Abstract
Arabidopsis (Arabidopsis thaliana) overexpressing glycolate oxidase (GO) in chloroplasts accumulates both hydrogen peroxide (H2O2) and glyoxylate. GO-overexpressing lines (GO plants) grown at 75 μmol quanta m−2 s−1 show retarded development, yellowish rosettes, and impaired photosynthetic performance, while at 30 μmol quanta m−2 s−1, this phenotype virtually disappears. The GO plants develop oxidative stress lesions under photorespiratory conditions but grow like wild-type plants under nonphotorespiratory conditions. GO plants coexpressing enzymes that further metabolize glyoxylate but still accumulate H2O2 show all features of the GO phenotype, indicating that H2O2 is responsible for the GO phenotype. The GO plants can complete their life cycle, showing that they are able to adapt to the stress conditions imposed by the accumulation of H2O2 during the light period. Moreover, the data demonstrate that a response to oxidative stress is installed, with increased expression and/or activity of known oxidative stress-responsive components. Hence, the GO plants are an ideal noninvasive model system in which to study the effects of H2O2 directly in the chloroplasts, because H2O2 accumulation is inducible and sustained perturbations can reproducibly be provoked by exposing the plants to different ambient conditions.
Reactive oxygen species (ROS) include partially reduced forms of oxygen such as hydrogen peroxide (H2O2), hydroxyl radical (OH·), and superoxide anion (O2−·) and the excited state of the oxygen molecule, singlet oxygen (1O2; Apel and Hirt, 2004). They are inevitable by-products of aerobic chemical reactions that take place during normal cell metabolism (e.g. in chloroplasts, mitochondria, and peroxisomes). Examples are the generation of superoxide radicals by one-electron reduction of molecular oxygen by PSI and their rapid conversion to H2O2 by superoxide dismutase (SOD) or the generation of H2O2 by the peroxisomal glycolate oxidase reaction linked to photorespiration.
Production of ROS is dramatically enhanced in response to adverse environmental conditions, often resulting in an excessive demand of the cellular antioxidant machinery, for example, when plants are exposed to sudden increases in light intensity. Under such conditions, the electron carriers are overreduced and the triplet state of P680 is favored, leading to photoinhibition of PSII and the production of 1O2 (Hideg et al., 2002). In response to high light stress but also to wounding or infection, Arabidopsis (Arabidopsis thaliana) accumulates H2O2 specifically in the vascular bundles, where the major source of it is the chloroplast (Orozco-Cárdenas et al., 2001; Fryer et al., 2003; Chang et al., 2004).
ROS can cause oxidative damage to proteins, DNA, and lipids, leading to irreversible damage and ultimately to tissue necrosis (Halliwell, 2006). Plant cells evolved nonenzymatic and enzymatic mechanisms to limit the action of ROS. Nonenzymatic scavenging systems include ascorbate, glutathione, tocopherols, flavonoids, alkaloids, and carotenoids, with ascorbate being the most important reducing substrate for H2O2 detoxification (Noctor and Foyer, 1998; Asada, 1999). Enzymatic antioxidant mechanisms include SOD, ascorbate peroxidase (APX), glutathione peroxidase, and catalase (CAT). SOD, APX, and glutathione peroxidase are encoded by multiple genes in Arabidopsis, and the isoforms are localized to different subcellular compartments (Kliebenstein et al., 1998; Apel and Hirt, 2004; Iqbal et al., 2006). In contrast, CAT is basically found in peroxisomes (Apel and Hirt, 2004).
On the other hand, ROS can act as signaling molecules. At high concentrations, H2O2 induces cell death (Dat et al., 2003), while at low concentrations, it can act as a messenger molecule involved in acclimatory signaling (Karpinski et al., 1999; Dat et al., 2000). Some of these studies pointed to changes in the transcriptome and proteome of tobacco (Nicotiana tabacum) and Arabidopsis, indicating that a flow of information may exist from the site of ROS action to the nucleus (Desikan et al., 2001; Pnueli et al., 2003; Vandenabeele et al., 2003; Bechtold et al., 2008). In addition, chloroplast-generated O2·− resulting from the application of methyl viologen (MV) during active photosynthesis in Arabidopsis leaves caused a strong induction of genes encoding various transcription factor families and signaling pathways, including receptor-like kinases (Scarpeci et al., 2008).
As mentioned above, peroxisomes are the major site of H2O2 generation in C3 plants in the context of the C2 oxidative photosynthetic cycle, also known as the photorespiratory cycle or photorespiration (Tolbert, 1997; Wingler et al., 2000). Due to the oxygenase activity of Rubisco, glycolate 2-phosphate is formed within the chloroplasts, which is dephosphorylated and subsequently oxidized to glyoxylate by glycolate oxidase (GO) in the peroxisomes, with the concomitant production of H2O2 (Nishimura et al., 1983). H2O2 is then detoxified by the action of the peroxisomal CAT.
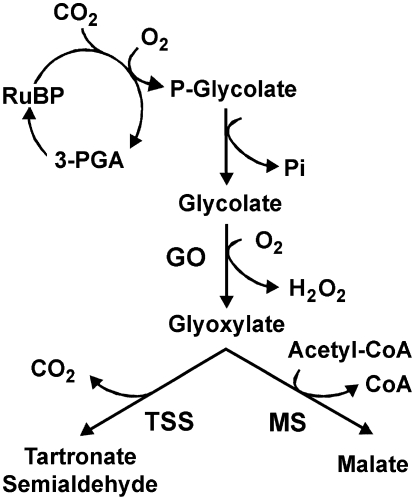
To assess the effects of metabolically generated H2O2 in chloroplasts, transgenic Arabidopsis plants were generated in which the peroxisomal GO was targeted to the chloroplasts (GO plants, Fig. 1). An exhaustive characterization of the GO plants indicated that the antioxidant machinery of chloroplasts is swamped by the light-induced production of H2O2 in these plants and that its accumulation is responsible for the observed GO phenotype. Moreover, the changes in transcript levels and activities of known oxidative stress-responsive components prompt us to propose the GO lines as an inducible system in which to study oxidative stress phenomena. Finally, the amount of H2O2 produced in the GO plants could be controlled by changing the conditions of growth, thus making the GO plants a challenging model in which to study the action of plastid-produced H2O2 as a signal molecule.
Figure 1.
Metabolic generation of H2O2 in chloroplasts through the action of GO. The further metabolization of glyoxylate by TSS or MS is also shown. 3-PGA, 3-Phosphoglycerate.
RESULTS AND DISCUSSION
Generation of Transgenic Lines Expressing GO in Chloroplasts
GO-overexpressing lines were produced by transforming Arabidopsis plants with a plasmid encoding GO without the peroxisomal targeting sequence but containing a transit peptide to direct the protein to chloroplasts. GO plants showed between 20% and 50% higher GO activities than wild-type plants (Fig. 2A). Southern-blot analysis indicated that the lines GO5, GO16, and GO20 contained one transgene insertion, while the line GO14 contained two insertions (data not shown). All transgenic lines analyzed in this work were nonsegregating T3 plants.
Figure 2.
Enzymatic activities in leaves of transgenic and wild-type (wt) plants. A and B, GO (A) and MS (B) activities. The values presented are means ± se of at least eight plants in each case. All transgenic lines showed significant differences from wild-type values calculated by Student's t test (P < 0.05). C, The presence of the TSS protein in TSS and GO-TSS lines is confirmed by western-blot analysis using specific antibodies. Ec, E. coli extract expressing recombinant TSS.
Generation of GO-MS- and GO-TSS-Overexpressing Lines
Line GO5 was further transformed with a plasmid encoding malate synthase (MS) from pumpkin (Cucurbita maxima) cotyledons without the putative C-terminal peroxisomal targeting sequence or, alternatively, a bacterial tartronate-semialdehyde synthase (TSS). Both constructs contained an N-terminal plastid targeting signal sequence to direct the corresponding proteins to chloroplasts (see “Materials and Methods”). MS and TSS catalyze the conversion of glyoxylate, the product of the GO reaction, into malate and tartronate-semialdehyde, respectively (Fig. 1). In the double transformants, GO activities were in the range of the parental GO line (Fig. 2A). The line GO-MS2 contained one MS transgene insertion, while lines GO-MS11 and GO-MS14 contained two insertions (data not shown). MS activity was detected in all transgenic lines, and, as expected, no activity was found in wild-type leaves (Fig. 2B).
All GO-TSS lines contained one TSS gene insertion. The TSS activity could not be measured; thus, the presence of the overexpressed protein was confirmed by western-blot analysis using specific antibodies raised against recombinant TSS. As shown in Figure 2C, a band of the expected apparent molecular mass could be detected in the transgenic lines. It is worth mentioning that this band had the same apparent molecular mass as the purified recombinant mature protein (approximately 65 kD; Fig. 2C), indicating that the precursor protein containing the transit peptide is correctly cleaved in vivo, leading to the mature protein inside the plastids. In addition, an in vitro stromal processing assay revealed that the GO and TSS precursor proteins are cleaved by stromal proteases; thus, they possess functional plastid transit sequences (Supplemental Fig. S1A). Moreover, MS was highly active in isolated chloroplasts of MS-expressing plants, while negligible levels were determined in wild-type plants, demonstrating the correct localization of the MS protein (Supplemental Fig. S1B).
H2O2 Accumulation Is Responsible for the GO Phenotype
Homozygous GO plants growing at moderate photon fluxes (75 μmol quanta m−2 s−1) and at ambient CO2 concentration (380 μL L−1) were smaller than wild-type plants, presenting a reduced rosette diameter (Figs. 3A and 4A) and pale-green rosettes (Fig. 3A). Under these conditions, the GO plants presented a retarded flowering time, except for GO5 plants, which showed a similar flowering time as the wild type (Fig. 4B). The level of glyoxylate was increased in the GO plants compared with the wild-type plants (Fig. 3B). For H2O2 detection, 3,3′-diaminobenzidine (DAB) staining was performed in leaves of wild-type and transgenic lines. In contrast to the wild type, a clear H2O2 accumulation was observed in lines overexpressing GO after exposure to 200 μmol quanta m−2 s−1 (Fig. 3C), while no differences in DAB staining patterns were observed in leaves of plants grown at low photon fluxes (30 μmol quanta m−2 s−1; data not shown).
Figure 3.
Phenotypes of GO, GO-TSS, and GO-MS lines. A, Rosette phenotypes of plants grown at ambient atmospheric CO2 concentration (380 μL L−1) in different light conditions. wt, Wild type; μE, μmol quanta m−2 s−1. B, Glyoxylate content in leaf extracts of transgenic and wild-type plants. FW, Fresh weight. C, DAB staining of transgenic and wild-type leaves to assess H2O2 accumulation. Plants were grown for 14 d at 75 μmol quanta m−2 s−1 and transferred for 10 d to 200 μmol quanta m−2 s−1 during the light period. Brown deposits under bright-field illumination indicate the presence of H2O2 in all lines expressing GO.
Figure 4.
Growth and photosynthetic parameters of the GO lines. A and B, Rosette diameter (A) and flowering time (B) of plants grown at 75 μmol quanta m−2 s−1 and at ambient atmospheric CO2 concentration (380 μL L−1). C, ETR of plants grown for 35 d at 75 μmol quanta m−2 s−1 and at ambient atmospheric CO2 concentration (380 μL L−1). D, ETR of plants grown for 14 d at 75 μmol quanta m−2 s−1 and at ambient atmospheric CO2 concentration (380 μL L−1) and then transferred to 4,000 μL L−1 CO2 for 21 d. The values presented in A, C, and D are means ± se of at least eight plants each. The values presented in B were obtained by counting the number of bolted plants (70 plants per line in total) at the end of each week. Asterisks indicate significant differences from wild-type (wt) values calculated by Student's t test (P < 0.05). A representative experiment from two biological replicates is presented in each case.
Chlorophyll fluorescence indicated that the photosynthetic electron transport rate (ETR; Fig. 4C) was markedly decreased in GO plants. In addition, the Fv/Fm parameter, which indicates the maximum quantum efficiency of PSII (thus, a decrease in this ratio is an indicator of photoinhibition of photosynthesis), was estimated from chlorophyll fluorescence. No differences in Fv/Fm were observed between the genotypes at moderate light intensities of growth (0.81 ± 0.07, 0.76 ± 0.02, and 0.79 ± 0.01 for the wild-type, GO5, and GO-MS11 plants, respectively). On the other hand, GO plants grown at low light intensities (30 μmol quanta m−2 s−1) and subsequently exposed for 6 h to high light intensities (600 μmol quanta m−2 s−1) showed enhanced photoinhibition, with Fv/Fm of 0.67 ± 0.03, 0.55 ± 0.02, and 0.52 ± 0.03 for the wild-type, GO5, and GO-MS11 plants, respectively. Before high light treatment, the Fv/Fm ratios obtained were similar for all genotypes (0.77 ± 0.01, 0.78 ± 0.01, and 0.77 ± 0.01 for the wild-type, GO5, and GO-MS11 plants, respectively). Moreover, under high light conditions, the GO plants showed bleaching and developed severe oxidative lesions after long-term exposure to high light (Fig. 3A).
In order to suppress the flux through the GO by inhibiting photorespiration, 14-d-old plants grown under normal conditions (75 μmol quanta m−2 s−1 and 380 μL L−1 CO2) were transferred to high CO2 conditions (4,000 μL L−1). After 7 d of growth at high CO2, the GO plants were as green as the wild-type plants, and after another 14 d, the rosette diameter of the GO plants (Fig. 4A) and the photosynthetic ETRs (Fig. 4D) resembled those of the wild type. Furthermore, growth at a low light intensity (30 μmol quanta m−2 s−1 and 380 μL L−1 CO2) resulted in neither apparent phenotypic differences between GO and wild-type plants (Fig. 3A) nor in the accumulation of H2O2 (data not shown). Interestingly, the GO-TSS and GO-MS lines presented the same phenotypic characteristics as the GO plants under all conditions (Fig. 3, A and C), but the glyoxylate content of all double overexpression lines was similar to that of the wild type (Fig. 3B). These results strongly indicate that TSS and MS were able to further metabolize glyoxylate produced by the action of GO and that the observed damages were due to the accumulation of H2O2 rather than that of glyoxylate.
GO Plants Accumulate Less Starch in High Light Conditions
We then evaluated the capacity of the transformants to accumulate starch at the end of the light period. No evident differences in starch accumulation among the GO, GO-MS, and wild-type plants were observed when plants were grown under moderate light intensity and ambient CO2 concentration (380 μL L−1; Fig. 5A). On the contrary, the GO and GO-MS plants showed decreased starch accumulation under high light conditions (Fig. 5B), a feature that was not observed when plants were grown under nonphotorespiratory conditions (i.e. high CO2 concentrations; Fig. 5C). These results and the lower growth rates of the GO and GO-MS plants in conditions at which the oxygenase activity of Rubisco becomes important (Fig. 3A) are probably due to enhanced photoinhibition. In addition to this, the repression of genes encoding photosynthetic components by H2O2 (Desikan et al., 2001; Vandenabeele et al., 2003; Scarpeci and Valle, 2008) and the direct damage or inhibition of enzyme activities involved in CO2 assimilation and energy metabolism by H2O2 (Verniquet et al., 1991; Palatnik et al., 1999; Asada, 2006; Baxter et al., 2007) can also explain these observations. Moreover, Scarpeci and Valle (2008) showed that in plants treated with MV, most of the genes involved in starch degradation through the phosphorolytic pathway, the triose-phosphate/phosphate translocator, and genes involved in starch and Suc synthesis were repressed, while genes involved in starch breakdown by the hydrolytic pathway and those involved in Suc degradation were induced.
Figure 5.
Accumulation of starch in leaves of transgenic and wild-type (wt) plants. A, Plants were grown for 42 d at 75 μmol quanta m−2 s−1 and at a CO2 concentration of 380 μL L−1. B, Plants were grown for 35 d at 75 μmol quanta m−2 s−1 and at a CO2 concentration of 380 μL L−1 and then transferred for 7 d to 600 μmol quanta m−2 s−1. C, Plants were grown for 42 d at 75 μmol quanta m−2 s−1 and at a CO2 concentration of 4,000 μL L−1. In all cases, plants were assayed for starch content using iodine staining 6 h after the onset of the light period. μE, μmol quanta m−2 s−1.
GO Plants Possess Enhanced Antioxidant Enzyme Activities
The antioxidant machinery of GO plants was analyzed by determining APX and CAT activities in leaf extracts and SOD activity by native-PAGE. In comparison with wild-type plants, the GO plants showed a significantly enhanced total APX activity by approximately 26% and 10% when grown at moderate light (75 μmol quanta m−2 s−1) and after 6 h of exposure to higher light intensities (200 μmol quanta m−2 s−1), respectively (Fig. 6A). No significant change in total CAT activity was observed at 75 and 200 μmol quanta m−2 s−1 in GO plants compared with wild-type plants (Fig. 6B). It is worth mentioning that Miyake and Asada (1996) showed that APX is rapidly inactivated by traces of H2O2 in the absence of ascorbate. These contrasting results can be reconciled considering that their results were obtained with a purified APX isoform from spinach (Spinacia oleracea) thylakoids, while in our study the total APX activity was determined in Arabidopsis leaves. On the other hand, in chloroplasts, ascorbate contents are high (approximately 20–300 mm; Smirnoff, 2000), which is the most likely reason why APX in GO plants is not so effectively inactivated by the H2O2 generated by GO.
Figure 6.
Analysis of antioxidant enzyme activities in wild-type (wt) and GO5 plants. A and B, APX (A) and CAT (B) activity quantification of leaf extracts from 21-d-old plants grown at 75 μmol quanta m−2 s−1 or exposed for 6 h to 200 μmol quanta m−2 s−1. Plants were grown under ambient atmospheric CO2 concentration (380 μL L−1). The values presented are means ± se of over three replicate experiments. Significant differences in the mean values from the wild type according to the Student's t test are indicated with asterisks (P < 0.05). μE, μmol quanta m−2 s−1. C, In gel test for SOD activity using 15 μg of total protein leaf extract from wild-type and GO5 plants grown at 75 μmol quanta m−2 s−1. chl, Chloroplastic; cyt, cytosolic.
When foliar extracts were subjected to native-PAGE and stained for SOD activity, bands corresponding to the manganese SOD (MnSOD; slowest mobility; Kliebenstein et al., 1998), iron SOD (FeSOD; next slowest mobility; Kliebenstein et al., 1998), and copper-zinc SOD (CuZnSOD; the fastest migrating as a diffuse doublet; Kliebenstein et al., 1998) isoforms were detected in wild-type and GO5 plants grown under 75 μmol quanta m−2 s−1 (Fig. 6C). The intensity of the bands corresponding to MnSOD and FeSOD was similar in both genotypes, whereas the intensity of the CuZnSOD doublet band was enhanced in the GO5 plants (Fig. 6C). Thus, the GO plants exhibited an enhanced activity of both the plastidic (lower mobility of the doublet; Kliebenstein et al., 1998) and the cytosolic (faster mobility of the doublet; Kliebenstein et al., 1998) CuZnSOD isoforms. Oxidative conditions provoked by growing these plants at 200 μmol quanta m−2 s−1 did not produce a further increment in total SOD activity (data not shown).
As Gln synthetase (GS) and 2-Cys peroxiredoxin (2-Cys Prx) were shown to be sensitive to oxidative conditions (Palatnik et al., 1999; Scarpeci et al., 2008), these enzymes were followed by western-blot analysis in leaf extracts of GO plants. Protein levels of GS1 (cytosolic), GS2 (chloroplastic), and 2-Cys Prx (chloroplastic) remained constant at moderate light intensity and after high light treatment in all GO lines assayed compared with the respective controls, indicating that these ROS target proteins remained unaltered in GO plants in these light conditions (Supplemental Fig. S2). In agreement with the higher activity of CuZnSOD determined in GO plants with respect to the wild type at moderate photon fluxes, the amount of CuZnSOD (chloroplastic) determined by western-blot analysis was also increased in the GO plants in comparison with control MS plants (Supplemental Fig. S2), indicating that the increased activity followed by the in gel assays is at least a consequence of higher CuZnSOD accumulation.
Taken together, these results indicate that the GO phenotype is a direct consequence of the transgenic GO activity. Production of glycolate in the chloroplasts only takes place during the light period as a result of the oxygenase activity of Rubisco. Under nonphotorespiratory conditions (e.g. very low light intensities or high CO2 concentrations), the production of glycolate inside the chloroplasts is minimized and the contents of H2O2 and glyoxylate should remain at very low levels because no substrate for GO is available, thus explaining the normal development of GO plants under these conditions. On the contrary, as under moderate light and normal ambient CO2 concentrations, photorespiration is established and GO plants accumulate H2O2 and, thus, show a retarded growth phenotype, a characteristic of plants with enhanced ROS accumulation, like transformants with decreased thylakoid-bound APX activity (Tarantino et al., 2005) or the knockout mutant of chloroplastic CuZnSOD (Rizhsky et al., 2003). Moreover, under stress situations in which photorespiration is exacerbated (e.g. high light intensities or high temperature), the huge production of H2O2 causes extensive oxidative damage to GO plants. In this way, it is possible to modulate the production of H2O2 inside the chloroplast by exposing the GO plants to defined growth conditions.
The data obtained also indicate that the GO plants sense a higher level of H2O2, since antioxidative enzyme activities were enhanced with respect to the wild type but these increments were not high enough to efficiently scavenge the excess H2O2 produced in situ. The GO phenotype and the observation that the coexpression of MS or TSS with GO reduced the glyoxylate levels to wild-type levels (Fig. 2B) but did not alleviate the GO phenotype demonstrate that the GO phenotype is a direct consequence of H2O2 accumulation and that the chloroplastic endogenous machinery in charge of detoxifying H2O2 was overloaded.
Anthocyanin Biosynthetic Genes Are Repressed in GO Plants
In order to determine the effects of high light exposure, 21-d-old plants growing at 75 μmol quanta m−2 s−1 were transferred to 600 μmol quanta m−2 s−1 and the phenotypic changes were recorded during the following 24 d. As a response to a long-term high light exposure, the leaves of wild-type plants developed a brown-purple coloration due to the accumulation of anthocyanins, and a high level of expression of selected genes involved in the phenylpropanoid biosynthetic pathway (e.g. PHENYLALANINE AMMONIA-LYASE1 [PAL1], CHALCONE SYNTHASE [CHS], and DIHYDROFLAVONOL REDUCTASE [DFR]) was observed after 6 h of exposure to high light (Fig. 7). On the contrary, the GO plants showed intense bleaching and photooxidative lesions. Moreover, the accumulation of anthocyanins was not visible before 15 d of treatment (Fig. 7, A and B). Accordingly, GO plants showed only low expression levels of the genes involved in the phenylpropanoid biosynthetic pathway in response to high light (Fig. 7C). No differences in the accumulation of these transcripts between wild-type and GO plants became apparent after 10 d in high light (data not shown). The GO-TSS and GO-MS lines responded similarly to long-term high light stress (data not shown).
Figure 7.
Responses of wild-type (wt) and GO lines to high light treatment. A and B, Phenotypes of the wild-type and GO plants (A) and quantification of the anthocyanin levels (expressed as corrected A535 per milligram fresh weight) at different times during growth at 600 μmol quanta m−2 s−1 and ambient CO2 concentration (380 μL L−1; B). The values are means ± se of three measurements of at least six plants each. FW, Fresh weight. C, Semiquantitative RT-PCR analysis of PAL1, CHS, and DFR transcript levels in leaves of wild-type, GO, and GO-MS plants after 6 h of high light exposure. PCR products of 251 bp (PAL1), 448 bp (CHS), and 450 bp (DFR) were amplified using 33 cycles. As a loading control, a 521-bp ACTIN2 cDNA fragment was amplified using 29 cycles.
Anthocyanins have been shown to act as a “sunscreen,” protecting cells from high light damage by absorbing blue-green light, thereby protecting the tissues from photoinhibition (Steyn et al., 2002; Nagata et al., 2003). In agreement with this, the retarded accumulation of anthocyanins of the GO plants can also contribute to the almost arrested growth of these plants during the first 14 d in high light. Nevertheless, they were able to flower and produced viable seeds.
Recently, Vanderauwera et al. (2005) showed that a transcriptional cluster including anthocyanin regulatory and biosynthetic pathway genes was strongly and rapidly induced by high light in wild-type Arabidopsis plants, while this induction was delayed in CAT-deficient plants that accumulate photorespiratory H2O2. Taking these observations into account, the retarded production of anthocyanin pigments in the GO plants can be explained by the impairment of the induction of genes involved in the biosynthesis of anthocyanins caused by the accumulation of H2O2. The molecular mechanism of this regulatory action of H2O2, however, remains to be elucidated.
Changes in the Transcription of Oxidative Stress Markers
As shown above, photorespiratory conditions like exposure to high light intensities for 6 h resulted in increased levels of H2O2 in GO plants. To analyze if transcriptional changes of the genes involved in oxidative stress occurred in the GO and GO-MS plants under these conditions, some marker genes were selected based on evidence from expression profiles obtained after MV treatment (Scarpeci et al., 2008). Those authors showed that within the first 2 h of 50 μm MV treatment, several genes were differentially expressed. Among them were the small heat shock protein (Hsp17.6B-CI or sHSP; At2g29500), the transcription factor WRKY30 (At5g24110), and the chloroplastic localized ferritin (Fer1; At5g01600). Expression analysis by quantitative real time (RT)-PCR indicated a clear accumulation of these transcripts in GO plants at high light intensities, in contrast to the control plants expressing MS (Fig. 8). These results also showed that the H2O2 produced after 6 h of high light exposure was sufficient to induce changes in the gene expression of GO plants.
Figure 8.
Responses of GO, GO-MS, and MS plants to high light treatment. Changes in the expression levels of selected H2O2-induced markers were analyzed by quantitative RT-PCR in 21-d-old plants grown under 75 μmol quanta m−2 s−1 (moderate light [ML]) and at ambient atmospheric CO2 concentration (380 μL L−1) and exposed for 6 h to 600 μmol quanta m−2 s−1 (high light [HL]) relative to control plants kept at 75 μmol quanta m−2 s−1. Data are means ± se of three individual experiments run in triplicate. Asterisks indicate that values are significantly different from those of the control MS plants as determined by Student's t test (P < 0.05).
CONCLUSION
GO plants show retardation in growth and flowering time. Similar features were observed in cat2 and apx1 mutants (Pnueli et al., 2003; Queval et al., 2007). It is feasible that the enhanced levels of H2O2 in these plants affected the expression of transcription factors involved in the regulation of plant growth and flowering. Because the growth suppression in GO plants was dependent on photorespiratory conditions, it is feasible that this effect is directly linked to H2O2 accumulation in cells. On the other hand, GO plants showed a reduced expression of genes involved in anthocyanin biosynthesis, such as PAL1, CHS, and DFR. This could be related to a suppression of the senescence program in which anthocyanin levels are elevated (Hoch et al., 2001). Both effects could be part of a mechanism that drives the acclimatory resistance in plants exposed to sublethal doses of H2O2 that did not affect the viability of Arabidopsis plants but may play a regulatory role in the cellular network, leading to altered gene expression and the reconfiguration of metabolism in order to adapt to oxidative conditions.
ROS are key molecules in the regulation of plant development, stress responses, and programmed cell death. Previous studies indicated that, depending on the type of ROS or its subcellular production site, different cellular responses are provoked (Gadjev et al., 2006). The proposed selective signaling by specific ROS remained difficult to tackle experimentally, because no suitable experimental systems were available to modulate the levels of a specific ROS at a particular subcellular location and at a given time. Previously reported H2O2 transcriptome studies relied on the use of cell suspensions and exogenously applied H2O2 (Desikan et al., 2001). External H2O2 application may activate additional signal transduction pathways, which could complicate the analysis. Later, transgenic plants with deficiencies in antioxidant enzymes were used to address this question (Gadjev et al., 2006). Pnueli et al. (2003) described changes in the transcriptome of Arabidopsis plants deficient in cytosolic APX, and Vandenabeele et al. (2004) used CAT-deficient plants in which H2O2 is generated in peroxisomes under photorespiratory conditions. The GO plants are unique in that H2O2 is generated inside the chloroplast even in the absence of biotic or abiotic stress or oxidants. Furthermore, the amount of H2O2 produced in these lines could be controlled by modulating light intensities, CO2 levels, or both.
It has been shown that the high antioxidant capacity of the cell ensures that ROS signaling is a localized event under physiological conditions and that even if H2O2 can diffuse freely inside the cell, the molecule has to interact with sensors in close proximity to the site of generation (Buchanan and Balmer, 2005). Moreover, it was proposed that limiting the accumulation of H2O2 to its site of production allows the initiation of distinct signals depending on its subcellular origin (Mullineaux and Karpinski, 2002). By contrast, breakdown in the integrity of this spatial component, such that H2O2 diffuses into other subcellular compartments, would promote oxidative stress and trigger signaling associated with cell death (Mullineaux et al., 2006). Therefore, we demonstrate that GO plants represent an excellent and challenging tool in which to study the action of H2O2 as a signal molecule in chloroplasts as well as the consequences of oxidative stress from a holistic approach.
MATERIALS AND METHODS
PCR Amplification of the Transgenes
The cDNAs corresponding to GO from Arabidopsis (Arabidopsis thaliana) leaves (At3g14420) and MS from pumpkin (Cucurbita maxima) cotyledons (X56948) and the gene corresponding to the bacterial TSS (NP_415040) were amplified using Platinum Pfx DNA polymerase (Invitrogen) and cloned into pGEM T-Easy (Promega) or, alternatively, pCR-Blunt II-TOPO (Invitrogen). In the case of GO and MS, the nucleotides encoding the last amino acids (A/SRL), which presumably represent peroxisomal targeting signals (Horng et al., 1995), were omitted. The following primer combinations were used: GOfow (5′-TACAATTGGAGATCACTAACGTTACCGAGT-3′) and GOrev1 (5′-TGGGACACTCCACGTCCTTAGTCTAGACTAGTA-3′), MSfow (5′-ACACCGGTCGCTGGGAATGTATTCTGAATCGGCA-3′) and MSrev1 (5′-CACATAGGCATACATCATCCCAGGTGAGTCGACGTT-3′), and TSSfow (5′-TAGAATTCGCAAAAATGAGAGCCGTTGAC-3′) and TSSrev (5′-CGGTCGACTTATTCATAGTGCATGAAGCAGGT-3′). To follow the cloning strategy, primers were designed to introduce unique MunI and SpeI sites at the 5′ and 3′ ends of GO, AgeI and SalI sites at the 5′ and 3′ ends of MS, and a unique EcoRI restriction site at the 5′ end of TSS. In order to target GO to chloroplasts, the cDNA corresponding to the stromal targeting presequence from the Arabidopsis phosphoglucomutase (At5g51820; 225 bp) was amplified by PCR using the following primers: PGMfow (5′-TAGGTACCCAATCAACAATGACGTCGACCTAC-3′) and PGMrev (5′-GAGATTAAATCGTTGCCGACGAAGCAATTGTA-3′). The oligonucleotides were designed to introduce unique KpnI and MunI restriction sites at the 5′ and 3′ ends. The fragment obtained was cloned upstream of the GO cDNA. To target MS to the chloroplast, a fragment containing the tomato (Lycopersicon esculentum)-rbcS3C (Rubisco small subunit; X66072) promoter (715 bp) and transit peptide (172 bp) was amplified by PCR using genomic DNA and the following primers: rbcS3Cfow (5′-ACGAGCTCATCCAGAATTGGCGTTGGATTA-3′) and rbcS3Crev (5′-AGCAACGGTGGAAGAGTCAGTTGCAACCGGTAT-3′). The primers were designed to introduce unique SacI and AgeI restriction sites at the 5′ and 3′ ends. The fragment obtained was inserted upstream of the MS coding regions. In order to target TSS to the chloroplast, the cDNA fragment coding for the stromal targeting presequence from soybean (Glycine max)-rbcs (Rubisco small subunit; V00458; 258 bp) was amplified by PCR using the following primers: TPfow (5′-TAGGTACCAGCTTGGATATCTGGCAGCAGAAA-3′) and TPrev (5′-TACAATTGCATAGAAGCCATCATGCATT-3′). The oligonucleotides were designed to introduce a unique MunI restriction site at the 3′ end. The fragment obtained was cloned upstream of the TSS coding region. All plasmid constructions were sequenced using the PRISM fluorescent dye-terminator system (Applied Biosystems) to exclude any possible mutation that could be created by the polymerase action.
Construction of Binary Vectors, Transformation of Arabidopsis, and Selection of Transformants
To direct the expression in Arabidopsis, the DNAs encoding the plastidic precursor of the enzymes were cloned into a modified version of the binary vector pGreenII (Hellens et al., 2000; Fahnenstich et al., 2007) using different strategies. In the case of MS, the cauliflower mosaic virus 35S promoter was cut out of the vector and the tomato-rbcS3C promoter was used to direct the expression. Selection marker genes that confer kanamycin (Kan) or, alternatively, hygromycin (Hyg) resistance were cloned into the StuI site within the basic pGreenII vector. GO was cloned into the pGreenII 35S-Kan vector, MS into the pGreenII rbcS3C-Hyg vector, and TSS into the pGreenII 35S-Hyg vector. The resulting plasmids, named 35S:GO, rbcS3C:MS, and 35S:TSS, were electroporated into Agrobacterium tumefaciens GV3101 bearing the helper plasmid pSoup and used to transform Arabidopsis plants (ecotype Colombia) via vacuum infiltration (Bechtold et al., 1993). Transformed seeds were selected by resistance to either kanamycin or hygromycin. Plants containing the transgenes were allowed to self-pollinate. All analyses were performed with nonsegregating homozygous T3 transgenic lines.
Plant Growth Conditions and Stress Treatment
Seeds of Arabidopsis ecotype Columbia and the transgenic lines were sown on soil and kept in darkness for 4 d at 4°C to synchronize germination. Seedlings were then transferred to pots containing three parts soil (Gebr. Patzer) and one part vermiculite (Basalt Feuerfest) and grown under a 16-h-light/8-h-dark regime at photosynthetically active photon flux densities (PPFDs) of 30, 75, or alternatively 200 μmol quanta m−2 s−1 at 22°C day/18°C night temperatures. Growth under high light conditions was conducted using a PPFD of 600 μmol quanta m−2 s−1. For (RT)-PCR and western-blot analyses, plants grown for 21 d at 75 μmol quanta m−2 s−1 were exposed for 6 h to high light (600 μmol quanta m−2 s−1) or kept at 75 μmol quanta m−2 s−1 (control plants). For APX and CAT activities, plants grown for 21 d at 75 μmol quanta m−2 s−1 were exposed for 6 h to 200 μmol quanta m−2 s−1 or kept at 75 μmol quanta m−2 s−1 (control plants). After the treatments, samples were immediately frozen in liquid nitrogen and stored at −80°C until use. For growth at high CO2 concentrations, 14-d-old plants grown at 75 μmol quanta m−2 s−1 and ambient CO2 conditions were transferred to a chamber with a CO2 concentration of 4,000 μL L−1 and the same PPFD.
The selection of transgenic plants was conducted on solid Murashige and Skoog medium containing 50 μg mL−1 (w/v) kanamycin or 30 μg mL−1 hygromycin at a PPFD of 75 μmol quanta m−2 s−1. After 14 d of growth, the selected seedlings were transferred to soil.
Heterologous Expression of TSS, Purification of the Recombinant Proteins, and Preparation of Antiserum against TSS
The bacterial gene encoding TSS was amplified by PCR using Escherichia coli DH5α genomic DNA as template. The following primer combinations, which introduced unique NdeI and BamHI restriction sites at the 5′ and 3′ ends, respectively, were used: TSSpETfow (5′-GTCATATGGCAAAAATGAGAGCCGTTGACG-3′) and TSSpETrev (5′-GAGGATCCTTATTCATAGTGCATGAAGCAG-3′). The amplified products were cloned into pCR-Blunt II-TOPO (Invitrogen) and sequenced using the PRISM fluorescent dye-terminator system (Applied Biosystems). The plasmids were digested with NdeI and BamHI and subcloned into the pET16b expression vector (Novagen).
Protein expression was induced with isopropyl β-d-thiogalactopyranoside. The expressed proteins were purified by nickel-nitrilotriacetic acid agarose chromatography (Qiagen) followed by 10% SDS-PAGE. Gel-purified proteins were used as antigens for the production of polyclonal antibodies in rabbits by Eurogentec.
Protein Detection by Western-Blot Analysis
Protein extracts were prepared from 21-d-old plants by grinding leaf material in liquid nitrogen and resuspending it in 100 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 2 mm EDTA, 10% (v/v) glycerol, and 10 mm 2-mercaptoethanol in the presence of a protease inhibitor cocktail (Sigma-Aldrich). The homogenates were clarified by centrifugation, and the supernatants were separated by 10% SDS-PAGE (Laemmli, 1970). Proteins were electroblotted onto polyvinylidene difluoride membrane (Bio-Rad) and detected using specific polyclonal antibodies against TSS, GS (Scarpeci et al., 2007), chloroplastic Hsp70, 2-Cys Prx, and chloroplastic CuZnSOD. Bound antibodies were located by linking to alkaline phosphatase-conjugated goat anti-rabbit IgG according to the manufacturer's instructions (Sigma-Aldrich). Alkaline phosphatase activity was detected colorimetrically.
Stromal Protease in Vitro Processing Assay
Intact chloroplasts were prepared from 100 mg of 28-d-old leaves of pea (Pisum sativum). Small pieces of leaves were homogenized in medium A (330 mm sorbitol, 5 mm ascorbate, 2 mm EDTA, 1 mm MgCl2, 1 mm MnCl2, 0.5 mm KH2PO4, 4 mm Cys, 50 mm MES/KOH, pH 6.1, and 0.05% [w/v] bovine serum albumin). The homogenate was filtered with Miracloth filters (Calbiochem) and centrifuged for 1 min at 4,000g at 4°C. The precipitate was resuspended and washed twice in medium B (330 mm sorbitol, 50 mm HEPES-KOH, pH 7.8, and 0.1 mm phenylmethylsulfonyl fluoride), and the chloroplast pellet was resuspended in 20 mm HEPES, pH 8.0, and incubated for 15 min at 4°C. After a 20-min centrifugation step at 10,000g at 4°C, the supernatant containing the stromal proteases was ready for the in vitro digestion assay. The complete coding sequences of the GO and TSS cloned into pGEM T-Easy were transcribed and translated using the TNT coupled reticulocyte lysate system (Promega) and [35S]Met as labeled amino acid according to the manufacturer's instructions. The in vitro processing assay with stromal extract (20 μL final volume) contained 10 mm HEPES, pH 8.5, 0.4% chloramphenicol, 2 mm Met, and 4 μL of stromal extract. The reactions were allowed to proceed for 3 h at 28°C. The aliquots taken at different incubation times were analyzed by SDS-PAGE and fluorography. The gels were exposed on PhosphorScreen membranes for 5 d (Kodak, Sigma-Aldrich) and were analyzed in the PhosphorImager (Storm 860; Molecular Dynamics).
Preparation of Leaf Extracts, Chloroplast Isolation, and Enzymatic Measurements
GO
Leaf material was homogenized in the presence of liquid nitrogen, resuspended in 100 mm HEPES (pH 7.2), 1 mm EDTA, and 10 mm 2-mercaptoethanol, and the homogenate was clarified by centrifugation. Aliquots of 10 μL were used for the enzymatic assays following the method of Yamaguchi and Nishimura (2000) with the following modifications. The reaction medium contained 100 mm triethanolamine (pH 7.8), 3 mm EDTA, 0.75 mm oxidized glutathione, and 4 mm phenylhydrazine. The reaction was started by the addition of 2.3 mm sodium glycolate and followed spectrophotometrically at 320 nm. One unit is defined as the amount of enzyme catalyzing the production of 1 μmol glyoxylate-phenylhydrazone min−1 calculated from the extinction coefficient for phenylhydrazone at 324 nm of 16.8 mm−1 cm−1.
MS
Leaf material was homogenized in the presence of liquid nitrogen and resuspended in extraction buffer consisting of 50 mm Tris-HCl (pH 8.0) and 1 mm MgCl2, and the homogenate was clarified by centrifugation. Intact chloroplasts were isolated as described by Kunst et al. (1988). Pelleted chloroplasts were resuspended in 0.33 m sorbitol, 50 mm Tris-HCl (pH 8.0), and 1 mm MgCl2. Aliquots of 10 μL of the leaf extract and chloroplasts were used for the enzymatic assays. MS activity was determined following the procedure described by Smith et al. (2003). The reaction medium contained 50 mm Tris-HCl (pH 8.0), 5 mm MgCl2, 3 mm acetyl-CoA, and 1.6 mm 5,5-dithiobis 2-nitrobenzoic acid. The reaction was started by the addition of 4 mm glyoxylate and followed spectrophotometrically at 410 nm at 25°C. One unit is defined as the amount of enzyme catalyzing the production of 1 μmol 4-nitrothiolate min−1 calculated from the extinction coefficient for 4-nitrothiolate at 410 nm of 13.7 m−1 cm−1.
SOD
Extraction of leaf material for SOD activity was carried out according to a published procedure (Gupta et al., 1993). The supernatant was recovered at 4°C by centrifugation (20,000g, 20 min), and equal amounts of soluble protein (15 μg) were subjected to native-PAGE. SOD in gel activity was determined as described previously (Beauchamp and Fridovich, 1971).
Measurements of Glyoxylate Content
The determination of glyoxylate was conducted using a modification of the protocol described by Häusler et al. (1996). Leaf material (30–100 mg) was homogenized in the presence of liquid nitrogen, 500 μL of 100 mm HCl, and 0.1% phenylhydrazine and incubated at 80°C for 5 min. After chilling on ice, the samples were centrifuged at 10,000g for 2 min, and 200 μL of the supernatant was mixed with 750 μL of 18.5% HCl and 50 μL of 4% (w/v) K3Fe(CN)6. The mixture was centrifuged at 10,000g for 2 min, and the absorbance of the supernatant was measured at 520 nm exactly 8 min after the addition of K3Fe(CN)6. As a control, K3Fe(CN)6 was omitted.
RNA Isolation and PCR Analysis
Total RNA was isolated from 100 mg of leaves using the TRIzol reagent (Gibco-BRL). RNA was converted into first-strand cDNA using SuperScript II reverse transcriptase (Invitrogen). Semiquantitative RT-PCR analysis of transcripts of selected anthocyanin biosynthetic enzymes (PAL1 [At2g37040], CHS [At5g13939], and DFR [At5g42800]) was conducted in a final volume of 10 μL using 0.5 μL of the transcribed product and Taq DNA polymerase (Qiagen). To achieve specific amplification products, the primers were designed in a way that at least one intron was spanned in the genomic DNA. The pairs of primers used were as follows: PAL1fow (5′-ACACAAGAGCAACGGAGGAGGA-3′) and PAL1rev (5′-CTATTGGTAACAGTGTGAAGGTGG-3′), CHSfow (5′-ATGGTGATGGCTGGTGCTTCTTCT-3′) and CHSrev (5′-TGCCTGGTGCTGACTACCAGCTCA-3′), and DFRfow (5′-TCATCGGTTCATGGCTAGTGATG-3′) and DFRrev (5′-TGACAGGATGGATGTATTTCGTGT-3′). As control, the ACTIN2 gene was amplified using the primers Actin2for (5′-TGTACGCCAGTGGTCCTACAACC-3′) and Actin2rev (5′-GAAGCAAGAATGGAACCACCG-3′) and the following amplification conditions: 3 min of denaturation at 94°C; 29 to 33 cycles at 94°C for 30 s, 57°C for 40 s, and 72°C for 30 s; followed by 5 min at 72°C. PCR products were resolved on a 1.5% (w/v) agarose gel. Quantitative RT-PCR analyses of WRKY30 (At5g24110), sHSP (At2g29500), and Fer1 (At5g01600) transcripts were performed using the following primers: W30L (5′-CGCTGGACGATGGATTCAGTTGGAGA-3′) and W30R (5′-TCGGTTCGAGGTTTTGTATCGGCATTG-3′), sHSPL (5′-GCGATCGTGAACGCACGTGTGGA-3′) and sHSPR (5′-TCCATCTTCACATTCTCCGGCAACC-3′), and FerF (5′-CACCGCCGCTAATCCCGCTCTGTCTCC-3′) and FerR (5′-AAACTCCACCCTATCGTCTCACCTATC-3′). Tubulin transcripts were measured as internal control using the primers TubF (5′-GAGAATGCTGATGAGTGCATGG-3′) and TubR (5′-CAGGGAACCTCAGACAGCAAGT-3′). cDNAs were amplified using the Mastercycler ep Realplex2 thermocycler (Eppendorf). PCR conditions were 1 min at 95°C and 40 cycles of 15 s at 95°C, 30 s at 55°C, and 40 s at 72°C. Following amplification, products were denatured by heating from 60°C to 95°C to check amplification specificity. RT-PCR was performed using a SYBR Green fluorescence-based assay. Gene-specific cDNA amounts were calculated from threshold cycle (Ct) values, expressed relative to controls and normalized with respect to tubulin cDNA, used as internal reference. Values were normalized by an internal reference (Ctr) according to the equation ΔCt = Ct − Ctr and quantified as 2−ΔCt. A second normalization by a control (Ctc) ΔΔCt = Ct − Ctc produces a relative quantification: 2−ΔΔCt (Livak and Schmittgen, 2001).
Chlorophyll Fluorescence Parameters
Measurements of chlorophyll a fluorescence of the upper leaf surface were performed with a PAM-2000 pulse amplitude modulation chlorophyll fluorometer (Walz; Schreiber et al., 1986). Basal fluorescence (F0) was measured with modulated weak red light using leaves of plants that were dark adapted for at least 10 min. Maximal fluorescence in the dark-adapted state (Fm) and during illumination (Fm′) was induced with a saturating white light pulse (5,000 μmol m−2 s−1; duration, 0.8 s). According to Genty et al. (1989), the effective quantum yield of PSII (YPSII) was calculated as YPSII = (Fm′ − Fs)/Fm′ (where Fs is steady-state fluorescence). Based on this, the absolute rates of electron transport around PSII at a given PPFD were assessed by the formula ETR = YPSII × PPFD × 0.84 × 0.5. The factors are based on the assumptions that 84% of the incident quanta are absorbed by the leaf (factor 0.84) and that the transport of one electron by the two photosystems requires the absorption of two quanta (factor 0.5). The Fv/Fm index was calculated as (Fm − F0)/Fm.
Qualitative Assay of Starch and H2O2
Starch accumulation was determined by the iodine staining method as described by Caspar et al. (1986). Detection of H2O2 was performed by infiltrating leaves with a solution of 1 mg mL−1 DAB in MES buffer (pH 6.5) as described by Thordal-Christensen et al. (1997). H2O2 was visualized as a reddish-brown coloration. Prior to imaging, chlorophyll was removed from leaves with 70% (v/v) ethanol.
Anthocyanin Measurements
Leaf material (50–100 mg) was frozen in liquid nitrogen, and the pigments were extracted in 500 μL of 1% (v/v) HCl and 18% (v/v) 1-propanol during 3 min at 95°C. After an overnight incubation at room temperature, the samples were centrifuged for 20 min at 20,000g. The absorbance of the supernatant was measured spectrophotometrically at 535 and 650 nm, and the quantity of anthocyanins was determined by correcting the absorption at 535 nm using the Raleigh formula [A535 (corrected) = A535 − A650; Lange et al., 1971) and normalized to the fresh weight of each sample.
Statistical Analysis
Significance was determined according to Student's t test using Excel software (Microsoft).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. In vitro stromal protease digestion analyzed by SDS-PAGE and fluorography (A) and MS activity in isolated chloroplasts of transgenic plants overexpressing MS and wild-type (wt) plants (B).
Supplemental Figure S2. Western-blot analysis of cytosolic GS1 (cyt), chloroplastic GS2 (chl), chloroplastic 2-Cys Prx (chl), and chloroplastic CuZnSOD (chl) using leaf extracts of GO5, GO-MS11, and control MS4 plants grown under 75 μmol quanta m−2 s−1 (ML) and after exposing the plants for 6 h to 600 μmol quanta m−2 s−1 (HL).
Supplementary Material
Acknowledgments
We thank Andreas Weber for helpful discussions. For the generous gifts of antisera, we thank Ricardo Wolosiuk (2-Cys Prx), Eduardo Ceccarelli (chloroplastic Hsp70), and Ramiro Lascano (chloroplastic CuZnSOD).
This work was supported by the Deutsche Forschungsgemeinschaft (award to V.G.M.) and the National Agency for the Promotion of Science and Technology, Argentina (grant no. 13549 to E.M.V.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Verónica G. Maurino (v.maurino@uni-koeln.de).
The online version of this article contains Web-only data.
References
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–659 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their function. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter CJ, Redestig H, Schauer N, Repsilber D, Patil KR, Nielsen J, Selbig J, Liu J, Fernie AR, Sweetlove LJ (2007) The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiol 143 312–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44 276–287 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris Life Sci 316 1194–1199 [Google Scholar]
- Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux P (2008) Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J Exp Bot 59 121–133 [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56 187–220 [DOI] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1986) Alterations in growth, photosynthesis and respiration in a starchless mutant of Arabidopsis thaliana (L.) Heynh deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM (2004) Induction of ASCORBATE PEROXIDASE 2 expression in wounded Arabidopsis leaves does not involve known wound-signaling pathways but is associated with changes in photosynthesis. Plant J 38 499–511 [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57 779–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Beeckman T, Kangasjärvi J, Langebartels C, Inzé D, Van Breusegem F (2003) Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J 33 621–632 [DOI] [PubMed] [Google Scholar]
- Desikan R, Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnenstich H, Saigo M, Niessen M, Zanor MI, Andreo CS, Fernie AR, Drincovich MF, Flügge UI, Maurino VM (2007) Alteration of organic acid metabolism in Arabidopsis thaliana overexpressing the maize C4-NADP-malic enzyme causes accelerated senescence during extended darkness. Plant Physiol 145 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Bill L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33 691–705 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F (2006) Transcriptomic footprinting discloses specificity of reactive oxygen species signalling in Arabidopsis. Plant Physiol 141 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Gupta AS, Webb RP, Holaday AS, Allen RD (1993) Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol 103 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (2006) Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol 141 312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Bailey KJ, Lea PJ, Leegood RC (1996) Control of photosynthesis in barley mutants with reduced activities of glutamine synthetase and glutamate synthase. Planta 200 388–396 [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42 819–832 [DOI] [PubMed] [Google Scholar]
- Hideg É, Barta C, Kalai T, Hideg K, Asada K (2002) Detection of singlet oxygen and superoxide with fluorescent sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol 43 1154–1164 [DOI] [PubMed] [Google Scholar]
- Hoch WA, Zeldin EL, McCown BH (2001) Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol 21 1–8 [DOI] [PubMed] [Google Scholar]
- Horng JT, Behari R, Burke LECA, Baker A (1995) Investigation of the energy requirement and targeting signal for the import of glycolate oxidase into glyoxysomes. Eur J Biochem 230 157–163 [DOI] [PubMed] [Google Scholar]
- Iqbal A, Yabuta Y, Takeda T, Nakano Y, Shigeoka S (2006) Hydroxyperoxide reduction by thioredoxin-specific glutathione peroxidase isozymes of Arabidopsis thaliana. FEBS J 27 5589–5597 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen J, Mullineaux PC (1999) Systemic signaling and acclimatation in response to excess excitation energy in Arabidopsis. Science 284 654–657 [DOI] [PubMed] [Google Scholar]
- Kliebenstein D, Monde RA, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol 118 637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA 85 4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Lange H, Shropshire W, Mohr H (1971) An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol 47 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K (1996) Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37 423–430 [Google Scholar]
- Mullineaux PM, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5 43–48 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence of hydrogen peroxide-directed signalling in light-stressed plants. Plant Physiol 141 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du ZJ, Kikuchi S (2003) Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. J Agric Food Chem 51 2992–2999 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Akhmedov YD, Strzalka K, Akazawa T (1983) Purification and characterization of glycolate oxidase from pumpkin cotyledons. Arch Biochem Biophys 222 397–402 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49 249–279 [DOI] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vàsquez J, Ryan CA (2001) Hydrogen peroxide acts as second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Carrillo N, Valle EM (1999) The role of photosynthetic electron transport in the oxidative degradation of chloroplastic glutamine synthetase. Plant Physiol 121 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Liang H, Rozenberg M, Mittler R (2003) Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J 34 187–203 [DOI] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52 640–657 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278 38921–38925 [DOI] [PubMed] [Google Scholar]
- Scarpeci TE, Marro ML, Bortolotti S, Boggio SB, Valle EM (2007) Plant nutritional status modulates glutamine synthetase levels in ripe tomatoes (Solanum lycopersicum cv. Micro-Tom). J Plant Physiol 164 137–145 [DOI] [PubMed] [Google Scholar]
- Scarpeci TE, Valle EM (2008) Rearrangement of carbon metabolism in Arabidopsis thaliana subjected to oxidative stress condition: an emergency survival strategy. Plant Growth Regul 54 133–142 [Google Scholar]
- Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM (2008) Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol 66 361–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10 51–62 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photorespiration. Philos Trans R Soc Lond B Biol Sci 355 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CV, Huang CC, Miczak A, Russell DG, Sacchettini JC, Höner zu Bentrup K (2003) Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis. J Biol Chem 278 1735–1743 [DOI] [PubMed] [Google Scholar]
- Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155 349–361 [DOI] [PubMed] [Google Scholar]
- Tarantino D, Vannini C, Bracale M, Campa M, Soave C, Murgia I (2005) Antisense reduction of thylakoidal ascorbate peroxidase in Arabidopsis enhances paraquat-induced photooxidative stress and nitric oxide-induced cell death. Planta 221 757–765 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Tolbert NE (1997) The C2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48 1–25 [DOI] [PubMed] [Google Scholar]
- Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39 45–58 [DOI] [PubMed] [Google Scholar]
- Vandenabeele S, Van der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, et al (2003) A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Natl Acad Sci USA 100 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inze D, Van Breusegem F (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verniquet F, Gaillard J, Neuburger M, Douce R (1991) Rapid inactivation of plant aconitase by hydrogen peroxide. Biochem J 276 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 255 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Nishimura M (2000) Reduction to below threshold levels of glycolate oxidase activities in transgenic tobacco enhances photoinhibition during irradiation. Plant Cell Physiol 41 1397–1406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.