Abstract
Recombinant viral genomes carrying a selectable drug resistance marker have been constructed by insertion of a hybrid gene for neomycin resistance into the helper-independent adenovirus vector, delta E1/X. The hybrid gene consists of sequences coding for the aminoglycoside 3'-phosphotransferase II from Tn5, under the control of the simian virus 40 early promoter, and renders mammalian cells resistant to the neomycin analog, G-418. Most of adenovirus early region 1 is deleted from delta E1/X (nucleotides 455 to 3330), and recombinant viral genomes carry the hybrid gene in its place. The large and small XbaI fragments of delta E1/X were ligated to the hybrid gene, and the mixture was transfected into 293 cells. Single plaques were isolated and subsequently passaged in 293 cells to produce virus stocks. The recombinant viruses efficiently rendered cultured rat (Rat2) and simian (CV1) cells resistant to G-418. Cloned cell lines selected for resistance to G-418 contained viral DNA integrated into the host cell genome, demonstrating that early region 1 is not essential for integration of the viral genome. Southern transfer experiments revealed that (i) the sites of integration in the host genome were not unique; (ii) in general, transformed CV1 cell lines contained single-copy, full-length viral genomes, colinear with the infecting virus; (iii) transformed Rat2 cell lines generally contained one to several copies of full-length viral genomes integrated colinearly with the infecting viral DNA; and (iv) three of these five lines of transformed Rat2 cell lines contained tandemly repeated viral DNA sequences in which the right and left ends of the viral genome were joined to each other.
Full text
PDF

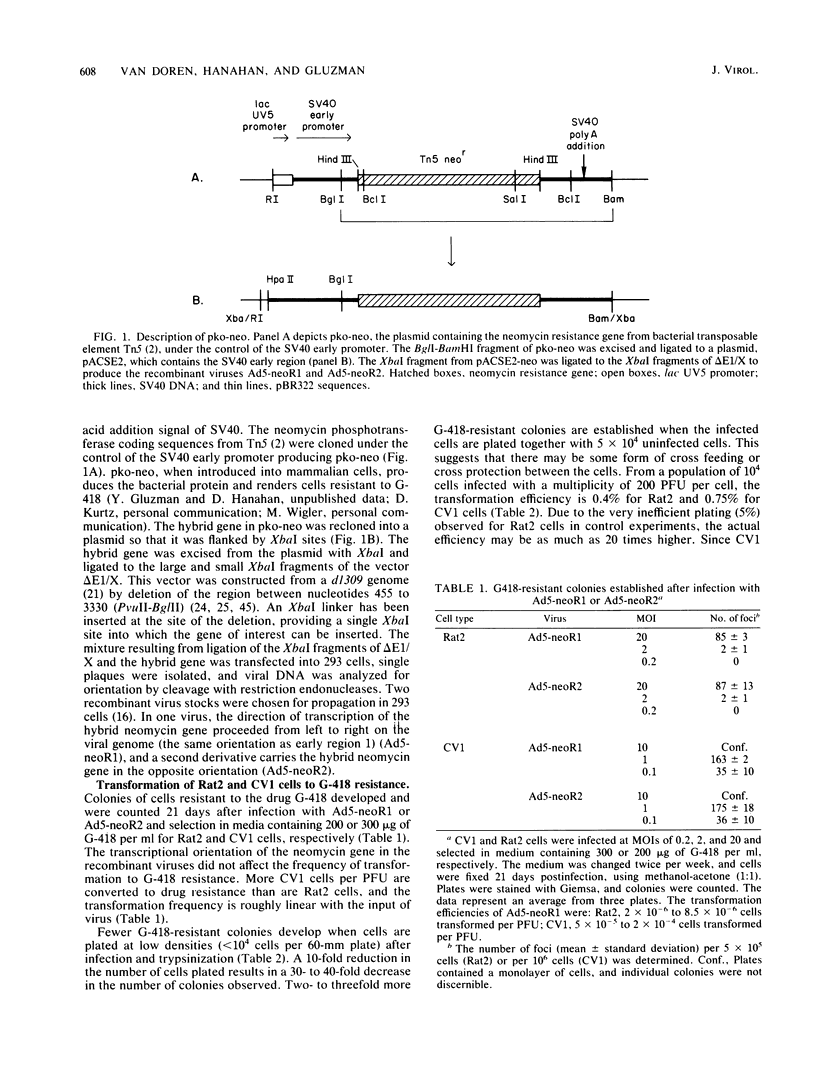




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss L. E., Ginsberg H. S., Fisher P. B. Cold-sensitive expression of transformation by a host range mutant of type 5 adenovirus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1352–1356. doi: 10.1073/pnas.80.5.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite A. W., Murray J. D., Bellett A. J. Alterations to controls of cellular DNA synthesis by adenovirus infection. J Virol. 1981 Aug;39(2):331–340. doi: 10.1128/jvi.39.2.331-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
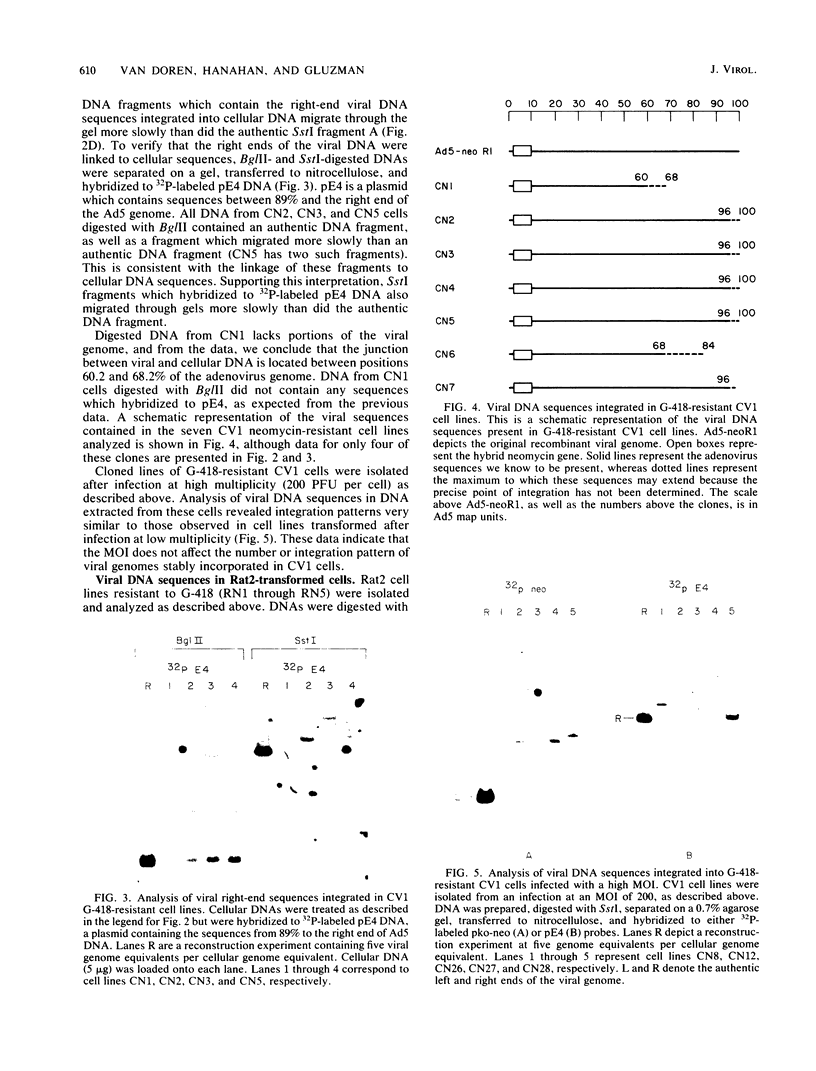
- Cheetham B. F., Bellett A. J. A biochemical investigation of the adenovirus-induced G1 to S phase progression: thymidine kinase, ornithine decarboxylase, and inhibitors of polyamine biosynthesis. J Cell Physiol. 1982 Feb;110(2):114–122. doi: 10.1002/jcp.1041100203. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van der Feltz M. J., van der Eb A. J. Transformation of primary rat kidney cells by DNA fragments of weakly oncogenic adenoviruses. J Virol. 1979 Dec;32(3):943–950. doi: 10.1128/jvi.32.3.943-950.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Uptake, fixation, and expression of foreign DNA in mammalian cells: the organization of integrated adenovirus DNA sequences. Curr Top Microbiol Immunol. 1982;101:127–194. doi: 10.1007/978-3-642-68654-2_6. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Fisher P. B., Weinstein I. B., Ginsberg H. S. Patterns of viral DNA integration in cells transformed by wild type or DNA-binding protein mutants of adenovirus type 5 and effect of chemical carcinogens on integration. J Virol. 1980 May;34(2):305–314. doi: 10.1128/jvi.34.2.305-314.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. B., Babiss L. E., Weinstein I. B., Ginsberg H. S. Analysis of type 5 adenovirus transformation with a cloned rat embryo cell line (CREF). Proc Natl Acad Sci U S A. 1982 Jun;79(11):3527–3531. doi: 10.1073/pnas.79.11.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Ensinger M. J., Kauffman R. S., Mayer A. J., Lundholm U. Cell transformation: a study of regulation with types 5 and 12 adenovirus temperature-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):419–426. doi: 10.1101/sqb.1974.039.01.054. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Van Doren K. Palindromic adenovirus type 5-simian virus 40 hybrid. J Virol. 1983 Jan;45(1):91–103. doi: 10.1128/jvi.45.1.91-103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Ibelgaufts H., Doerfler W., Scheidtmann K. H., Wechsler W. Adenovirus type 12-induced rat tumor cells of neuroepithelial origin: persistence and expression of the viral genome. J Virol. 1980 Jan;33(1):423–437. doi: 10.1128/jvi.33.1.423-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Lo C. W. Transformation by iontophoretic microinjection of DNA: multiple integrations without tandem insertions. Mol Cell Biol. 1983 Oct;3(10):1803–1814. doi: 10.1128/mcb.3.10.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat J., Van Ormondt H. The nucleotide sequence of the transforming HindIII-G fragment of adenovirus type 5 DNA. The region between map positions 4.5 (HpaI site) and 8.0 (HindIII site). Gene. 1979 May;6(1):75–90. doi: 10.1016/0378-1119(79)90086-6. [DOI] [PubMed] [Google Scholar]
- Maat J., van Beveren C. P., van Ormondt H. The nucleotide sequence of adenovirus type 5 early region E1: the region between map positions 8.0 (HindIII site) and 11.8 (SmaI site). Gene. 1980 Jun;10(1):27–38. doi: 10.1016/0378-1119(80)90140-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. J., Ginsberg H. S. Persistence of type 5 adenovirus DNA in cells transformed by temperature-sensitive mutant, H5ts125. Proc Natl Acad Sci U S A. 1977 Feb;74(2):785–788. doi: 10.1073/pnas.74.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. K., Temin H. M. High-efficiency ligation and recombination of DNA fragments by vertebrate cells. Science. 1983 May 6;220(4597):606–609. doi: 10.1126/science.6301012. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Naujokas M., Hassell J. A. Homologous recombination between transfected DNAs. Mol Cell Biol. 1983 Sep;3(9):1680–1685. doi: 10.1128/mcb.3.9.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Transformation of rodent cells by DNA extracted from transformation-defective adenovirus mutants. J Virol. 1983 Jun;46(3):1039–1044. doi: 10.1128/jvi.46.3.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. L. Integration and expression of viral DNA in cells transformed by host range mutants of adenovirus type 5. J Virol. 1982 Feb;41(2):674–685. doi: 10.1128/jvi.41.2.674-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben M., Bacchetti S., Graham F. Covalently closed circles of adenovirus 5 DNA. Nature. 1983 Jan 13;301(5896):172–174. doi: 10.1038/301172a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Greene R., Stringer J., Mitchison T., Hu S. L., Botchan M. Analysis of the sites of integration of viral DNA sequences in rat cells transformed by adenovirus 2 or SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):569–584. doi: 10.1101/sqb.1980.044.01.059. [DOI] [PubMed] [Google Scholar]
- Small J., Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology. 1969 Dec;39(4):653–665. doi: 10.1016/0042-6822(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Van Ormondt H., Maat J., De Waard A., Van der Eb A. J. The nucleotide sequence of the transforming HpaI-E fragment of adenovirus type 5 DNA. Gene. 1978 Dec;4(4):309–328. doi: 10.1016/0378-1119(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Houweling A. Transformation with specific fragments of adenovirus DNAs. II. Analysis of the viral DNA sequences present in cells transformed with a 7% fragment of adenovirus 5 DNA. Gene. 1977;2(3-4):133–146. doi: 10.1016/0378-1119(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Doerfler W. Patterns of integration of viral DNA in adenovirus type 2-transformed hamster cells. J Mol Biol. 1981 Apr 5;147(2):227–246. doi: 10.1016/0022-2836(81)90439-3. [DOI] [PubMed] [Google Scholar]
- Visser L., Reemst A. C., van Mansfeld A. D., Rozijn T. H. Nucleotide sequence analysis of the linked left and right hand terminal regions of adenovirus type 5 DNA present in the transformed rat cell line 5RK20. Nucleic Acids Res. 1982 Apr 10;10(7):2189–2198. doi: 10.1093/nar/10.7.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., Wassenaar A. T., van Maarschalkerweerd M. W., Rozijn T. H. Arrangement of integrated viral DNA sequences in cells transformed by adenovirus types 2 and 5. J Virol. 1981 Sep;39(3):684–693. doi: 10.1128/jvi.39.3.684-693.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., van Maarschalkerweerd M. W., Rozijn T. H., Wassenaar A. D., Reemst A. M., Sussenbach J. S. Viral DNA sequences in adenovirus-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):541–550. doi: 10.1101/sqb.1980.044.01.056. [DOI] [PubMed] [Google Scholar]
- Younghusband H. B., Tyndall C., Bellett A. J. Replication and interaction of virus DNA and cellular DNA in mouse cells infected by a human adenovirus. J Gen Virol. 1979 Nov;45(2):455–467. doi: 10.1099/0022-1317-45-2-455. [DOI] [PubMed] [Google Scholar]