Abstract
Initiation of leaves at the flanks of the shoot apical meristem occurs at sites of auxin accumulation and pronounced expression of auxin-inducible PIN-FORMED1 (PIN) genes, suggesting a feedback loop to progressively focus auxin in concrete spots. Because PIN expression is regulated by auxin response factor activity, including MONOPTEROS (MP), it appeared possible that MP affects leaf formation as a positive regulator of PIN genes and auxin transport. Here, we analyze a novel, completely leafless phenotype arising from simultaneous interference with both auxin signaling and auxin transport. We show that mp pin1 double mutants, as well as mp mutants treated with auxin-efflux inhibitors, display synergistic abnormalities not seen in wild type regardless of how strongly auxin transport was reduced. The synergism of abnormalities indicates that the role of MP in shoot meristem organization is not limited to auxin transport regulation. In the mp mutant background, auxin transport inhibition completely abolishes leaf formation. Instead of forming leaves, the abnormal shoot meristems dramatically increase in size, harboring correspondingly enlarged expression domains of CLAVATA3 and SHOOTMERISTEMLESS, molecular markers for the central stem cell zone and the complete meristem, respectively. The observed synergism under conditions of auxin efflux inhibition was further supported by an unrestricted PIN1 expression in mp meristems, as compared to a partial restriction in wild-type meristems. Auxin transport-inhibited mp meristems also lacked detectable auxin maxima. We conclude that MP promotes the focusing of auxin and leaf initiation in part through pathways not affected by auxin efflux inhibitors.
Plants continuously produce lateral organs, primarily leaves and flowers, at the flanks of shoot apical meristems (SAMs). Considerable advances have been made over the past 10 years on the understanding of the genetic basis of meristem maintenance, proliferation, and lateral organ formation (for review, see Williams and Fletcher, 2005; Carraro et al., 2006; Shani et al., 2006; Tucker and Laux, 2007). At the center of the meristem, a central zone (CZ) of generally less frequently dividing cells provides cells for the more frequently dividing surrounding peripheral zone (PZ) and underlying rib zone (RZ; Reddy et al., 2004). Together, the CZ, PZ, and RZ make up the SAM. The meristem provides cells for lateral organ (i.e. leaf and flower formation) and underlying pith formation. The size of the CZ is regulated by a feedback loop involving the CLAVATA (CLV) genes and the WUSCHEL (WUS) gene (Fletcher et al., 1999; Schoof et al., 2000; Clark, 2001). The meristem is specified and maintained by the SHOOTMERISTEMLESS (STM) gene (Long et al., 1996; Muday and DeLong, 2001; Kumaran et al., 2002), along with other members of the same gene family, primarily BREVIPEDICELLUS (BP)/KNAT1 and KNAT2 (Chuck et al., 1996; Ori et al., 2000; Muday and DeLong, 2001; Byrne et al., 2002). STM appears to carry out this function at least in part by preventing the expression of ASYMMETRIC LEAVES1 (AS1) in the meristem (Byrne et al., 2000; Long and Barton, 2000). AS1, in turn, promotes lateral organ formation at the flanks of the PZ by down-regulating BP/KNAT1 and KNAT2 at the sites of lateral organ formation (Byrne et al., 2000, 2002). Lateral organ formation also depends on the AINTEGUMENTA (ANT) gene, which promotes cell proliferation in these structures (Mizukami and Fischer, 2000). Both ANT and AS1 have been used as early molecular markers for the formation of lateral organs (Long and Barton, 1998; Byrne et al., 2000; Vernoux et al., 2000). Although some of the interactions of meristem-organizing genes have been documented, clear evidence of how primordia-specific genes become expressed at the sites of lateral organ formation remain elusive. The separation of the emerging lateral organs is promoted by several genes, most notably the CUP-SHAPED COTYLEDON genes, adding another level of regulation involved in lateral organ formation (Aida et al., 1997, 1999; Hibara et al., 2003; Vroemen et al., 2003; Koyama et al., 2007).
It has long been known that the formation of lateral organs can be influenced by the plant hormone auxin (Reinhardt et al., 2000, and refs. therein). Application of auxin, as well as auxin efflux inhibitors, results in a range of phenotypes from altered numbers and positions of flowers and leaves to a complete block of flower formation from reproductive SAMs (Wardlaw, 1949; Meicenheimer, 1981; Okada et al., 1991; Mattsson et al., 1999). Recent advances suggest that auxin accumulation is required for lateral organ initiation and that auxin is transported to these sites by membrane-bound efflux transport proteins that polarly localize to apical or basal ends of cells (Benkova et al., 2003; Reinhardt et al., 2003; Friml et al., 2004; Heisler et al., 2005; Petrasek et al., 2006). A key component in this process is PIN-FORMED1 (PIN1), a member of the PIN family of membrane-bound auxin efflux proteins (Okada et al., 1991; Galweiler et al., 1998). Loss-of-function mutations in the PIN1 gene result in reduced auxin transport and defective cotyledon and flower formation (Okada et al., 1991). Petrasek et al. (2006) have recently shown that PIN auxin efflux proteins are sufficient to facilitate auxin efflux in yeast (Saccharomyces cerevisiae) cells, suggesting that directionality of auxin flow can be regulated by the subcellular localization of PIN proteins. The PINOID (PID) gene, encoding a protein Ser/Thr kinase, acts as a positive regulator of polar auxin transport (PAT) by regulating the subcellular localization of PIN1 (Bennett et al., 1995; Benjamins et al., 2001; Friml et al., 2004; Lee and Cho, 2006). Loss-of-function pid mutants display defects in lateral organ formation similar to pin1 mutants, consistent with its role in regulating PIN1-mediated auxin efflux.
Auxin transport is promoted by the activity of the MONOPTEROS (MP) gene (Wenzel et al., 2007), which belongs to the auxin response factor (ARF) family of transcription factors (Guilfoyle et al., 1998; Hardtke and Berleth, 1998). Members of this family are posttranslationally activated in response to auxin via auxin-mediated degradation of members of the AUX/IAA family of nuclear repressor proteins that bind to ARFs and inhibit ARF dimerization and subsequently target gene transcription (Kim et al., 1997; Ulmasov et al., 1997, 1999; Leyser and Berleth, 1999; Dharmasiri and Estelle, 2002; Liscum and Reed, 2002). Not only mutations in PIN1 and PID, but also in the MP gene, interfere with lateral organ formation on inflorescence meristems (Przemeck et al., 1996). Local auxin application can restore flower formation on the flanks of pin1 and pid, but not mp mutant inflorescences (Reinhardt et al., 2000, 2003), suggesting that in mp mutants, not the local supply of auxin, but auxin sensitivity, is diminished. Similarly, cotyledon response assays show that mp mutants are more resistant to the effects of exogenous auxin treatments than the strong auxin-resistant mutant allele axr1-12, demonstrating that mp mutants are severely defective in auxin signaling (Mattsson et al., 2003).
Recent reports show that ARFs, including MP, may regulate the expression of PIN genes (Sauer et al., 2006; Wenzel et al., 2007). To test whether MP exerts its effect on lateral organ formation exclusively as a regulator of PIN genes and auxin transport, we created mp pin1 double mutants and also grew mp mutants on medium supplemented with auxin efflux inhibitors. Here, we show that mp pin1 double mutants, as well as mp mutants treated with auxin efflux inhibitors, display strong synergistic abnormalities. These mutants fail to develop any lateral organs and the SAM develops into a leafless dome. The appearance of a synergistic defect indicates that the role of MP in shoot meristem organization is not limited to the regulation of auxin transport and the novel meristem phenotype implicates auxin transport and signaling in the regulation of meristem size.
RESULTS
mp pin1 Double Mutants Fail to Form Leaves
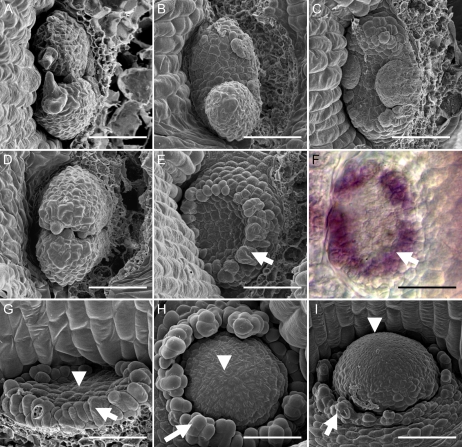
The shoot meristems of both pin1 and mp single mutants produce a functional rosette of leaves from the vegetative SAM, but are highly defective in the analogous process of flower formation from the reproductive SAM (Okada et al., 1991; Przemeck et al., 1996; Fig. 1, A and B). To assess whether MP function in shoot organization acts exclusively through the regulation of auxin transport, we generated mp pin1 double mutants. Analysis of progeny from a cross between heterozygous mp and pin1 plants resulted in the identification of a fraction of mp-like plants that had formed a leafless dome from the SAM (Fig. 1, C and D). The segregation ratio of this novel phenotype was not significantly different from an expected theoretical value based on χ2 analysis (P = 0.75; Supplemental Table S1), supporting the notion that the individuals were double mutants. The domes had a smooth surface and lacked differentiated epidermal, trichome, and stomata cells (Fig. 1D). After 2 to 3 weeks of culture in short-day conditions, the majority of the putative double mutants had developed additional leafless dome structures arising from the base of the initial dome (Fig. 1E). Such domes were never observed in single mp or pin1 mutant populations. The appearance of a novel phenotype in the absence of both gene activities leads us to conclude that MP and PIN1 act, at least in part, in separate pathways (see “Discussion”).
Figure 1.
Development of leafless domes from mp meristems. A, Wild-type rosette of leaves at 14 DAG compared to mp at 21 DAG (B). C and D, Photograph (C) and scanning electron micrograph (D) of mp pin1 double mutant at 40 DAG. E to I, mp pin1 double mutants at 60 DAG (E and F) and 75 DAG (G and H). Multiple leafless domes (E) and example of extreme fasciation leading to leafless flattened structures (F). Examples of filamentous projections (G and H) sometimes ending in pistil-like structures (I). J, mp pid double mutant at 50 DAG. K, Single fused leaf and no cotyledons in a pid pin1 double mutant at 14 DAG. L, A tubular third leaf in a 21-DAG wild-type seedling treated with 10 μm NPA. M to O, mp grown on medium with 10 μm NPA at 50 DAG (M), 40 μm 9-hydroxyfluorene-9-carboxylic acid at 35 DAG (N), and 40 μm 2,3,5-triiodobenzoic acid at 35 DAG (O). P, Leafless dome formation in Pro35S:MP plant grown on medium with 10 μm NPA for 40 DAG. Scale bars = 1 mm (A and B); 500 μm (C, E–H, J–P); 100 μm (D); 50 μm (I).
Phenotypes of mp pin1 double-mutant plants ranged from highly fasciated domes (Fig. 1F) to single or multiple dome formation and in the vast majority of all plants, leaf formation was absent. After 3 to 4 weeks of culture, many of the domes had formed one or more filament-like projections from its surface. A large number of these projections were formed after prolonged culture (Fig. 1, G and H). We interpreted these as inflorescences because they sometimes produced pistil-like or petal-like structures at their apices (Fig. 1I; data not shown). We found further evidence that MP acts on another pathway distinct from the regulation of PIN1 by the evaluation of mp pid and pin1 pid double mutants. The PID gene is known to be required for subcellular localization of PIN1 in plant cells transporting auxin (Friml et al., 2004) and may thus be thought to act in the same pathway as PIN1. Consistent with this interpretation, the mp pid double mutants produced phenotypes that were indistinguishable from the mp pin1 phenotype (Fig. 1J; Supplemental Table S1). Further, as previously reported (Furutani et al., 2004), the pin1 pid double mutants were characterized by a variable degree of wide or fused leaves, but did not produce the leafless dome phenotype observed in mp pin1 or mp pid double mutants. (Fig. 1K). The fact that the pin pid double mutant displays defects that are qualitatively similar to those of both single mutants is consistent with PIN1 and PID acting in the same pathway, in line with molecular evidence (Friml et al., 2004). In summary, mp pin1 and mp pid double mutants produced an identical, novel synergistic phenotype, suggesting that MP function in shoot meristem organization goes beyond the regulation of auxin transport processes (see “Discussion”).
Reduction of Auxin Transport Does Not Abolish Lateral Organ Formation
The phenotypes from the mp pin1 and mp pid double mutants suggest that, in the mp mutant background, leaf initiation becomes extremely sensitive to reduction of auxin transport. To assess this possibility, we grew mp seedlings on medium supplemented with the polar auxin efflux inhibitor 1-N-naphthylphthalamic acid (NPA). The observed defects very much resembled the phenotype of mp pin1 and mp pid double mutants (Fig. 1M). In addition, a large part of the heterogeneity observed in double mutants was lost at NPA concentrations at or above 10 μm NPA, suggesting that the heterogeneity was due to a comparatively weaker reduction in auxin transport in pin1 or pid mutants. Similar phenotypes were obtained with other, chemically distinct, auxin efflux inhibitors (i.e. 9-hydroxyfluorene-9-carboxylic acid [HFCA] and 2,3,5-triiodobenzoic acid [TIBA]; Fig. 1, N and O) when applied to mp mutants.
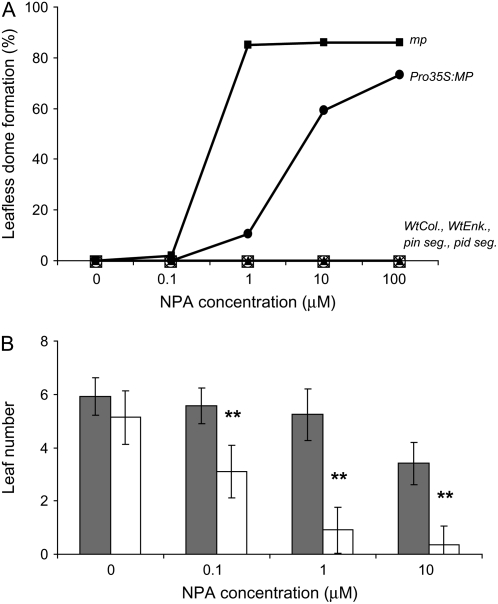
Because auxin transport is reduced in mp mutants (Przemeck et al., 1996), we next asked whether the leafless dome phenotype could simply be a consequence of particularly weak auxin transport. To this end, we grew wild-type plants and mp mutants in the presence of increasing NPA concentrations. As shown in Figure 2A, leaf formation in wild type, but also in pin1 and pid shoots, could not be abolished by any concentration of NPA, not even at 100 μm NPA, an eventually lethal concentration. Upon exposure to NPA, wild type, pin1, and pid3 mutants developed leaf fusions or tubular leaves, but never formed leafless domes (Figs. 1L and 2A). In wild-type plants, 0.1 and 1 μm NPA had no significant effect on the numbers of leaves produced by 21 d after germination (DAG; Fig. 2B). In contrast, in mp mutants, NPA concentrations as low as 0.1 μm resulted in a dramatic decrease in leaf initiation (Fig. 2B) and, at concentrations of 1 μm NPA and higher, the majority of mp mutants developed leafless domes. The novel leafless domes continued to grow, demonstrating that their inability to produce leaves was not the expression of a general growth defect. We conclude that MP, in addition to promoting auxin transport, must stimulate another activity that leads to the actual formation and growth of leaf primordia (see “Discussion”).
Figure 2.
Frequency of leafless dome formation in response to NPA. A, Wild-type Columbia, wild-type Enkheim, pin1(Enk) segregating population, pid segregating population, Pro35S:MP, and mp plants were grown on a series of medium containing 0 to 100 μm NPA. All genotypes were scored at 35 DAG and leafless dome formation was judged by the presence of leafless dome structure. Between 42 and 178 plants were scored for each genotype and treatment. B, Wild-type Columbia (gray bars) and mp (white bars) plants were grown on medium as in A and were scored for number of leaf primordia visible under a dissecting microscope at 21 DAG. **, Significant difference between NPA-grown wild type and mp mutants within the respective NPA treatment as determined by Student's t test analysis; P < 0.05. Error bars = sd.
Plants with Ectopic Expression of MP Display Similar NPA Hypersensitivity
The above results suggest that a loss of MP function is required for the formation of the leafless dome phenotype in the presence of NPA. Because MP is expressed specifically in leaf anlagen and primordia at the flanks of the meristem (Hardtke and Berleth, 1998; Wenzel et al., 2007), we tested whether altered expression of MP would suffice to interfere with leaf formation under conditions of PAT inhibition. To this effect, we grew plants misexpressing MP from the constitutive cauliflower mosaic virus 35S promoter on medium supplemented with NPA. Growth of Pro35S:MP plants in the presence of NPA did result in the frequent formation of leafless domes (Figs. 2A and 1P). The response of Pro35S:MP plants to NPA was intermediate between wild type and mp mutants because leaf formation was abolished at 10 μm NPA in the majority of mp plants. Therefore, not only the expression of MP per se, but also its restriction to distinct domains, appears critical for the initiation of leaf primordia under conditions of reduced PAT.
SAM Enlarges during Leafless Dome Formation
The strict requirement of defects in MP activity for the formation of leafless domes led us to have a more careful look at the mp meristem and its ability to form leaves in the absence of PAT inhibition. We found various defects in phyllotaxy and growth of mp primordia compared to wild type (Fig. 3, A–C), suggesting that the mp meristem is already labile in this process. We also observed an immediate response of mp meristems to NPA. In the presence of 10 μm NPA, wild-type meristems initiated a normal first pair of leaf primordia, whereas mp meristems did not form any visible leaf primordia (Fig. 3, D and E). Instead, in mp mutants, the cells immediately surrounding the meristem appeared to elongate, forming a ring of elongated cells around the meristem (Fig. 3, E–G). Subsequently, the meristem region began to enlarge to initiate the formation of the leafless dome (Fig. 3, H and I). The ring of cells initially expressed the leaf founder cell marker AS1 (Fig. 3F), but expression of AS1 and growth of these cells ceased by 6 DAG (Supplemental Fig. S1). After approximately 4 to 6 DAG, all subsequent growth came from the meristem (Fig. 3, G–I) and the resulting leafless dome structure is derived entirely from this region.
Figure 3.
Phyllotactic defects in the mp meristem and the initiation of leafless domes. A to C, The first leaf primordia in wild-type seedlings (A) and mp mutants (B and C). mp mutants with two opposite cotyledons were used for analysis to preclude any effects of cotyledon placement on subsequent leaf primordia formation. D to I, Wild type (D) and mp mutants (E–I) grown on medium with 10 μm NPA. The cells in the peripheral region of the NPA-grown mp SAM elongate to form a collar of cells (E–I, arrows), which have leaf cell fate as judged by in situ hybridization with an AS1 antisense probe (F). Central region of meristem indicated by arrowheads in G to I. All samples are 2 DAG, except H (4 DAG) and I (9 DAG). Scale bars = 50 μm. [See online article for color version of this figure.]
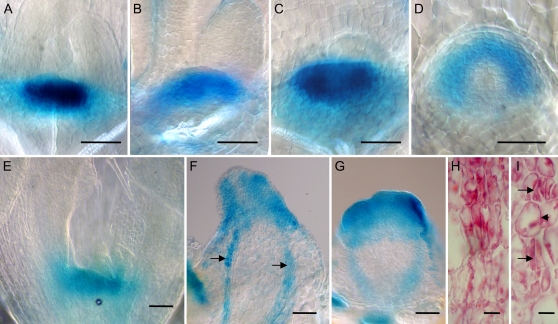
To determine the extent and organization of the meristem domain in leafless domes, we assessed the expression conferred by the STM gene promoter in these structures. Figure 4, A to D, shows a comparison of ProSTM:GUS meristem expression in wild-type and mp plants grown in the presence or absence of 10 μm NPA after 7 d of growth. Whereas the size of the meristem in 7-d-old wild-type, mp plants, and wild-type plants grown in the presence of NPA appeared comparable, the ProSTM:GUS expression domain was more curved and visibly wider in NPA-grown mp plants (Fig. 4D). After 21 d of growth, a distinct leafless dome structure had developed in NPA-grown mp plants. The ProSTM:GUS expression was localized at the apex of these structures (Fig. 4, F and G) and, although highly variable in size, appeared both wider and deeper than the corresponding expression domain in wild-type plants grown in parallel on the same medium (Fig. 4E). In NPA-grown mp plants, the leafless domes also expressed ProSTM:GUS in thin strands along the apical-basal axis (Fig. 4F, arrows). Upon closer inspection, these strands appeared to consist of elongated and narrow cells typical of procambial strands (Fig. 4H). Similar procambial expression of STM has previously been reported in the pith meristem of wild-type plants (Long et al., 1996). The procambial strands, however, are frequently interrupted and never differentiate into vascular tissues (Fig. 4I; data not shown).
Figure 4.
Expression of STM in leafless domes and procambial defects. Expression of ProSTM:GUS in wild type (A), mp (B), wild type grown on medium with 10 μm NPA (C and E), and mp grown on medium with 10 μm NPA (D, F–I). Arrows in F indicate expression of ProSTM:GUS in elongated procambial cell types orientated along the longitudinal axis. Longitudinal medial sections of leafless domes shows procambial strands (H and I) that are frequently interrupted (arrows in I). A to D, 7 DAG; E to I, 21 DAG. Size bars = 50 μm (A–G) and 10 μm (H and I).
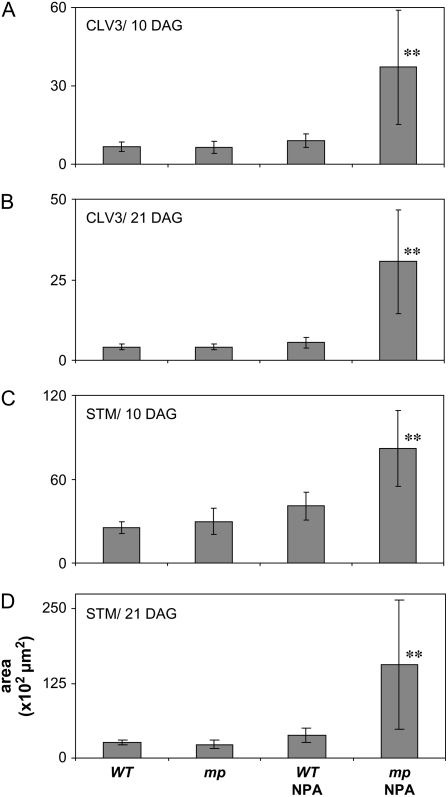
To further explore the enlarged SAMs in NPA-grown mp plants, we quantified the area of expression of the CZ marker, CLV3, and the meristem marker STM at 10 and 21 DAG using ProCLV3:GFP and ProSTM:GUS, respectively (Fig. 5; representative imaged areas shown in Supplemental Fig. S2). After 10 d, the average areas of CLV3 and STM expression were significantly larger in NPA-grown mp mutants. Although highly variable, the area of CLV3 expression was on average 4.1 times and the STM expression 2.0 times as large in NPA-grown mp plants as compared to NPA-grown wild-type plants, thereby illustrating that leafless domes have enlarged CZs and meristem identity, respectively (Fig. 5, A and C). After 21 d of growth, the differences had increased further, with 5.5 times larger area of CLV3 expression and 4.2 times larger area of STM expression in NPA-grown mp plants as compared to NPA-grown wild-type plants (Fig. 5, B and D). In summary, the leafless domes appear to have the organization of an enlarged shoot apex, comprising an apical meristem, and a basal radially organized stem region, but the CZ, as well as the entire meristem region, is enlarged and the basal region shows limited internal and external cellular differentiation.
Figure 5.
Quantification of CZ and meristem areas. Wild-type or mp plants were grown in the absence or presence of 10 μm NPA and the areas based on ProCLV3:GFP:ER expression domains at 10 (A) and 21 DAG (B), and ProSTM:GUS expression domains at 10 (C) and 21 DAG (D). The y axis shows measured areas in 102 μm2. Bars represent average of measured areas from six to 13 meristems; error bars are sd. **, Significant difference between NPA-grown mp mutants compared to all other genotypes and treatments, as determined by Student's t test analysis; P < 0.05. Representative images of measured areas are shown in Supplemental Figure S2.
Leafless Domes Fail to Focus PIN1 Expression and Auxin
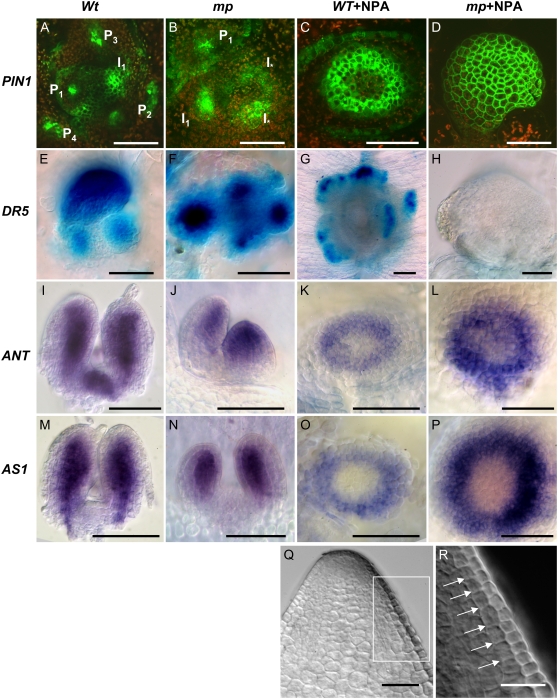
Previous studies have reported that PIN1 expression is up-regulated at sites of flower primordia formation in the reproductive SAM (Heisler et al., 2005). We used a ProPIN1:PIN1:GFP marker to visualize PIN1 expression in vegetative SAMs defective in mp and/or auxin transport functions. Our analysis showed that PIN1 expression was most pronounced in discrete epidermal spots on the surface of vegetative wild-type SAMs and internal procambial midveins of young primordia (Fig. 6A), in agreement with previous findings from the reproductive SAM. In mp meristems, PIN1 expression domains were more diffuse, occurred in defective phyllotactic patterns, and expression appeared spuriously in cells that are normally not involved in primordia formation (Fig. 6B). PIN1 expression in NPA-grown wild-type seedlings was very weak or absent in the CZ area of the meristem, thereby forming a ring of high expression in the PZ, possibly predicting the future formation of a tubular leaf (Fig. 6C). Remarkably, in NPA-grown mp plants, PIN1 expression was not even restricted to the PZ and, instead, expression was evenly distributed throughout the entire surface of young domes, including the CZ and more basal parts of the leafless dome (Fig. 6D). To assess whether the lack of PIN1 focus formation in NPA-grown mp plants is accompanied by a lack of auxin maxima formation, we analyzed the expression of the auxin-responsive ProDR5:GUS marker. In wild-type seedlings, ProDR5:GUS is expressed initially at the apices of emerging leaf primordia and also internally in leaf primordia in conjunction with the formation of procambial tissues, but ProDR5:GUS expression is not found in the CZs and PZs of the SAM (Fig. 6E; Mattsson et al., 2003). In mp seedlings, the ProDR5:GUS expression in leaf primordia apices was always more diffuse than in wild-type seedlings (Fig. 6F). Wild-type plants responded to NPA with a considerable delay in leaf primordia formation and, when leaf primordia emerged, the ProDR5:GUS expression was found at the margins of the circular or close to circular leaf primordia (Fig. 6G). At no point did we observe localized ProDR5:GUS expression at the flanks of NPA-grown mp meristems (Fig. 6H). In summary, the leafless dome meristems of NPA-grown mp mutants show defects in the focusing of PIN1 expression and do not form local auxin-response maxima as judged by ProDR5:GUS.
Figure 6.
Marker analysis of leafless dome meristems. Material grown on medium supplemented with 10 μm NPA indicated as +NPA. A to D, ProPIN1:PIN1:GFP expression in wild type (A), mp (B), wild type + NPA (C), and mp NPA (D). Order of leaf primordia, present and incipient, is indicated in wild type (A) and mp (B). Aberrant expression is indicated by I*. E to H, ProDR5:GUS expression in wild type (E), mp (F), wild type + NPA (G), mp + NPA (H) at 21 DAG. I to L, ANT antisense probe at 3 DAG in wild type (I), mp (J), and 21-DAG wild type + NPA (K) and mp + NPA (L). M to P, AS1 antisense probe at 3 DAG in wild type (M), mp (N), and 21-DAG wild type + NPA (O) and mp + NPA (P). Q and R, Apex of 21-DAG leafless dome (Q) and higher magnification of marked area in R. Arrows in R indicate cell walls produced by anticlinal cell divisions. All size bars = 50 μm, except for H (100 μm) and R (25 μm).
Leaf Founder Cell Markers Are Expressed in Leafless Dome Meristems
The synergistic phenotype in mp pin1 double mutants suggests that MP acts not only through regulation of PAT in the process of leaf formation, but may separately promote the growth of leaf primordia. Potential target genes could be involved in leaf founder cell fate specification or associated with subsequent organ outgrowth. The ANT and AS1 genes are expressed in leaf founder cell populations and subsequently during outgrowth of leaf primordia (Elliott et al., 1996; Long and Barton, 1998; Byrne et al., 2000). We used the expression of these genes to assess whether leaf founder cell populations are established at the flanks of the meristem in leafless domes. In wild-type plants, we found that the expression of these markers preceded the formation of leaf primordia and that they were expressed in outgrowing primordia (Fig. 6, I and M), in agreement with published results. The expression of ANT and AS1 in mp mutants appears identical to wild-type expression patterns (Fig. 6, J and N), except for the defects in phyllotaxy already described (Fig. 3, B and C). In response to NPA, wild-type plants expressed ANT and AS1 in a circular domain (Fig. 6, K and O) consistent with the subsequent formation of a tubular leaf. We observed a similar ring-shaped expression of ANT and AS1 near the apex of leafless domes in NPA-grown mp plants (Fig. 6, L and P). Thus, leaf founder cell populations appear to be specified in the PZs of wild-type and mp plants treated with NPA, but this specification is not sufficient for leaf formation in the latter. The failure to form leaves in leafless domes appears to be due to a defect in outgrowth of leaf primordia. In wild-type plants, early leaf initiation can be detected by a switch from anticlinal to periclinal cell divisions in the L2 layer (Medford et al., 1992). We screened longitudinal medial sections of more than 15 leafless domes without finding any indications of periclinal divisions in the L2 layer. Instead, we observed smooth surfaces of the PZ, and a pattern of cell walls in the L2 layer that indicated strict anticlinal cell division planes (Fig. 6, Q and R).
In summary, we conclude that the defect in leaf primordia formation in NPA-grown mp plants does not involve a block in the formation of leaf founder cells, but appears to involve a block of subsequent periclinal divisions in the process of leaf outgrowth, which appears to depend on MP activity.
DISCUSSION
Several lines of evidence have indicated that PIN gene expression is auxin (Heisler et al., 2005; Vieten et al., 2005, 2007; Scarpella et al., 2006; Wenzel et al., 2007) and ARF dependent (Sauer et al., 2006; Wenzel et al., 2007), suggesting that MP functions in leaf initiation by mediating PIN gene expression. In this case, however, one would expect that loss of MP function should not matter in plants severely compromised in auxin transport. Here, we observed that mp mutants of various allele strengths are hypersensitive to NPA treatment and display synergistic defects in double mutants with pin1. These findings provide strong evidence for an involvement of MP in a process beyond the control of auxin transport. Importantly, the synergistic defects cannot be mimicked by applying increased concentration of NPA to wild-type or pin1 plants, further supporting that MP regulates further, hitherto unexplored, processes to promote leaf initiation. As one of those processes, we propose that MP has a role in promoting the actual outgrowth of leaves and flowers. Notably, it has also been suggested that, activating ARFs, including MP, could bind to the promoters of auxin-regulated leaf specification genes, thereby promoting leaf formation in the PZ of the meristem, whereas interaction with other ARFs limits this action in the CZ of the meristem (Leyser, 2006). Given this scenario, ARFs like MP would therefore be implicated in also having functions in conferring differential properties to zones in the SAM.
Reinhardt et al. (2003) have formulated a model in which leaf primordia form at sites of elevated epidermal auxin concentration. Preexisting primordia are thought to influence the position of new primordia by depleting the vicinity of auxin through auxin transport. Thus, new leaf primordia would only form at sites far enough away from existing primordia to allow new auxin maxima to form. This mechanism would not only explain the dependence of leaf formation on auxin maxima, but also the phyllotactic pattern of leaves and how it is influenced by the position of preexisting primordia. Mathematical modeling of leaf initiation based on these findings postulated a positive feedback loop concentrating auxin into concrete spots on the surface of the SAM because of positive influence of auxin on the amount and orientation of PIN1 efflux carriers in neighboring cells (Jonsson et al., 2006; Smith et al., 2006). Mutants in the PIN1 gene, as well as NPA-treated plants, fail to focus auxin through convergent PIN1 polarity (Heisler et al., 2005) and do not form flowers from the inflorescence meristem (Okada et al., 1991; Vernoux et al., 2000). This is evidenced by the fact that, in NPA-treated plants, the concentration and polarization of PIN1-GFP toward individual spots is much reduced (Heisler et al., 2005). These interpretations are also consistent with the fact that flowers can be formed in both pin1 mutant and NPA-treated inflorescence meristems upon local application of auxin. Apparently, the local application bypasses the need for auxin transport-driven focusing of auxin toward flower initiation sites.
MP is another likely component of the postulated mechanism because mp mutants also fail to form flowers from the inflorescence meristem and have reduced auxin transport capacity (Przemeck et al., 1996). Further, MP encodes an ARF (Ulmasov et al., 1997; Hardtke and Berleth, 1998), which might be involved in the auxin-dependent regulation of PIN expression (Sauer et al., 2006; Wenzel et al., 2007). No flowers can be induced by local auxin application on the flanks of mp inflorescence meristems (Reinhardt et al., 2003), suggesting that it is not only auxin transport and auxin accumulation that are defective in mp mutants, but also a failure to trigger lateral organ outgrowth even when auxin is locally provided (Reinhardt et al., 2003). Thus, published auxin application experiments already hint to a role of MP in controlling auxin responses in lateral organ outgrowth.
The inhibition of auxin transport in mp mutant backgrounds generates an unprecedented type of abnormal SAM development, which not only completely obstructs the formation of lateral organs, but also vastly expands the shoot apex. Marker gene expression indicates that the enlarged apical dome is composed of expanded STM and CLV3-expressing domains surrounded by a wide circular PZ, marked by ANT and AS1. Although no leaf primordia are formed under these conditions, there seems to be some dispersed growth because the ANT and AS1 expression domains are extremely wide.
Under conditions of normal auxin transport, ARFs acting redundantly to mp appear to be sufficient for triggering organ formation from the vegetative, yet not from the reproductive, SAM as mp mutants produce leaves. Conversely, inhibition of auxin transport seems to allow for sufficient auxin focusing in the epidermis to trigger vegetative leaf initiation as long as MP is functional. However, poorly defined leaf initiation points seem to be insufficient to trigger organ outgrowth through redundantly acting ARFs when MP is not functional. Whereas failed leaf initiation may thus be explained as the superimposition of defects in two interdependent steps, the reasons for the enlargement of the CZ seem to reflect other, unknown levels of control. It has been proposed that the restriction of leaf-initiating auxin focusing to the PZ reflects auxin sensitivity zones due to the specific expression domains of competing ARFs (Leyser, 2006). In this interpretation, it is plausible that the removal of an important ARF may destabilize the zoning sufficiently to promote cell proliferation also in the CZ. In this context, it is remarkable that we observed equally strong PIN1-GFP expression in the PZ and CZ uniquely in NPA-exposed mp mutants. Formally, it is also possible that the expansion of the CZ could be a necessary consequence of defective lateral organ formation. Several levels of mutually antagonistic gene activities have been implicated in the control of stem cell pool size of the shoot meristem (for review, see Clark, 2001; Williams and Fletcher, 2005; Carraro et al., 2006; Tucker and Laux, 2007) in which some negative regulators originate from the PZ. Because there are no other leafless genotypes available, we cannot genetically separate leaflessness from SAM expansion. However, it should be noted that, in the inflorescences of pin1 mutants devoid of lateral flowers, the size of the meristem and its constituent zones have been described as normal (Vernoux et al., 2000), arguing against a mechanism where signals negatively regulating shoot meristem size are derived from concrete flower or leaf primordia.
The sizes of SAMs vary considerably across the plant kingdom (Steeves and Sussex, 1989) and the influences of new regulators on SAM size are continuously being revealed (Chaudhury et al., 1993; Clark et al., 1993, 1995; Running et al., 2004; Green et al., 2005; Chiu et al., 2007). The discovery of highly abnormally sized SAMs as a consequence of simultaneous interference with auxin transport and ARF function may provide an entry point in the genetic analysis of auxin's role in this process.
MATERIALS AND METHODS
Plant Material and Growth
The mpG12, G33, Tu399, pid3, and pin1-1 mutant alleles used for double- and single-mutant analysis have been described previously by Okada et al. (1991), Berleth and Jurgens (1993), Hardtke and Berlet, (1998), Christensen et al. (2000), and Benjamins et al. (2001). All MP alleles used in this study are characterized as strong alleles and no differences were observed between different alleles and subsequent treatments or double-mutant generation. The 35S∷MP line was generated as described by Hardtke et al. (2004) and overexpression of MP transcripts was confirmed by quantitative PCR using a Rotor-Gene 3000 real-time quantitative thermocycler (Corbett Life Sciences) and the Platinum SYBER Green quantitative PCR SuperMix (Invitrogen), with the primers MP-RT-F (CGATTTGGATCCGTTGAGAT) and MP-RT-R (ACCCCATTCAGTTTCACCAG; Hardtke et al., 2004; data not shown). The ProDR5∷GUS (Ulmasov et al., 1997), ProPIN1:PIN1:GFP (Benkova et al., 2003), ProSTM:GUS (Kirch et al., 2003), and ProCLV3:GFP:ER (Lenhard and Laux, 2003) transgenes were crossed into the mp mutant background. Attempts to introgress a ProDR5∷GFP construct from the Arabidopsis Biological Resource Center into mp mutant background failed, possibly as a consequence of repulsion due to linkage. Surface-sterilized seeds were grown on ATS medium (Lincoln et al., 1990) and exposed to NPA as described (Mattsson et al., 1999). For quantification of CLV3 and STM expression domains in meristems, images were taken and subsequently analyzed using ImageJ, version 1.37, software (National Institutes of Health). mp seedlings germinate approximately 1 d after wild type, most likely due to lack of hypocotyl and root. Comparable developmental stages were chosen for each dataset defined by the wild type; for example, the 3-DAG stage is defined as 3-DAG wild-type plants and 4-DAG mp plants, with similar sizes of leaf primordia.
In Situ Hybridization, Histology, and GUS Assays
All gene fragments were amplified from cDNA generated from total RNA extracted from 14-d-old wild-type seedlings using TRIzol reagent (Invitrogen) and subsequently reverse transcribed using RevertAid Moloney murine leukemia virus reverse transcriptase (Fermentas) and cloned into pBluescript II SK(−) (Stratagene). The ANT and AS1 fragments were generated as described (Long and Barton, 1998; Byrne et al., 2000). Whole-mount in situ hybridization procedure was as described (Zachgo et al., 2000) with some modifications, including overnight fixation and agitation in a fresh solution containing 0.1 m triethanolamine (pH 8) and 0.5% (v/v) acetic anhydride for 15 min, followed by two washes in 1× phosphate-buffered saline solution prior to hybridization for 2 d at 60°C. For histological analysis, plant material was fixed and sectioned as described by Ruzin (1999). Localization of GUS activity was carried out as described by Mattsson et al. (2003).
Microscopy
A Zeiss LSM 410 was used to image ProPIN1:PIN1:GFP and ProCLV3:GFP:ER using a 488-nm excitation filter and 500- to 530-nm emission filter combination. Background red autofluorescence was detected using a 568-nm excitation filter and an LP 580 emission filter set. Digital interference contrast images were taken on a Nikon Eclipse 600 microscope using a Canon D30 digital camera and tissue clearing and preparation were performed as described by Mattsson et al. (1999). Samples for scanning electron micrographs were fixed in 2.5% glutaraldehyde in 0.05 m cacodylate buffer, dehydrated in a graded ethanol series before being critically point dried, and mounted on stubs. Samples were then coated with gold-palladium in a scanning electron microscope Prep2 sputter coater (Nanotech) and imaged using a Hitachi S-2600N VP-SEM.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Time series of AS1 expression in leafless domes.
Supplemental Figure S2. Quantification of CZ and meristem areas.
Supplemental Table S1. Segregation of double mutants.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center and Dr. Steven Chatfield (University of Guelph) for supplying marker lines, and the University of British Columbia bioimaging facility for excellent service. We also thank Dr. Sherryl Bisgrove and the reviewers of the manuscript for providing helpful suggestions to improve the text.
This work was supported by Natural Sciences and Engineering Research Council (NSERC) discovery grants (to J.M. and T.B.) and an NSERC graduate student fellowship (to M.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jim Mattsson (mattsson@sfu.ca).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9 841–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126 1563–1570 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8 505–520 [Google Scholar]
- Berleth T, Jurgens G (1993) The role of the Monopteros gene in organizing the basal body regions of the Arabidopsis embryo. Development 118 575–587 [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408 967–971 [DOI] [PubMed] [Google Scholar]
- Byrne ME, Simorowski J, Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals KNOX gene redundancy in Arabidopsis. Development 129 1957–1965 [DOI] [PubMed] [Google Scholar]
- Carraro N, Peaucelle A, Laufs P, Traas J (2006) Cell differentiation and organ initiation at the shoot apical meristem. Plant Mol Biol 60 811–826 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Dennis ES (1993) amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis, and precocious flowering. Plant J 4 907–916 [Google Scholar]
- Chiu WH, Chandler J, Cnops G, Van Lijsebettens M, Werr W (2007) Mutations in the TORNADO2 gene affect cellular decisions in the peripheral zone of the shoot apical meristem of Arabidopsis thaliana. Plant Mol Biol 63 731–744 [DOI] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478 [DOI] [PubMed] [Google Scholar]
- Chuck G, Lincoln C, Hake S (1996) KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 8 1277–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE (2001) Cell signalling at the shoot meristem. Nat Rev Mol Cell Biol 2 276–284 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067 [Google Scholar]
- Dharmasiri S, Estelle M (2002) The role of regulated protein degradation in auxin response. Plant Mol Biol 49 401–409 [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283 1911–1914 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al (2004) A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306 862–865 [DOI] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M (2004) PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131 5021–5030 [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230 [DOI] [PubMed] [Google Scholar]
- Green KA, Prigge MJ, Katzman RB, Clark SE (2005) CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17 691–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ, Ulmasov T, Hagen G (1998) The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol Life Sci 54 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T (2004) Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131 1089–1100 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15 1899–1911 [DOI] [PubMed] [Google Scholar]
- Hibara K, Takada S, Tasaka M (2003) CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation. Plant J 36 687–696 [DOI] [PubMed] [Google Scholar]
- Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E (2006) An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 103 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A (1997) Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA 94 11786–11791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch T, Simon R, Grunewald M, Werr W (2003) The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell 15 694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Furutani M, Tasaka M, Ohme-Takagi M (2007) TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran MK, Bowman JL, Sundaresan V (2002) YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 14 2761–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Cho HT (2006) PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18 1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard M, Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130 3163–3173 [DOI] [PubMed] [Google Scholar]
- Leyser O (2006) Dynamic integration of auxin transport and signalling. Curr Biol 16 R424–433 [DOI] [PubMed] [Google Scholar]
- Leyser O, Berleth T (1999) A molecular basis for auxin action. Semin Cell Dev Biol 10 131–137 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Reed JW (2002) Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 49 387–400 [PubMed] [Google Scholar]
- Long J, Barton MK (2000) Initiation of axillary and floral meristems in Arabidopsis. Dev Biol 218 341–353 [DOI] [PubMed] [Google Scholar]
- Long JA, Barton MK (1998) The development of apical embryonic pattern in Arabidopsis. Development 125 3027–3035 [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 66–69 [DOI] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126 2979–2991 [DOI] [PubMed] [Google Scholar]
- Medford JI, Behringer FJ, Callos JD, Feldmann KA (1992) Normal and abnormal development in the Arabidopsis vegetative shoot apex. Plant Cell 4 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meicenheimer RD (1981) Changes in Epilobium phyllotaxy induced by N-1-naphthylphthalamic acid and a-4-chlorophenoxyisobutyric acid. Am J Bot 68 1139–1154 [Google Scholar]
- Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6 535–542 [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Chuck G, Bowman JL, Hake S (2000) Mechanisms that control KNOX gene expression in the Arabidopsis shoot. Development 127 5523–5532 [DOI] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312 914–918 [DOI] [PubMed] [Google Scholar]
- Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T (1996) Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200 229–237 [DOI] [PubMed] [Google Scholar]
- Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM (2004) Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development 131 4225–4237 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426 255–260 [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S (2004) Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA 101 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin SE (1999) Plant Microtechnique and Microscopy. Oxford University Press, New York
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644 [DOI] [PubMed] [Google Scholar]
- Shani E, Yanai O, Ori N (2006) The role of hormones in shoot apical meristem function. Curr Opin Plant Biol 9 484–489 [DOI] [PubMed] [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P (2006) A plausible model of phyllotaxis. Proc Natl Acad Sci USA 103 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM (1989) Patterns in Plant Development. Cambridge University Press, Cambridge, UK
- Tucker MR, Laux T (2007) Connecting the paths in plant stem cell regulation. Trends Cell Biol 17 403–410 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ (1999) Dimerization and DNA binding of auxin response factors. Plant J 19 309–319 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J (2000) PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127 5157–5165 [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12 160–168 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132 4521–4531 [DOI] [PubMed] [Google Scholar]
- Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MA, de Vries SC (2003) The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell 15 1563–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw CW (1949) Experiments on organogenesis in ferns. Growth 13 93–131 [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J (2007) Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J 49 387–398 [DOI] [PubMed] [Google Scholar]
- Williams L, Fletcher JC (2005) Stem cell regulation in the Arabidopsis shoot apical meristem. Curr Opin Plant Biol 8 582–586 [DOI] [PubMed] [Google Scholar]
- Zachgo S, Perbal MC, Saedler H, Schwarz-Sommer Z (2000) In situ analysis of RNA and protein expression in whole mounts facilitates detection of floral gene expression dynamics. Plant J 23 697–702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.