inoculated with HpNoks1 after 72 h incubation under light (light gray bars), 24 h incubation in the dark followed by 48 h incubation under light (white bars), or 72 h incubation in the dark (dark gray bars), and the number of conidiophores per leaf was determined after 7 d incubation under light.
To more directly test the influence of UV-induced DNA damage on pathogen resistance, we inoculated UV-sensitive Arabidopsis mutants with H. parasitica 24 h postirradiation. These mutants exhibit different degrees of UV sensitivity due to defects in CPD photolyase (uvr2), 6-4PP photolyase (uvr3), homologs of the NER 5′ (ercc1-1, uvh1-2) or 3′ (uvr1-1) endonuclease proteins, or chalcone isomerase (tt5), which is required for synthesis of UV-absorbing flavonoids (Britt et al., 1993; Li et al., 1993; Jiang et al., 1997a; Fidantsef et al., 2000; Liu et al., 2000, 2001; Hefner et al., 2003). The uvr2 and uvr3 mutants fail to photoreactivate CPDs or 6-4PPs, respectively, and uvh1-2 and uvr1-1 prevent NER of UV photoproducts, but ERCC1-1 has not been shown to be involved in DNA damage removal (Britt et al., 1993; Li et al., 1993; Jiang et al., 1997a; Fidantsef et al., 2000; Hefner et al., 2003). However, the interaction of human ERCC1 and XPF (UVH1 homolog) to form the NER 5′ endonuclease (Friedberg et al., 2006), plus the requirement of UVH1 (Arabidopsis XPF homolog) for NER of UV photoproducts in planta (Fidantsef et al., 2000; Liu et al., 2000), argues that the UV sensitivity of the ercc1-1 mutant is probably due to NER inactivation.
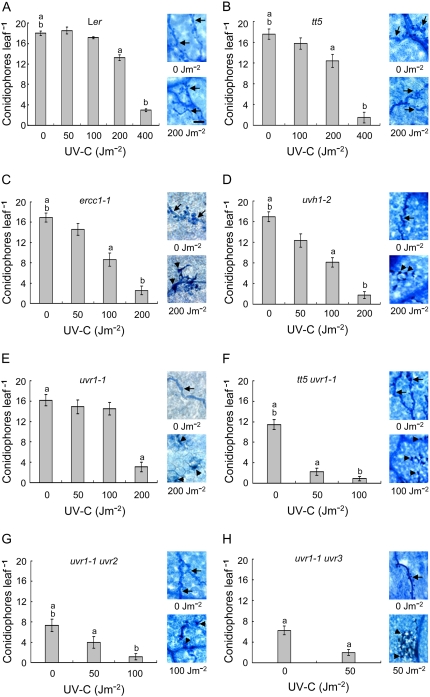
Although UV induced resistance to H. parasitica in the tt5 mutant (P < 0.01 or 0.001 for 0 Jm−2 compared to 200 Jm−2 or 400 Jm−2, respectively), the tt5 mutation did not increase UV-induced pathogen resistance over that observed for the Ler wild type (Fig. 5, A and B; P > 0.05 at each common dose). This may reflect the relatively low UV doses used and the modest UV sensitivity of the tt5 mutant in our hands, which is consistent with the poor absorption of 254-nm radiation by flavonoids (Lois, 1994; Stapleton and Walbot, 1994). Because of growth inhibition by higher UV doses, the ercc1-1, uvh1-2, and uvr1-1 mutants were treated with doses ≤ 200 Jm−2. Even so, pathogen resistance was more pronounced for these mutants after UV exposure relative to the levels of resistance induced in Ler by the same UV doses (Fig. 4, C–E; P < at least 0.05 for each comparison except for 100 Jm−2 with the uvr1-1 mutant, P > 0.05). Conidiophore production was decreased, hyphal spread was diminished, and HR lesions were apparent in inoculated tissues at doses where little or no evidence of resistance was detectable in the wild type. Interestingly, with the exception of 100 Jm−2, the levels of resistance were similar in the three mutants, all of which likely are defective in the incision step of NER. Combining the uvr1-1 NER mutation with the tt5 shielding defect or the CPD or 6-4PP photolyase mutation further sensitized the plants to UV and enhanced UV-induced pathogen resistance over that seen in the single NER mutants at the same doses (Fig. 4, F–H; P < 0.0001 in all cases). We did not assess the effects of the uvr2 and uvr3 mutations alone. uvr2 and uvr3 were isolated as secondary mutations in the uvr1-1 background (Jiang et al., 1997a) but were not separable from uvr1-1 (A.B. Britt, personal communication). By searching the SALK Institute Genome Analysis Laboratory (http://signal.salk.edu/cgi-bin/tdnaexpress) and Arabidopsis Information Resource (http://www.arabidopsis.org/) databases, we found UVR2- and UVR3-disrupted T-DNA insertion lines for Col-0 and Wassilewskija but not Ler. The resistance of Col-0 and Wassilewskija to HpHind4 precluded the use of the T-DNA insertion mutants for strictly comparative studies with the Ler mutants. We note, however, that photoreactivation of CPDs and 6-4PPs is much more efficient than their removal by NER, which allows the influence of photoreactivation in the uvr1-1 background to be easily discerned (Britt et al., 1993; Jiang et al., 1997a; Tanaka et al., 2002). Clearly, as the capacity of the plant to repair CPDs and 6-4PPs decreased, progressively lower UV doses were required to counter pathogen attack, thereby linking UV photoproducts to the induction of pathogen resistance. The data also suggest that repair of CPDs or 6-4PPs does not constitute or generate an inducing signal, because preventing their repair increased UV-induced pathogen resistance rather than diminishing it.
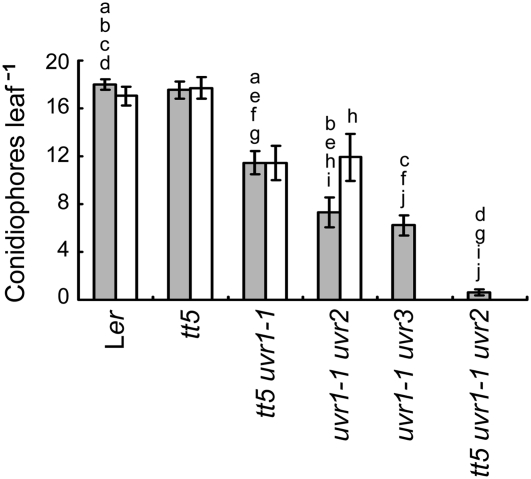
Figure 5.
Effects of UV shielding and DNA repair defects on resistance of unirradiated plants to H. parasitica. Leaves of wild-type (Ler), shielding defective (tt5), repair defective (uvh1-2, ercc1, uvr1-1, uvr1-1 uvr2, uvr1-1 uvr3), or shielding and repair defective (tt5 uvr1-1, tt5 uvr1-1 uvr2) plants were inoculated with HpHind4 24 h after mock UV exposure, and the number of conidiophores per leaf (light gray bars) was determined 7 d after inoculation. White bars show the number of conidiophores per leaf for plants that were grown from seed through the end of the experiment under Mylar. Only the wild-type, tt5, tt5 uvr1-1, and uvr1-1 uvr2 plants were tested this way. Data taken from Figure 4 are shown here for ease of comparison. Each column represents the mean ± se of four independent experiments.
Figure 4.
Effects of UV shielding and DNA repair defects on UV-induced resistance to H. parasitica. Leaves of Ler wild-type (A), shielding defective (B), NER defective (C–E), shielding and NER defective (F), NER and CDP photolyase defective (G), or NER and 6-4PP photolyase defective (H) plants were inoculated with HpHind4 24 h after mock treatment or UV exposure, and the number of conidiophores per leaf was determined 7 d after inoculation. On the right of each section, representative samples of leaves that were inoculated with HpHind4 24 h after mock UV treatment or UV irradiation, incubated for 7 d, and then stained with lactophenol-trypan blue are shown. Hyphae-bearing haustoria or HR lesions are indicated by black arrows or arrowheads, respectively. Bar in A (bottom right) = 50 μm. [See online article for color version of this figure.]
DNA Repair Defects Confer Resistance to H. parasitica in Unirradiated Plants
In the absence of UV treatment, the tt5, ercc1-1, uvh1-2, and uvr1-1 mutations alone had no effect on resistance to H. parasitica (Fig. 4). Surprisingly, compared to the Ler wild type, the tt5 uvr1-1 double mutant exhibited moderate resistance to the pathogen (P < 0.001), the uvr1-1 uvr2 and uvr1-1 uvr3 double mutants were even more resistant than the tt5 uvr1-1 double mutant (P < 0.05 or 0.01, respectively), and a triple tt5 uvr1-1 uvr2 mutant was almost completely resistant (Fig. 5; P < 0.001 for the triple mutant compared to all other mutants). Previously, it was determined that CPDs could be detected in alfalfa (Medicago sativa) seedlings raised in growth chambers and were eliminated by placing a filter that absorbed wavelengths below 400 nm between the chamber lamps and the plants (Quaite et al., 1992b). These findings, plus the direct relationship we observed between the degree of pathogen resistance and the UV sensitivity of the mutants, led us to inquire whether DNA damage induced by our incubator lamps might be involved. These lamps emit very low levels of UV-B and UV-A (320–400 nm) radiation (see “Materials and Methods”), and although the plants were grown in covered plastic containers that reduce UV transmission, the containers do not completely block all UV wavelengths. Given that CPD and 6-4PP induction declines markedly between 260 and 310 nm (Rosenstein and Mitchell, 1987; Matsunaga et al., 1991; Quaite et al., 1992a), pathogen resistance might have been stimulated in the double and triple repair-deficient mutants by accumulation of DNA damage resulting from exposure to shorter UV-B wavelengths during the 26-d pretreatment growth period. To assess this possibility, plants were incubated continuously from seed under Mylar, which absorbs ≥92% of UV radiation below 310 nm but transmits 8% to 63% of longer UV-B wavelengths and ≥63% of UV-A radiation. Due to limited seed stocks, not all the multiple mutants could be tested this way. The response of the wild type, tt5 and tt5 uvr1-1 mutants to H. parasitica was not altered by growth under Mylar (Fig. 5), whereas the susceptibility of the uvr1-1 uvr2 mutant increased to the level of the tt5 uvr1-1 mutant (P < 0.05). These observations indicate that short wavelength UV-B radiation emitted by the incubator lamps contributed to, but was not entirely responsible for, the pathogen resistance of the uvr1-1 uvr2 mutant. They also suggest that the difference in the responses of the tt5 uvr1-1 and uvr1-1 uvr2 mutants to incubation under Mylar was due to the CPD photolyase defect in the latter mutant, again implicating CPDs in the activation of resistance.
DISCUSSION
In this study, we demonstrate that UV-C treatment of Arabidopsis induced persistent, dose-dependent resistance to the oomycete pathogen H. parasitica. This was not a direct effect of UV on the pathogen itself, because resistance occurred when plants were inoculated 24 h or more postirradiation. UV treatment also did not prevent penetration by the pathogen, as indicated by the formation of HR lesions when irradiated plants were subsequently inoculated with H. parasitica. We did not examine the kinetics of resistance induction. However, resistant plants normally mount an immune response after hyphae have formed and penetrated the epidermis, which takes 12 to 18 h (Koch and Slusarenko, 1990; Donofrio and Delaney, 2001). So the occurrence of resistance in plants inoculated with the pathogen immediately after irradiation suggested that resistance developed within approximately 12 h of UV treatment. This may be an overestimate given that recent profiling of plant transcriptomes indicates UV can increase expression of plant defense genes in under 1 h (Casati and Walbot, 2004; Ulm et al., 2004; Molinier et al., 2005; Swindell, 2006). Withholding photoreactivating light postirradiation but prior to pathogen inoculation, or mutational inactivation of photoreactivation and/or NER, further enhanced UV-induced resistance. Finally, in the absence of UV treatment, mutants with defects in NER and UV shielding or photoreactivation exhibited increased resistance to the pathogen, which, in the photoreactivation-deficient NER mutant, was partly attributable to UV-B wavelengths emitted by incubator lights. We conclude that UV induces resistance to H. parasitica in normally susceptible plants, and CPDs and 6-4PPs play a key role in this response. Reactive oxygen species (ROS) have been implicated in the establishment of pathogen resistance (Torres et al., 2006), and UV-C can produce oxidative DNA damage in naked DNA in solution, probably via photosensitized reactions that generate ROS (Doetsch et al., 1995; Wei et al., 1998). However, UV-C doses up to two orders of magnitude higher than used in our study are required, and the oxidative damage yields are much lower (≤4%) than for CPDs. It seems unlikely that the UV-C doses we used could have induced enough oxidative damage via ROS to make a significant contribution to resistance relative to that made by CPDs. Nonetheless, we cannot exclude the possibility that UV-induced oxidative DNA damage may have contributed in a minor way to UV-C-induced pathogen resistance.
Innate plant immunity consists of a least two components. Pathogen-associated molecular pattern-triggered immunity is activated by transmembrane receptors recognizing extracellular pathogen molecules, whereas effector-triggered immunity (EFI) involves recognition of intracellular pathogen effectors by plant R proteins (Chisholm et al., 2006; Dodds et al., 2006; Jones and Dangl, 2006). Resistance of Arabidopsis to H. parasitica is mediated through pathogen effector-R protein recognition (Allen et al., 2004; Sohn et al., 2007), and so is of the EFI type, which usually induces HR lesions (Koch and Slusarenko, 1990). Consistent with this scenario, resistance of Ler to HpNoks1 and Col-0 to HpHind4 was accompanied by formation of HR lesions. This observation, plus the susceptibility of Ler and Col-0 to HpHind4 and HpNoks1, respectively, is consistent with Ler having an R protein that recognizes the HpNoks1 but not the HpHind4 effector, and vice versa for Col-0. Given that plants were inoculated with H. parasitica 24 h or more after UV treatment, it seems unlikely that UV irradiation offset the ability of the pathogen to suppress a resistance response. Consequently, UV-induced resistance to HpHind4 in Ler and to HpNoks1 in Col-0 suggests that UV can initiate EFI in the absence of H. parasitica effector recognition by Arabidopsis R proteins.
Unlike the wild-type plants, Arabidopsis NER mutants defective in flavonoid biosynthesis and/or photoreactivation displayed resistance to H. parasitica without deliberate prior UV treatment. Pretreatment growth of plants under Mylar to filter out wavelengths ≤310 nm reduced the resistance of the uvr1-1 uvr2 mutant, which cannot excise CPDs or 6-4PPs or photoreactivate CPDs (Jiang et al., 1997a). This suggests that failure to repair UV photoproducts induced by low-fluence, short-wavelength UV-B radiation from the incubator lamps led to an accumulation of DNA damage over the 26 d of pretreatment growth that activated resistance in the mutants deficient in both photoreactivation and NER. In wild-type plants, UV-B-induced expression of genes characteristic of stress responses is associated with high fluence rates, whereas low fluence rates are linked to metabolic and developmental changes (Frohnmeyer and Staiger, 2003; Stratmann, 2003; Suesslin and Frohnmeyer, 2003; Ulm and Nagy, 2005; Jenkins and Brown, 2007). Although fluence rate plays a substantial role in determining UV-B responses, our results suggest the capacity of the plant to repair DNA damage may be an important factor in determining the type(s) of response elicited by different fluence rates.
Incubation under Mylar primarily screens out radiation below 310 nm but did not reduce the pathogen resistance of the tt5 uvr1-1 mutant, which can photoreactivate CPDs and 6-4PPs (Jiang et al., 1997a). This observation suggests another form(s) of DNA damage might also promote resistance to H. parasitica. Longer wavelength UV-B and UV-A that penetrates Mylar can damage DNA indirectly via oxidative stress, but much higher fluences than those emitted by the growth chamber lamps are required (Peak et al., 1987; Foyer et al., 1994), making it less likely that lamp-induced oxidative damage was responsible. However, oxidative damage can also occur as a consequence of endogenous metabolism. Because the tt5 mutation confers a deficiency in flavonoid production and flavonoids are free radical scavengers (Landry et al., 1995; Rice-Evans et al., 1997; De Beer et al., 2002), tt5 mutants are more sensitive to ROS than wild-type plants (Filkowski et al., 2004). Furthermore, some oxidative DNA lesions are substrates for NER (Satoh et al., 1993; Demple and Harrison, 1994; Reardon et al., 1997; Scott et al., 1999), the human UVR1 homolog XPG participates in repair of oxidative damage (Dianov et al., 2000; Le Page et al., 2000), and the Arabidopsis uvr1-1 mutant is sensitive to hydrogen peroxide (Liu et al., 2001). In addition, photolyases can potentiate NER in the dark (Sancar, 1990; Sancar et al., 2000), though whether they influence NER of oxidative damage is unknown. These findings, plus our results, suggest accumulation of endogenous oxidative DNA damage over the pre-inoculation growth period might also have contributed to pathogen resistance in the double and triple mutants. If so, the tt5 and single NER mutants may have exhibited no increase in resistance relative to the wild-type plants because of their better capacity (than the multiple mutants) to repair incubator lamp-induced UV photoproducts and endogenous oxidative DNA damage or neutralize naturally occurring ROS, respectively.
The uvr1-1 mutation confers a premature senescence phenotype (Liu et al., 2001). An interaction between uvr1-1 and the other mutations present in the multiple mutants may have accounted for our difficulty in recovering seeds from some of these plants. Conceivably, such a pleiotropic interaction might also have played a role in the pathogen resistance of one or more of the multiple mutants not deliberately treated with UV. This role would have to be a modest one at best. It would be difficult otherwise to reconcile pathogen resistance increasing with the UV sensitivity of the mutants and being decreased in the uvr1-1 uvr2 mutant by Mylar screening.
Systemic acquired resistance (SAR) can develop upon attack by necrotizing pathogens and provide a long-lasting, enhanced resistance response to subsequent pathogen incursion in cells of the originally infected as well as uninoculated tissues (Durrant and Dong, 2004; Conrath, 2006). Thus, through SAR, plants can rapidly and effectively mount defenses against pathogens. The UV-induced resistance we observed was accompanied by a HR in the presence of H. parasitica, and SAR can arise as part of a pathogen-induced HR. Furthermore, genes expressed during the establishment of SAR include a number whose expression is known to be UV inducible (Brederode et al., 1991; Ward et al., 1991; Van Loon and Van Strien, 1999; Durrant and Dong, 2004). Whether the signaling involved in plant responses to UV is localized or systemic is controversial. Failure to detect up-regulation of defense genes in covered leaves of UV-B-irradiated pea (Pisum sativum) and tobacco plants has been reported by some investigators (Green and Fluhr, 1995; Kalbin et al., 2001). Others have shown that UV-induced changes in gene expression can occur in unexposed tissues of maize (Zea mays) and tobacco (Yalpani et al., 1994; Casati and Walbot, 2004). In this study, we did not test for systemic resistance by inoculating covered leaves of irradiated plants or inoculating leaves that emerged in the 7-d postirradiation period. Reminiscent of SAR, however, we found that plants exhibited resistance when challenged with H. parasitica 7 d after irradiation with 500 Jm−2 UV. This likely represents persistence of the initial UV-induced defense response rather than bona fide pathogen-induced SAR. Pathogen resistance was still observed 7 d after irradiation only for the highest UV dose used, 500 Jm−2. This plus the diminished magnitude of resistance for 200 Jm−2 contrasts with the long-lasting, more rapid/more effective activation of defense associated with SAR. Thus, our results suggest that UV-induced DNA damage can initiate the defense mechanisms that deal directly with primary infection by H. parasitica but probably does not activate SAR.
MATERIALS AND METHODS
Growth Conditions
Seeds of Arabidopsis (Arabidopsis thaliana) Ler and Col-0, and the UV-sensitive Ler mutants ercc1-1, tt5, uvh1-2, uvr1, tt5 uvr1, uvr1 uvr2, uvr1 uvr3, and tt5 uvr1 uvr2 (Jiang et al., 1997a; Fidantsef et al., 2000; Hefner et al., 2003) were surface-sterilized, placed on Murashige and Skoog medium (Sigma) in covered 9-cm-diameter sterile plastic petri dishes, and vernalized (Foster and Chua, 1999). The dishes were kept at 21°C under a 10-h photoperiod using a bank of fluorescent lamps (F36W/33, General Electric) emitting a total average light intensity of 100 μmol m−2 s−1 plus approximately 5.4 × 10−4 Jm−2 s−1 UV-B and 9.4 × 10−3 Jm−2 s−1 UV-A (according to the manufacturer's test specifications). These fluences were below the limits of detection of our UV dosimeter (UVX Digital Radiometer, UVX-31 or UVX-36 sensors, UVP), which registers the UV in sunlight, indicating that the UV output of the growth chamber lamps is considerably lower than incident solar UV. The dishes filtered out wavelengths below 285 nm and decreased transmission of 300-nm, 310-nm, 320-nm, and UV-A radiation by 60%, 50%, 40%, and 40% to 10%, respectively (determined spectroscopically). After 19 d, seedlings were transferred to soil (Terracotta and Tub Mixture, Debco Pty) in pots and grown under the same conditions. To initially maintain plants under high humidity, the pots were kept within sealed transparent plastic containers for 2 d following transplantation. The plastic of the containers filtered out wavelengths below 275 nm and decreased transmission of 280-nm, 290-nm, 300-nm, 310-nm, 320-nm, and UV-A radiation by 92%, 80%, 75%, 72%, 70%, and 70% to 55%, respectively (determined spectroscopically). To reduce exposure to UV wavelengths emitted by the fluorescent lamps, plants were grown as described above under Mylar (DuPont), which eliminates wavelengths below 306 nm and reduces transmission of 310-nm, 315-nm, 320-nm, and UV-A radiation by 92%, 47%, 37%, and 37% to 23%, respectively (determined spectroscopically).
Phenotypic Confirmation of Mutant Seed Stock
The Ler mutants exhibit different degrees of UV sensitivity depending on the mutation(s) and postirradiation lighting conditions (Jiang et al., 1997a; Fidantsef et al., 2000; Hefner et al., 2003). The tt5 mutation also affects seed color (Koornneef, 1990; Shirley et al., 1995). Mutant phenotypes were verified by inspecting seed color and testing the ability of seedlings to withstand increasing UV doses in the presence or absence of photoreactivating light.
UV Treatment
Plants were irradiated 1 week after being transferred to soil (i.e. at 26 d of growth). The UV source was an 80-cm, germicidal tube emitting 94% of its radiant energy as UV-C at 254 nm (Australian Ultra Violet) set to an incident dose rate of 2 Jm−2 s−1 using a UV dosimeter (UVX Digital Radiometer, UVX-25 sensor). Following irradiation, plants were incubated under the same conditions used for routine growth unless stated otherwise.
Hyaloperonospora parasitica Maintenance, Inoculation Procedure, and Microscopy
H. parasitica isolates were obtained from E. Holub (Horticultural Research International-East Malling) as oospores in dried leaf material and were maintained by rubbing leaves bearing conidiophores against leaves of 3- to 4-week-old uninfected plants (Koch and Slusarenko, 1990). For inoculation, spore suspensions (105 spores mL−1) in distilled water were prepared from leaves infested with conidiophores (Dangl et al., 1992), and four leaves per plant were inoculated with 2 μL of spore suspension (Holub et al., 1994). Plants were then incubated for 7 d in sealed transparent plastic containers at 16°C to maintain the cool, damp conditions that favor spore formation (Channon, 1981), with the same lighting conditions as used for growth. Pathogen ingression was determined by condiophore counts (up to a maximum of 20 per leaf on 48 leaves across 12 plants per experiment; Warren et al., 1998) made with a dissecting microscope. For tissue examination, six representative leaves per UV dose were decolorized with three changes of 90% ethanol or methanol. Hyphal spread and HR lesions were visualized by microscopy after staining with lactophenol-trypan blue, which stains fungal tissue living at the time of fixation and necrotic plant cells (Koch and Slusarenko, 1990).
ELISA Assay
Groups of 16 plants were grown and mock treated or UV irradiated as described above. Four leaves per plant were excised at 0 and 24 h postirradiation, frozen in liquid nitrogen, and stored at −80°C. DNA was extracted from 100 mg pooled leaf tissue using a genomic DNA isolation kit (Aquapure, Bio-Rad Laboratories), the yield was determined by spectrophotometric A260, and the DNA resuspended in phosphate-buffered saline at 0.2 μg mL−1. CPDs were detected by an ELISA procedure using 96-well microtitre plates with four well replicates per sample, monoclonal anti-CPD antibodies (Mori et al., 1991; Kobayashi et al., 2001; TMD-2, MBL International), peroxidase-streptavidin, and spectrophotometric A492 according to the manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed using a software package (GraphPad Prism version 5.01, GraphPad Software) to run the following tests: Student's two-tailed t test (Fig. 2A); one-way ANOVA with Dunnett's multiple comparison post test (Figs. 2B and 4); two-way ANOVA with Bonferroni's multiple comparison to compare all means at 0 Jm−2 with all corresponding means at other UV doses (Figs. 2C, 3, and 5); or one-way ANOVA with Bonferroni's multiple comparison to compare means at a single UV dose. In all cases, values of P < 0.05 were considered significant.
Acknowledgments
We thank A.B. Britt for generously providing seeds of the UV-sensitive mutants and E. Holub for providing the H. parasitica isolates.
This work was supported by the Australian Research Council (D.M.C., B.A.K., P.M.S.) and by Deakin University (D.M.C., B.A.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David M. Cahill (david.cahill@deakin.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- A-H-Mackerness S, John CF, Jordan B, Thomas B (2001) Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett 489 237–242 [DOI] [PubMed] [Google Scholar]
- Allen RL, Bittner-Eddy PD, Grenville-Briggs LJ, Meitz JC, Rehmany AP, Rose LE, Beynon JL (2004) Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306 1957–1960 [DOI] [PubMed] [Google Scholar]
- Birrell GW, Giaever G, Chu AM, Davis RW, Brown JM (2001) A genome-wide screen in Saccharomyces cerevisiae for genes affecting UV radiation sensitivity. Proc Natl Acad Sci USA 98 12608–12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomelli A, Mercier L, Franchel J, Baillieul F, Benizri E, Mauro MC (2004) Response of grapevine defences to UV-C exposure. Am J Enol Vitic 55 51–59 [Google Scholar]
- Borie B, Jeandet P, Parize PA, Bessis R, Adrian M (2004) Resveratrol and stilbene synthase mRNA production in grapevine leaves treated with biotic and abiotic phytoalexin elicitors. Am J Enol Vitic 55 60–64 [Google Scholar]
- Brash DE (1988) UV mutagenic photoproducts in Escherichia coli and human cells: a molecular genetics perspective on human skin cancer. Photochem Photobiol 48 59–66 [DOI] [PubMed] [Google Scholar]
- Brederode FT, Linthorst HJ, Bol JF (1991) Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol 17 1117–1125 [DOI] [PubMed] [Google Scholar]
- Britt AB, Chen JJ, Wykoff D, Mitchell D (1993) A UV-sensitive mutant of Arabidopsis defective in the repair of pyrimidine-pyrimidinone (6-4) dimers. Science 261 1571–1574 [DOI] [PubMed] [Google Scholar]
- Britt AB, Fiscus E (2003) Growth responses of Arabidopsis DNA repair mutants to solar irradiation. Physiol Plant 118 183–192 [Google Scholar]
- Brosché M, Strid Å (2003) Molecular events following perception of ultraviolet-B radiation by plants. Physiol Plant 117 1–10 [Google Scholar]
- Brown JE, Lu TY, Stevens C, Khan VA, Lu JY, Wilson CL, Collins DJ, Wilson MA, Igwegbe ECK, Chalutz E, et al (2001) The effect of low dose ultraviolet light-C seed treatment on induced resistance in cabbage to black rot (Xanthomonas campestris pv. Campestris). Crop Prot 20 873–883 [Google Scholar]
- Casati P, Walbot V (2004) Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues. Genome Biol 5 R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon AG (1981) Downy mildew of Brassicas. In DM Spencer, ed, The Downy Mildews. Academic Press, London, pp 321–329
- Chen JJ, Mitchell DL, Britt AB (1994) A light-dependent pathway for the elimination of UV-induced pyrimidine (6-4) pyrimidinone photoproducts in Arabidopsis. Plant Cell 6 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124 803–814 [DOI] [PubMed] [Google Scholar]
- Conrath U (2006) Systemic acquired resistance. Plant Sign Behav 1 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Holub EB, Debener T, Lehnackers H, Ritter C, Crute IR (1992) Genetic definition of loci involved in Arabidopsis pathogen interactions. In C Koncz, N Chua, J Schell, eds, Methods in Arabidopsis Research. World Scientific Publishing, Singapore, pp 393–418
- Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411 826–833 [DOI] [PubMed] [Google Scholar]
- De Beer D, Joubert E, Gelderblom WCA, Manley M (2002) Phenolic compounds: a review of their possible role as in vivo antioxidants of wine. South African J Enol Viticul 23 48–61 [Google Scholar]
- de Capdeville G, Wilson CL, Beer SV, Aist JA (2002) Alternative disease control agents induce resistance to blue mold in harvested ‘red delicious’ apple fruit. Phytopathology 92 900–908 [DOI] [PubMed] [Google Scholar]
- Demple B, Harrison L (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem 63 915–948 [DOI] [PubMed] [Google Scholar]
- Dianov GL, Thybo T, Dianova II, Lipinski LJ, Bohr VA (2000) Single nucleotide patch excision repair is the major pathway for removal of thymine glycol from DNA in human cell extracts. J Biol Chem 275 11809–11813 [DOI] [PubMed] [Google Scholar]
- Dodds PN, Lawrence GJ, Catanzariti AM, The T, Wang CIA, Ayliffe MA, Kobe B, Ellis JG (2006) Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax avirulence genes. Proc Natl Acad Sci USA 103 8888–8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch PW, Zasatawny TH, Martin AM, Dizdaroglu M (1995) Monomeric base damage products from adenine, guanine and thymine induced by exposure of DNA to ultraviolet radiation. Biochemistry 34 737–742 [DOI] [PubMed] [Google Scholar]
- Donofrio NM, Delaney TP (2001) Abnormal callose response phenotype and hypersusceptibility to Peronospora parasitica in defense-compromised Arabidopsis nim1-1 and salicylate hydroxylase-expressing plants. Mol Plant Microbe Interact 14 439–450 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- El Ghaouth A, Wilson CL, Callahan AM (2003) Induction of chitinase, β-1,3-glucanase, and phenylalanine ammonia lyase in peach fruit by UV-C treatment. Phytopathology 93 349–355 [DOI] [PubMed] [Google Scholar]
- Fidantsef AL, Mitchell DL, Britt AB (2000) The Arabidopsis UVH1 gene is a homolog of the yeast repair endonuclease RAD1. Plant Physiol 124 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski J, Kovalchuk O, Kovalchuk I (2004) Genome stability of vtc1, tt4, and tt5 Arabidopsis thaliana mutants impaired in protection against oxidative stress. Plant J 38 60–69 [DOI] [PubMed] [Google Scholar]
- Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9 275–296 [Google Scholar]
- Foster R, Chua NH (1999) An Arabidopsis mutant with deregulated ABA gene expression: implications for negative regulator function. Plant J 17 363–372 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Descouvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ 17 507–523 [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2006) DNA Repair and Mutagenesis. American Society for Microbiology, Washington, DC
- Frohnmeyer H, Loyall L, Blatt MR, Grabov A (1999) Millisecond UV-B irradiation-evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J 20 209–227 [DOI] [PubMed] [Google Scholar]
- Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol 133 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego F, Fleck O, Li A, Wyrzykowska J, Tinland B (2000) AtRAD1, a plant homologue of human and yeast nucleotide excision repair endonucleases, is involved in dark repair of UV damages and recombination. Plant J 21 507–518 [DOI] [PubMed] [Google Scholar]
- Green R, Fluhr R (1995) UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 7 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera TS, Paul ND, Ayres PG (1997) The effects of ultraviolet-B (UV-B: 290-320nm) radiation on blister blight disease of tea (Camellia sinensis). Plant Pathol 46 179–185 [Google Scholar]
- Hefner E, Preuss SB, Britt AB (2003) Arabidopsis mutants sensitive to gamma irradiation include the homologue of the human repair gene ERCC1. J Exp Bot 54 669–680 [DOI] [PubMed] [Google Scholar]
- Holley SR, Yalamanchill RD, Moura DS, Ryan CA, Stratmann JW (2003) Convergence of signalling pathways induced by systemin, oligosaccharide elicitors, and ultraviolet-B radiation at the level of mitogen-activated protein kinases in Lycopersicon peruvianum suspension-cultured cells. Plant Physiol 132 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub EB, Beynon JL, Crute IR (1994) Phenotypic and genotypic characterisation of interactions between isolates of Peronospora parasitica and accessions of Arabidopsis thaliana. Mol Plant Microbe Interact 7 223–239 [Google Scholar]
- Jenkins GI, Brown BA (2007) UV-B perception and signal transduction. In GC Whitelam, KJ Halliday, eds, Light and Plant Development, Vol 30. Blackwell Publishing, Oxford, pp 155–182
- Jenkins ME, Harlow GR, Liu Z, Shotwell MA, Ma J, Mount DW (1995) Radiation-sensitive mutants of Arabidopsis thaliana. Genetics 140 725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CZ, Yee J, Mitchell DL, Britt AB (1997. a) Photorepair mutants of Arabidopsis. Proc Natl Acad Sci USA 94 7441–7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CZ, Yen CN, Cronin K, Mitchell D, Britt AB (1997. b) UV- and gamma-radiation sensitive mutants of Arabidopsis thaliana. Genetics 147 1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Kalbin G, Hidema J, Brosché M, Kumagai T, Bornman JF, Strid Å (2001) UV-B-induced DNA damage and expression of defence genes under UV-B stress: tissue-specific molecular marker analysis in leaves. Plant Cell Environ 24 983–990 [Google Scholar]
- Kobayashi N, Katsumi S, Imoto K, Nakagawa A, Miyagawa S, Furumura M, Mori T (2001) Quantitation and visualization of ultraviolet-induced DNA damage using specific antibodies. Pigment Cell Res 14 94–102 [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M (1990) Mutations affecting testa colour in Arabidopsis. Arabidopsis Inf Serv 28 1–4 [Google Scholar]
- Kucera B, Leubner-Metzger G, Wellmann E (2003) Distinct ultraviolet-signalling pathways in bean leaves. DNA damage is associated with β-1,3-glucanase gene induction, but not with flavonoid formation. Plant Physiol 133 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LG, Chapple CCS, Last RL (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LG, Stapleton AE, Lim J, Hoffman P, Hays JB, Walbot V, Last RL (1997) An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc Natl Acad Sci USA 94 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page F, Kwoh EE, Avrutskaya A, Gentil A, Leadon SA, Sarasin A, Cooper PK (2000) Transcription-coupled repair of 8-oxoguanine: requirement for XPG, TFIIH, and CSB and implications for Cockayne Syndrome. Cell 101 159–171 [DOI] [PubMed] [Google Scholar]
- Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hall JD, Mount DW (2001) Arabidopsis UVH3 gene is a homolog of the Saccharomyces cerevisiae RAD2 and human XPG DNA repair genes. Plant J 26 329–338 [DOI] [PubMed] [Google Scholar]
- Liu Z, Hossain GS, Islas-Osuna MA, Mitchell DL, Mount DW (2000) Repair of UV damage in plants by nucleotide excision repair: Arabidopsis UVH1 DNA repair gene is a homolog of Saccharomyces cerevisiae Rad1. Plant J 21 519–528 [DOI] [PubMed] [Google Scholar]
- Lois R (1994) Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis thaliana L. I. Mechanisms of UV-resistance in Arabidopsis. Planta 194 498–503 [Google Scholar]
- Maeda K, Kimura S, Demura T, Takeda J, Ozeki Y (2005) DcMYB1 as a transcriptional activator of the carrot phenylalanine ammonia-lyase gene (DcPAL1) in response to elicitor treatment, UV-B irradiation and the dilution effect. Plant Mol Biol 59 739–752 [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Hieda K, Nikaido O (1991) Wavelength dependent formation of thymine dimers and [6-4] photoproducts in DNA by monochromatic ultraviolet light ranging from 150 to 365 nm. Photochem Photobiol 54 403–410 [DOI] [PubMed] [Google Scholar]
- Mercier J, Baka M, Reddy B, Corcuff R, Arul J (2001) Short-wave ultraviolet irradiation for control of decay caused by Botrytis cinerea in bell pepper: Induced resistance and germicidal effects. J Am Soc Hortic Sci 126 128–133 [Google Scholar]
- Mercier J, Roussel D, Charles MT, Arul J (2000) Systemic and local responses associated with UV- and pathogen-induced resistance to Botrytis cinerea in stored carrot. Phytopathology 90 981–986 [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Jin J, Cleaver JE (1991) Relative induction of cyclobutane dimers and cytosine photohydrates in DNA irradiated in vitro and in vivo with ultraviolet-C and ultraviolet-B light. Photochem Photobiol 54 741–746 [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS (1989) The biology of the (6-4) photoproduct. Photochem Photobiol 49 805–819 [DOI] [PubMed] [Google Scholar]
- Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30 461–469 [DOI] [PubMed] [Google Scholar]
- Molinier J, Oakeley EJ, Niederhauser O, Kovalchuk I, Hohn B (2005) Dynamic response of plant genome to ultraviolet radiation and other genotoxic stresses. Mutat Res 571 235–247 [DOI] [PubMed] [Google Scholar]
- Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O (1991) Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol 54 225–232 [DOI] [PubMed] [Google Scholar]
- Nakajima S, Sugiyama M, Iwai S, Hitomi K, Otoshi E, Kim ST, Jiang CZ, Todo T, Britt AB, Yamamoto K (1998) Cloning and characterization of a gene (UVR3) required for photorepair of 6-4 photoproducts in Arabidopsis thaliana. Nucleic Acids Res 26 638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid-dependent signalling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Q, Hays JB (1991) UV-B inducible and temperature-sensitive photoreactivation of cyclobutane pyrimidine dimers in Arabidopsis thaliana. Plant Physiol 95 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ND (2000) Stratospheric ozone depletion, UV-B radiation and crop disease. Environ Pollut 108 343–355 [DOI] [PubMed] [Google Scholar]
- Peak MJ, Peak JG, Carnes BA (1987) Induction of direct and indirect single-strand breaks in human cell DNA by far- and near-ultraviolet radiations: action spectrum and mechanisms. Photochem Photobiol 45 381–387 [DOI] [PubMed] [Google Scholar]
- Perdiz D, Grof P, Mezzina M, Nikaido O, Moustacchi E, Sage E (2000) Distribution and repair of bipyrimidine photoproducts in solar UV-irradiated mammalian cells. J Biol Chem 275 26732–26742 [DOI] [PubMed] [Google Scholar]
- Quaite FE, Sutherland BM, Sutherland JC (1992. a) Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion. Nature 358 576–578 [Google Scholar]
- Quaite FE, Sutherland BM, Sutherland JC (1992. b) Quantitation of pyrimidine dimers in DNA from UVB-irradiated alfalfa (Medicago sativa L.) seedlings. Appl Theor Electrophor 2 171–175 [PubMed] [Google Scholar]
- Quaite FE, Takayanagi S, Ruffini J, Sutherland JC, Sutherland BM (1994) DNA damage levels determine cyclobutyl pyrimidine dimer repair mechanisms in alfalfa seedlings. Plant Cell 6 1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A (1997) In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci USA 94 9463–9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Papaga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2 152–159 [Google Scholar]
- Rosenstein BS, Mitchell DL (1987) Action spectra for the induction of pyrimidine (6-4) pyrimidone photoproducts and cyclobutane pyrimidine dimers in normal human skin fibroblasts. Photochem Photobiol 45 775–781 [DOI] [PubMed] [Google Scholar]
- Sancar A, Thompson C, Thresher RJ, Araujo F, Mo J, Ozgur S, Vagas E, Dawut L, Selby CP (2000) Photolyase/cryptochrome family blue-light photoreceptors use light energy to repair DNA or set the circadian clock. Cold Spring Harb Symp Quant Biol 65 157–171 [DOI] [PubMed] [Google Scholar]
- Sancar GB (1990) DNA photolyases: physical properties, action mechanism, and roles in dark repair. Mutat Res 236 147–160 [DOI] [PubMed] [Google Scholar]
- Satoh MS, Jones CJ, Woof RD, Lindahl T (1993) DNA excision-repair defect of xeroderma pigmentosum prevents removal of a class of oxygen free radical-induced base lesions. Proc Natl Acad Sci USA 90 6335–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sävenstrand H, Brosché M, Strid Å (2004) Ultraviolet-B signalling: Arabidopsis brassinosteroid mutants are defective in UV-B regulated defence gene expression. Plant Physiol Biochem 42 687–694 [DOI] [PubMed] [Google Scholar]
- Scott AD, Nelshabury M, Jones DH, Reed SH, Boiteux S, Waters R (1999) Spontaneous mutation, oxidative DNA damage, and the roles of base and nucleotide excision repair in the yeast Saccharomyces. Yeast 15 205–218 [DOI] [PubMed] [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggeman E, Koorneef M, Ausubel FM, Goodman HM (1995) Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J 8 659–671 [DOI] [PubMed] [Google Scholar]
- Sohn KH, Lei R, Nemri A, Jones JDG (2007) The downy mildew effectors proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell 19 4077–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton AE, Walbot V (1994) Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol 105 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Khan VA, Lu JY, Wilson CL, Pusey PL, Kabwe MK, Igwegbe ECK, Chalutz E, Droby S (1998) The germicidal and hormetic effects of UV-C light on reducing brown rot disease and yeast microflora of peaches. Crop Prot 17 75–84 [Google Scholar]
- Stevens C, Khan VA, Wilson Lu JY, Chalutz E, Droby S (2005) The effect of fruit orientation of postharvest commodities following low dose ultraviolet light-C treatment on host induced resistance to decay. Crop Prot 24 756–759 [Google Scholar]
- Stevens C, Liu J, Khan VA, Lu JY, Kabwe MK, Wilson CL, Igwegbe ECK, Chalutz E, Droby S (2004) The effects of low-dose ultraviolet light-C treatment on polygalacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Prot 23 551–554 [Google Scholar]
- Stratmann J (2003) Ultraviolet-B radiation co-opts defense signalling pathways. Trends Plant Sci 8 526–533 [DOI] [PubMed] [Google Scholar]
- Suesslin C, Frohnmeyer H (2003) An Arabidopsis mutant defective in UV-B light-mediated responses. Plant J 33 591–601 [DOI] [PubMed] [Google Scholar]
- Swindell WR (2006) The association among gene expression responses to nine abiotic stress treatments in Arabidopsis thaliana. Genetics 174 1811–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Sakamoto A, Ishigaki Y, Nikaido O, Sun G, Hase Y, Shikazono N, Tano S, Watanabe H (2002) An ultraviolet-B-resistant mutant with enhanced DNA repair in Arabidopsis. Plant Physiol 129 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Baumann A, Oravecz A, Máté Z, Ádám É, Oakeley EJ, Schäfer E, Nagy F (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci USA 101 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Ichimura K, Mizoguchi T, Peck SC, Zhu T, Wang X, Shinozaki K, Paszkowski J (2001) Distinct regulation of salinity and genotoxic stress responses by Arabidopsis MAP kinase phosphatase 1. EMBO J 21 6483–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Nagy F (2005) Signalling and gene regulation in response to ultraviolet light. Curr Opin Plant Biol 8 477–482 [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55 85–97 [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Métraux JP, Ryals JA (1991) Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Henk A, Mowery P, Holub E, Innes RW (1998) A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppress multiple bacterial and downy mildew resistance genes. Plant Cell 10 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth WM, Jiang Q, West CE, Nikaido M, Bray CM (2002) Characterization of Arabidopsis photolyase enzymes and analysis of their role in protection from ultraviolet-B radiation. J Exp Bot 53 1005–1015 [DOI] [PubMed] [Google Scholar]
- Wei H, Ca Q, Rahn R, Zhang X, Wang Y, Lebwohl M (1998) DNA structural integrity and base composition affect ultraviolet light-induced oxidative DNA damage. Biochemistry 37 6485–6490 [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell signalling during cold, drought and salt stress. Plant Cell 14 S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Enyedi AJ, León J, Raskin I (1994) Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193 372–376 [Google Scholar]