Abstract
Aquaporins are water channel proteins that facilitate the passage of water through biological membranes and play a crucial role in plant growth. We showed that ethylene treatment significantly reduced petal size, inhibited expansion of petal abaxial subepidermal cells, and decreased petal water content in rose (Rosa hybrida ‘Samantha’). Here, we report the isolation of a plasma membrane aquaporin (PIP) gene, Rh-PIP2;1, and characterized its potential role in ethylene-inhibited petal expansion. Rh-PIP2;1 is mainly localized on the plasma membrane and belongs to the class 2 subfamily of PIP proteins. We show that Rh-PIP2;1 is an active water channel. The transcripts of Rh-PIP2;1 are highly abundant in petal epidermal cells, especially in the abaxial subepidermal cells. The expression of Rh-PIP2;1 is highly correlated with petal expansion and tightly down-regulated by ethylene. Furthermore, we demonstrate that in Rh-PIP2;1-silenced flowers, petal expansion was greatly inhibited and anatomical features of the petals were similar to those of ethylene-treated flowers. We argue that Rh-PIP2;1 plays an important role in petal cell expansion and that ethylene inhibits petal expansion of roses at least partially by suppressing Rh-PIP2;1 expression.
The gaseous phytohormone ethylene modulates various plant developmental processes, including organ growth, flowering, fruit ripening, leaf senescence, and abscission (Abeles et al., 1992; Wang et al., 2002). Particularly, the inhibitory role of ethylene in organ growth has been well documented (Pierik et al., 2006). In Arabidopsis (Arabidopsis thaliana), reduction of hypocotyl elongation in dark-grown seedlings occurred merely 15 min after the plant was subjected to ethylene treatment (Binder et al., 2004). Ethylene-inhibited growth was also found in stems and petioles of Arabidopsis (Smalle and Van der Straeten, 1997), roots of Rumex and cucumber (Cucumis sativus; Visser et al., 1997; Pierik et al., 1999), and leaves of Poa species (Fiorani et al., 2002). In flower development, ethylene has been reported to be mainly involved in sexual determination (Yamasaki et al., 2003) and petal senescence (Woltering and van Doorn, 1988; van Doorn and van Meeteren, 2003; van Doorn and Woltering, 2008). Especially, several reports proved that ethylene also played an important role in floral organ abscission (Patterson and Bleecker, 2004; Butenko et al., 2006). In roses (Rosa spp.), Reid et al. (1989a, 1989b) showed that ethylene could promote or inhibit petal growth in a cultivar-dependent manner. However, little is known concerning the regulatory mechanisms of ethylene in petal development during the process of flower opening.
It has been reported that ethylene inhibits plant organ growth by regulating cell elongation and expansion processes. Ethylene impedes cell expansion in roots along the direction of the long axis but enhances cell radial expansion (Chadwick and Burg, 1967). Ethylene induces the formation of apical hypocotyl hooks by regulating asymmetrical cell elongation on the adaxial and abaxial sides of the apical hook region in Arabidopsis and pea (Pisum sativum; Lehman et al., 1996). Moreover, Arabidopsis ethylene constitutive response mutant plants (ctr1) are tiny and their leaf cell sizes are also much smaller (Kieber et al., 1993), while the ethylene-insensitive mutants (e.g. ers1 and etr1-1) are larger and their larger leaf area is thought to be caused by increased cell size (Bleecker et al., 1988; Hua et al., 1995). Cell expansion requires the synergistic action of cell wall loosening, deposition of cell wall materials, and water absorption. In Rumex, ethylene can influence petiole elongation by regulating expression of the genes related to cell expansion (Vreeburg et al., 2005). However, little is known about the mechanism of ethylene-regulated cell expansion.
Aquaporins (AQPs) are the primary channels of water transport across biological membranes. AQPs are a class of small (24–34 kD) transmembrane proteins and are localized on various cellular membranes (Maurel and Chrispeels, 2001). Plasma membrane intrinsic proteins (PIPs), which are plasma membrane-associated AQPs, can be classified into two major subgroups, PIP1 and PIP2. PIP2 has a shorter N terminus and a longer C terminus than PIP1 (Johansson et al., 2000). PIP2 usually has a higher channel activity than PIP1 (Daniels et al., 1994; Yamada et al., 1995; Moshelion et al., 2002; Fetter et al., 2004).
AQPs have been implicated in many developmental processes in plants, including cell expansion, organ movement, and elongation (for review, see Tyerman et al., 2002). In Arabidopsis, reduction of AtPIP1;2 (previously named AtPIP1b) mRNA levels decreased cellular permeability and increased root mass (Kaldenhoff et al., 1998). Overexpression of AtPIP1;2 in tobacco (Nicotiana tabacum) significantly increased plant growth rate and resulted in larger plants (Aharon et al., 2003). The transcriptional activity of a tobacco AQP, NtAQP1, was highly associated with cell expansion regions (e.g. young petioles and corolla; Siefritz et al., 2004). In tobacco, PIP1 and PIP2 showed distinct expression patterns during stigma and anther development, and they might contribute to the development of these organs in different ways (Bots et al., 2005a, 2005b). In Arabidopsis, eight PIP genes were detectable in flower buds, suggesting the involvement of PIPs in flower development (Quigley et al., 2002). In tulip (Tulipa spp.), temperature-dependent changes of a putative plasma membrane-associated AQP activity were associated with petal opening and closing (Azad et al., 2004). However, very limited direct evidence is available to support the role of PIPs in petal expansion.
Roses have been one of the most important ornamental crops in the floriculture industry for centuries, and flowering is one of the most important traits. Generally, flower opening requires programmatic expansion of petals. Considering that postmitotic development of petals mainly depends on water uptake-driven cell expansion, it is highly possible that AQPs play an important role in petal expansion.
Our previous studies showed that ethylene could accelerate the flower-opening process in roses and could result in irregular petal shape (Ma et al., 2006; Xue et al., 2008). In this study, we found that ethylene significantly reduced petal size and water content in roses and inhibited petal expansion by inhibiting cell expansion. Furthermore, we identified a rose AQP gene from the public EST collection and demonstrated that this gene was tightly down-regulated by ethylene and was involved in the regulation of rose petal expansion.
RESULTS
Ethylene Inhibits Petal Expansion
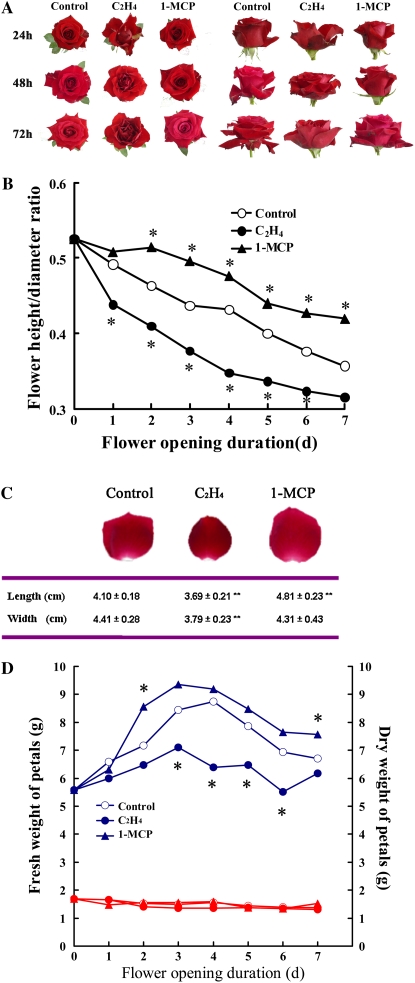
We have reported that ethylene plays a crucial role in the flower-opening process of roses (Ma et al., 2006; Xue et al., 2008). Here, we further investigated the effects of ethylene on petal expansion during flower opening. Our results showed that ethylene treatment resulted in vertically compressed flowers with decreased flower height-to-flower diameter ratio in rose (Rosa hybrida ‘Samantha’). An ethylene action inhibitor, 1-MCP (for 1-methylcyclopropene), was used to inhibit the ethylene effects because of its high specificity of action and lack of deleterious side effects (Serek et al., 1995; Sisler et al., 1996; Sisler and Serek, 1997; Cho and Cosgrove, 2002; Tanaka et al., 2005; Iwai et al., 2006). Expectably, 1-MCP increased the ratio significantly and resulted in vertically stretched flowers (Fig. 1, A and B). In addition, ethylene significantly decreased both petal length and width, while 1-MCP increased petal length significantly (Fig. 1C).
Figure 1.
Morphological changes of cut rose flowers in response to exogenous ethylene. A, Vertical (left) and side (right) views of flowers after ethylene and 1-MCP treatments. Flowers were exposed to air (control), 10 μL L−1 ethylene, or 2 μL L−1 1-MCP for 24 to 72 h, and the photographs were taken at the end of individual treatment times. Twenty-five flowers were used in each treatment, and representative results are shown. B, The ratio of flower height to flower diameter. Each data point represents the mean ± se (n = 15). Asterisks indicate significant differences (Student's t test; P < 0.05) between untreated control and ethylene- or 1-MCP-treated flowers. C, The size of petals after ethylene and 1-MCP treatments. The length and width of petals were measured on the 2nd d after 24-h treatments. One petal was chosen randomly from the second layer of each flower. Double asterisks indicate significant differences in length or width between untreated control and ethylene- or 1-MCP-treated flower petals (n = 15, P < 0.01). D, Changes of petal fresh weight (top blue lines) and dry weight (bottom red lines) after ethylene and 1-MCP treatments. Each data point represents the mean ± se (n = 15). Asterisks indicate significant differences (Student's t test; P < 0.05) between untreated control and ethylene- or 1-MCP-treated flower petals.
During normal flower opening, although the petals expanded significantly, their thickness significantly decreased, especially in the upper and middle regions (25% and 50% of the petal length from the top, respectively). Ethylene treatment significantly increased petal thickness in the upper and middle regions, while 1-MCP significantly decreased petal thickness in the upper region (Table I).
Table I.
Effect of ethylene on petal thickness (μm)
Petal thickness was measured at the upper, middle, and bottom regions (25%, 50%, and 75% of the petal length from the top, respectively). Flowers were treated with ethylene or 1-MCP on the first day of opening duration for 24 h. Day 0, The first day of flower opening. Day 3, The third day of flower opening. Data represents means from 15 replicates ±se. Different letters in the same column indicate significant differences according to Duncan's multiple range test (P < 0.05).
| Treatments | Upper (25%) | Middle (50%) | Bottom (75%) |
|---|---|---|---|
| Control (day 0) | 459.2 ± 10.3 a | 574.6 ± 21.4 a | 820.2 ± 56.2 a |
| Control (day 3) | 183.7 ± 26.5 b | 446.8 ± 15.1 b | 844.1 ± 65.8 a |
| Ethylene (day3) | 358.2 ± 38.2 a | 567.2 ± 14.3 a | 886.4 ± 35.0 a |
| 1-MCP (day 3) | 113.9 ± 34.3 c | 461.4 ± 10.8 b | 854.1 ± 37.6 a |
Ethylene Inhibits Cell Expansion of Petals
In general, petal cells cease division right before anthesis (Drews et al., 1992; Martin and Gerats, 1993; Guterman et al., 2002; Dafny-Yelin et al., 2005). Consequently, during flower opening, petal growth is driven mainly by cell expansion. Here, we determined the effects of ethylene on petal cell expansion.
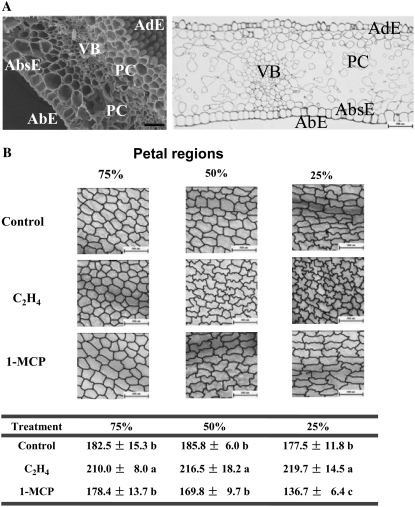
Scanning electron microscopy showed that the petal could be roughly divided into five sections anatomically: adaxial epidermis, parenchyma cell, vascular bundle, abaxial subepidermis (AbsE), and abaxial epidermis (Fig. 2A). Among the five sections, AbsE cells appeared to have a regular shape and to form a flat layer. The expansion of AbsE cells was tightly related to petal expansion (Supplemental Figs. S1 and S2). Therefore, we examined the size of AbsE cells by counting the number of cells in a 1,360- × 1,024-μm2 microscope visual area. We found that ethylene treatment significantly increased cell numbers per visual area, indicating a significant decrease in cell size, while 1-MCP significantly reduced cell numbers, especially in the upper region of petals, indicating a significant increase in cell size (Fig. 2B). Interestingly, ethylene treatment also resulted in interlocking AbsE cells of irregular shape, suggesting that normal cell polar expansion might be impeded by ethylene (Fig. 2B). These results indicated that ethylene inhibited petal expansion, at least partially, through the inhibition of AbsE cell expansion.
Figure 2.
Inhibition of the cell elongation of AbsE in petals by ethylene. A, The typical anatomic structure of a petal at flower full opening stage. Left, A scanning electron microscope image. Bar = 50 μm. Right, A light microscope image with semithin section. Bar = 200 μm. AbE, Abaxial epidermis; AdE, adaxial epidermis; PC, parenchyma cells; VB, vascular bundle. B, Traces of outlines of AbsE cells (top) and cell numbers (bottom) in different regions of petals. Bars = 200 μm. AbsE cells of petals were photographed using a Nikon IX-71 microscope. Petal samples were taken as a 0.5-cm × 0.4-cm slice at 25%, 50%, and 75% of the petal length from petal top on the 2nd d after 24-h treatments. The slices were fixed in formaldehyde and then cleared in ethanol. The traces were drawn using Photoshop 7.0 software. Fifteen flowers were used in each treatment, and representative results are shown. Cell numbers were counted using ImageJ software in a visual field of 1,360 × 1,024 μm2. Each data point represents the mean ± se (n = 15). Different letters in the same row indicate significant differences between different treatments according to Duncan's multiple range test (P < 0.05).
Ethylene Reduces Water Content of Petals
As indicated above, petal growth during flower opening is driven mainly by cell expansion, a process of rapid water uptake. In this study, we investigated petal water content during flower opening and upon ethylene or 1-MCP treatment. The fresh weight of petals in control flowers increased rapidly during the first 4 d of flower opening and then started to decrease, while the dry weight stayed at a steady level during the first 7 d of the flower-opening process (Fig. 1D). During flower opening, ethylene treatment substantially decreased the petal fresh weight, while 1-MCP significantly increased the fresh weight. However, no significant differences in dry weight were observed between the control flowers and those treated with ethylene or 1-MCP (Fig. 1D). This indicated that ethylene caused significant petal water loss while 1-MCP increased petal water content during flower opening.
Cloning and Characterization of Rh-PIP2;1
Since AQPs play important roles in regulating the water intake of plant cells and ethylene reduces the water content of rose petals, it is highly possible that ethylene impedes petal expansion of roses by regulating certain AQPs. To identify potential AQPs involved in petal expansion, we first searched the public rose EST database for sequences with similarity to known AQPs. Among the identified rose AQP-like ESTs, one (GenBank accession no. BQ104371) showed approximately 20-fold increase in expression in rose petals during the early period of flower opening, according to a microarray analysis (Guterman et al., 2002). Therefore, we characterized this EST for its functional role in rose petal expansion.
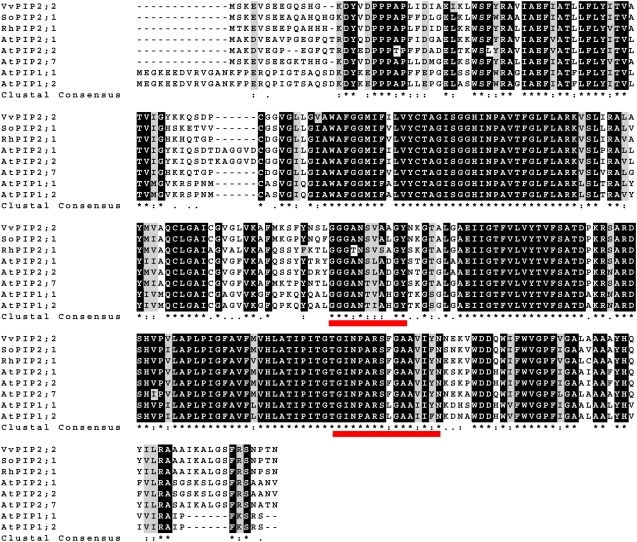
The full-length cDNA of the EST was isolated via RACE. The full transcript is 1,145 bp in length and contains an 846-bp open reading frame, a 91-bp 5′-untranslated region (UTR), and a 208-bp 3′-UTR. The deduced amino acid sequence contains the signature motifs of plasma membrane-associated AQPs (GGGANXXXXGY and TNPARSL/FGAAI/VI/VF/YN) and shares significant homology with plasma membrane AQPs from Arabidopsis, grape (Vitis vinifera), Brassica spp., and poplar (Populus spp.; Fig. 3). Based on the length of its C and N termini, this gene was classified as a class 2 subfamily PIP protein. Therefore, we named it Rh-PIP2;1.
Figure 3.
Alignment of deduced amino acid sequence of Rh-PIP2;1 with seven PIPs from other plants. The accession numbers of the used amino acid sequences are as follows: VvPIP2;2 (grape), AAF71820; SoPIP2;1 (spinach), AAA99274; AtPIP1;1 (Arabidopsis), NP_191702; AtPIP1;2 (Arabidopsis), CAB37860; AtPIP2;1 (Arabidopsis), NP_001030851; AtPIP2;2 (Arabidopsis), NP_181254; and AtPIP2;7 (Arabidopsis), P93004. Red lines under the putative amino acid sequence indicate signature sequences of plasma membrane-associated AQPs. [See online article for color version of this figure.]
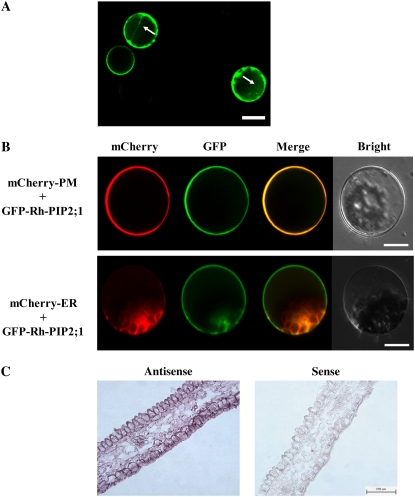
We then determined the localization of Rh-PIP2;1 by expressing a 35S∷GFP-Rh-PIP2;1 fusion gene in Arabidopsis. To determine a more detailed localization, we isolated protoplasts from the transgenic Arabidopsis harboring the 35S∷GFP-Rh-PIP2;1 cassette. We found that the green fluorescence was primarily distributed on the plasma membrane while also distributed on some internal membranes (Fig. 4A). Thus, we conducted colocalization of GFP-Rh-PIP2;1 with a mCherry (a red fluorescent protein mutant derived from mRFP1 that is a monomeric mutant of DsRed [Shaner et al., 2004])-labeled plasma membrane marker (PM-rk; The Arabidopsis Information Resource stock no. CD3-1007) and an endoplasmic reticulum marker (ER-rk; The Arabidopsis Information Resource stock no. CD3-959; Nelson et al., 2007), respectively. The results indicated that Rh-PIP2;1 was mainly distributed on the plasma membrane and much less on the endoplasmic reticulum (Fig. 4B).
Figure 4.
The intracellular localization of Rh-PIP2;1 and in situ hybridization of Rh-PIP2;1. A, The intracellular localization of Rh-PIP2;1 in Arabidopsis leaf mesophyll cell protoplast. The GFP-Rh-PIP2;1 fusion gene was inserted in pRTL2 and transformed into Arabidopsis leaf protoplast for transient expression. Imaging of the protoplast-expressed 35S∷GFP-Rh-PIP2;1 fusion was conducted with a laser scanning confocal microscope (Nikon C1). Arrows indicate internal membrane localization of Rh-PIP2;1. Bar = 20 μm. B, GFP-Rh-PIP2;1 localization is compared with a mCherry-labeled endoplasmic reticulum (ER) marker (ER-rk; CD3-959) and a mCherry-labeled plasma membrane (PM) marker (PM-rk; CD3-1007). At least 20 protoplasts were examined in expressing each marker. Bars = 10 μm. C, Pattern of Rh-PIP2;1 mRNA accumulation examined by in situ hybridization. Rh-PIP2;1 was detected by DIG-labeled 3′-UTR RNA antisense (left) and sense (right) probes in transverse sections of cut rose petals. Bar = 200 μm.
We also examined the spatial expression pattern of Rh-PIP2;1 in petals through in situ hybridization. A 216-bp fragment in the 3′ end of Rh-PIP2;1 was used to generate digoxigenin (DIG)-labeled RNA antisense and sense probes. In situ hybridization of Rh-PIP2;1 transcripts in petals at the early stages of flower opening showed that the expression of Rh-PIP2;1 was much stronger in AbsE than in adaxial epidermis, and the expression was also detected in parenchyma cells but at a relatively lower level (Fig. 4C).
Water Channel Activity of Rh-PIP2;1
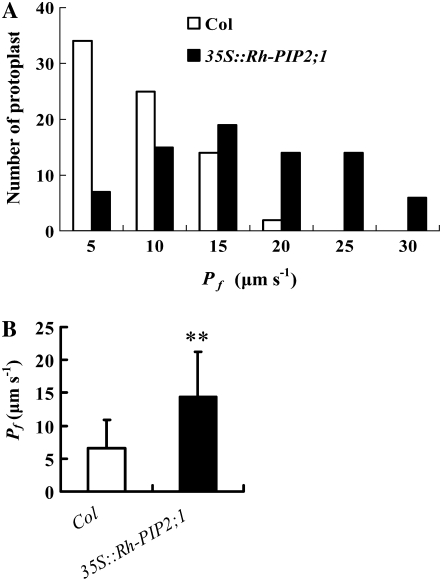
Plant PIP2 proteins usually have high water channel activities, whereas PIP1 proteins are often inactive or have low activities (Kaldenhoff and Fischer, 2006). Here, we tested the water channel activity of Rh-PIP2;1 by comparing osmotic water permeability (Pf) of leaf protoplasts between Arabidopsis wild-type (ecotype Columbia [Col]) plants and transgenic plants harboring the 35S∷Rh-PIP2;1 cassette. The number of leaf protoplasts with high Pf was significantly higher in transgenic plants (Fig. 5A). As shown in Figure 5B, on average, the Pf value of leaf protoplasts in transgenic plants was significantly higher than that in the wild type (14.4 and 6.6, respectively; P < 0.01). This indicates that Rh-PIP2;1 is an active water channel.
Figure 5.
Histogram of Pf of leaf protoplasts (A) and statistical analysis (B) from Arabidopsis wild-type (Col) and transgenic (35S∷Rh-PIP2;1) plants. Leaves of 20- to 22-d-old plants were harvested, and three different preparations of protoplasts were used. For each line, the Pf of 75 protoplasts was measured, and the initial diameter of protoplasts was about 60 μm. Error bars represent se (n = 75). The double asterisk indicates a significant difference in Pf between wild-type (Col) and transgenic (35S∷Rh-PIP2;1) plants (n = 75; t test; P < 0.01).
Regulation of Rh-PIP2;1 Expression by Ethylene
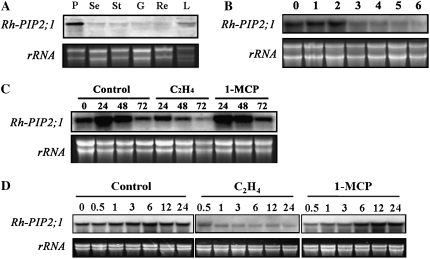
We determined the tissue-specific expression pattern of Rh-PIP2;1 by RNA gel-blot analysis. As shown in Figure 6A, Rh-PIP2;1 was mainly expressed in petals. We then determined its expression levels in petals over the course of flower opening. Rh-PIP2;1 was highly expressed within the first 2 d of flower opening, when petal expansion was the most active. The expression of Rh-PIP2;1 decreased dramatically after 3 d of flower opening, when the petal expansion process was almost completed (Fig. 6B). These results indicated that Rh-PIP2;1 expression was highly correlated with the petal expansion process.
Figure 6.
The expression of Rh-PIP2;1 in cut rose petals. A, Rh-PIP2;1 expression in different organs. P, Petal; Se, sepal; St, stamen; G, gynoecium; Re, receptacle; L, leaf. B, Rh-PIP2;1 expression in petals during normal flower opening. Lanes 0 to 6 indicate vase days. C and D, Rh-PIP2;1 expression in response to ethylene in petals. Flowers were exposed to air (control), 10 μL L−1 ethylene, or 2 μL L−1 1-MCP. Lanes 0 to 72 indicate treatment hours. Each lane contains 10 μg of total RNA. Ethidium bromide-stained rRNA was used as an internal control to normalize the amount of total RNA. Total RNA was isolated from three individual samples at each time point, and all of the hybridizations were repeated at least three times. Representative results are shown.
We further investigated the effects of ethylene and its action inhibitor, 1-MCP, on Rh-PIP2;1 expression in petals during the first 72 h of flower opening. Ethylene treatment significantly inhibited the expression of Rh-PIP2;1, while 1-MCP maintained its expression at a higher level for a longer time compared with the controls (Fig. 6C).
We then examined Rh-PIP2;1 expression in petals on a narrower time scale during the first 24 h of ethylene or 1-MCP treatment in order to understand how quickly Rh-PIP2;1 responds to ethylene. The results showed that the expression of Rh-PIP2;1 changed within 0.5 to 1 h upon ethylene treatment (Fig. 6D), suggesting a quick response of Rh-PIP2;1 expression to ethylene.
Characterization of Rh-PIP2;1-Silenced Flowers
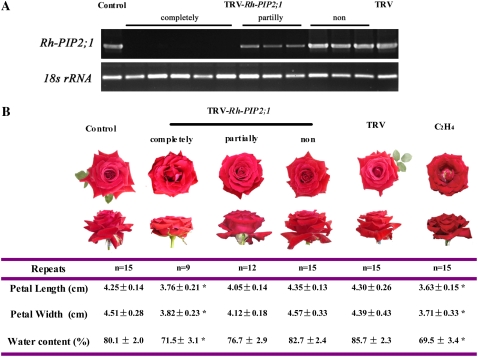
We functionally characterized the role of Rh-PIP2;1 in petal expansion by silencing Rh-PIP2;1 in rose flowers using a virus-induced gene silencing (VIGS) approach. The 290-bp fragment of Rh-PIP2;1 3′ end was used to construct the TRV-Rh-PIP2;1 silencing vector. We successfully silenced Rh-PIP2;1 in rose petals (Fig. 7A), although the efficiency of silencing (about 9:300) was much lower than that reported in tobacco and tomato (Solanum lycopersicum; Liu et al., 2002; Fu et al., 2005). Flowers with silenced Rh-PIP2;1 had petals with significantly decreased length and width as well as reduced water content (Fig. 7B), a phenotype similar to that of ethylene-treated flowers. Rh-PIP2;1 silencing also resulted in significantly increased cell numbers of AbsE per microscope visual area in the upper region of petals, indicating that the silencing reduced the cell volume; however, the cell shapes of AbsE were more regular than those of ethylene-treated petals (Fig. 8).
Figure 7.
Silencing of the Rh-PIP2;1 gene in rose petals by VIGS. Rose branches were infiltrated with Agrobacterium containing TRV alone (TRV, pTRV1 + pTRV2) or TRV carrying a fragment of Rh-PIP2;1 (TRV-Rh-PIP2;1, pTRV1 + pTRV2-Rh-PIP2;1). A, RT-PCR analysis of Rh-PIP2;1 in the Rh-PIP2;1-silencing flower petals. The first-strand cDNA was generated from 1 μg of total RNA and was used to amplify Rh-PIP2;1 and the 18S rRNA gene using gene-specific primers. The PCR cycles was 27 and 18 for Rh-PIP2;1 and 18S rRNA, respectively. Completely, Completely silenced flowers; partially, partially silenced flowers; non, no silenced flowers. B, The phenotype of Rh-PIP2;1-silencing flowers (top) and petal sizes and water content (bottom). The photographs were taken on the 7th d after infiltration. The petal size and water content were determined on the 7th d after infiltration as described in Figure 1. Each data point represents the mean ± se (n = 15 for control, TRV-Rh-PIP2;1 [non], and TRV; n = 9 for TRV-Rh-PIP2;1 [completely]; n = 12 for TRV-Rh-PIP2;1 [partially]).
Figure 8.
Silencing of Rh-PIP2;1 inhibits the cell elongation of the AbsE in petals. Petal samples were taken as a 0.5-cm × 0.4-cm slice at 25% of the length from petal top on the 7th d after infiltration and treated as described in Figure 2B. Bars = 200 μm. Cell numbers were counted as described in Figure 2C. Completely, Completely silenced flowers; partially, partially silenced flowers; non, no silenced flowers. Each data point represents the mean ± se (n = 15 for control, TRV-Rh-PIP2;1 [non], TRV, and ethylene treatment; n = 9 for TRV-Rh-PIP2;1 [completely]; n = 12 for TRV-Rh-PIP2;1 [partially]). Different letters indicate significant differences between different treatments according to Duncan's multiple range test (P < 0.05).
In addition, we also obtained 12 partially Rh-PIP2;1-silenced flowers, and their phenotypes were more similar to those of the wild-type flowers (Figs. 7 and 8).
DISCUSSION
Ethylene Influences Petal Expansion by Modulating AbsE Cells in Roses
In plant development, proliferative growth and postmitotic expansion are coordinated and modulated by phytohormones like auxin, brassinosteroids, GA, and ethylene (Mizukami, 2001; Ingram and Waites, 2006). In Arabidopsis, auxin, abscisic acid, GA, and brassinosteroids can enhance longitudinal expansion of many organs, such as leaves and roots, while ethylene increases lateral expansion of hypocotyls and roots (Saab et al., 1990, 1992; Spollen et al., 2000; for review, see Mizukami, 2001). Compared with wild-type plants, ethylene overexpression (eto) and constitutive response (ctr1) mutants have smaller organs, while ethylene-insensitive (etr) mutant plants appear to have larger leaves and floral organs (Ecker, 1995). Ethylene causes asymmetric cell elongation and results in formation of the apical hooks of pea (Peck et al., 1998). Treatment with ethylene or its precursor, 1-aminocyclopropane-1-carboxylic acid, greatly inhibits cell elongation in Arabidopsis roots (Le et al., 2001; De Cnodder et al., 2005). Thus, ethylene can play an important role in suppressing organ expansion in plants. Ethylene promotes flower opening in carnation (Dianthus caryophyllus; Jones and Woodson, 1997), orchid (Phalaenopsis spp.; Bui and O'Neill, 1998), and Petunia (Tang and Woodson, 1996). In rose, ethylene may promote or inhibit flower opening in a cultivar-dependent manner (Reid et al., 1989a, 1989b). In our previous work, we observed that ethylene promoted flower opening in Samantha rose (Cai et al., 2002; Ma et al., 2005, 2006; Xue et al., 2008). Despite these reports describing the effects of ethylene on ornamental plant flower opening, little is known about the effect of ethylene on petal expansion.
In this study, we show that ethylene treatment significantly reduced petal size (Fig. 1C) and resulted in vertically compressed flowers (Fig. 1, A and B). In addition, ethylene suppressed the expansion of AbsE cells and resulted in cells with irregular shapes (Fig. 2B). These findings strongly suggest that inhibited expansion of AbsE cells may contribute to the inhibition of petal expansion by ethylene.
Rh-PIP2;1 Is Involved in Petal Expansion of Rose
PIPs belong to a multigene family in higher plants, and 13 PIPs have been identified in both Arabidopsis (Johanson et al., 2001) and maize (Zea mays; Chaumont et al., 2001). It has been reported that high levels of PIP expression are usually associated with developmental processes requiring substantial water intake, including root elongation and expansion (Javot et al., 2003; Ranathunge et al., 2004), anther dehiscence (Bots et al., 2005a, 2005b), and petiole movement (Moshelion et al., 2002; Siefritz et al., 2004). During flower opening, petal expansion is mainly driven by cell expansion, a process of rapid water uptake (Drews et al., 1992; Martin and Gerats, 1993; Guterman et al., 2002). In this study, we show that petal fresh weight increased quickly and dry weight stayed at a steady level during petal expansion in rose, indicating that petal expansion should be primarily caused by water uptake-driven cell expansion in rose (Fig. 1D). In addition, we also showed that ethylene significantly inhibited petal expansion and reduced petal water content. Thus, we speculated that PIPs could be involved in the process of ethylene-inhibited petal expansion. Guterman et al. (2002) reported an AQP-like EST (BQ104371) that exhibited a 20-fold increase of mRNA concentration in petals of opened flowers compared with unopened buds, suggesting that it has a potential role in rose petal expansion. In this study, we further functionally characterized this EST, which we named Rh-PIP2; 1, by investigating its role in ethylene-inhibited petal expansion.
The deduced amino acid sequence of Rh-PIP2;1 shares high homology with members of the PIP2 subfamily from other plant species (Fig. 3). Rh-PIP2;1 was expressed predominantly in petals, and its transcript abundance was tightly correlated with the flower-opening process (Fig. 6, A and B). In addition, among petal tissues, Rh-PIP2;1 expression was stronger in AbsE, whose expansion is tightly related to the increase of petal size during flower opening (Fig. 4C). These results strongly suggest that Rh-PIP2;1 is involved in petal expansion.
In general, PIPs are localized on the plasma membrane. However, Siefritz et al. (2001) reported that a tobacco PIP2 protein, NtAQP1, was also localized on some endomembranes in protoplasts. We also found that Rh-PIP2;1 was mainly plasma membrane localized, but its presence was also observed in some internal membrane compartments. Furthermore, using a set of subcellular fluorescence-labeled markers (Nelson et al., 2007), we proved that the internal membrane where Rh-PIP2;1 was localized was the endoplasmic reticulum (Fig. 4, A and B). Mammalian AQPs and plant TIPs (for tonoplast intrinsic proteins) can shuttle between subcellular compartments in response to various stimuli (reviewed by Luu and Maurel, 2005). However, little is known concerning the dynamic localizations of plant PIPs. Recently, Zelazny et al. (2007) reported that PIP interaction could regulate their subcellular localization. Thus, further analysis would be necessary to understand the mechanisms of Rh-PIP2;1 dynamic localization.
Ethylene Regulates Rh-PIP2;1 Expression in Rose Petals
PIPs are likely to play divergent and unique roles in plants with fewer functional redundancies (Luu and Maurel, 2005). The expression of PIP genes can be regulated by developmental and environmental cues at both the transcriptional and posttranscriptional levels (Kjellbom et al., 1999; Hachez et al., 2006). At the transcriptional level, PIP gene expression is regulated by various environmental factors, including light (Cochard et al., 2007), drought (Aroca et al., 2006; Galmés et al., 2007), cold (Jang et al., 2004), salt (Jang et al., 2004; Boursiac et al., 2005), and phytohormones, including gibberellic acid (Phillips and Huttly, 1994; Suga et al., 2002), abscisic acid (Kaldenhoff et al., 1993; Zhu et al., 2005; Aroca et al., 2006), and brassinolide (Morillon et al., 2001).
At the posttranscriptional level, AQP channel activities are modulated by several mechanisms, including protein phosphorylation, pH, protein trafficking, and protein oligomerization (Luu and Maurel, 2005; Hachez et al., 2006). Phosphorylation at certain residues of some AQPs is thought to be an important modification mechanism for channel activity. For instance, the channel activities of an Arabidopsis α-TIP gene, AtTIP3;1, the spinach (Spinacia oleracea) SoPIP2;1 (PM28A), and the soybean (Glycine max) nodulin 26 are all regulated through protein phosphorylation (Maurel et al., 1995; Johansson et al., 1996; Guenther et al., 2003). Recently, Tornroth-Horsefield et al. (2006) reported that phosphorylation-induced structural remodeling led to channel gating of SoPIP2;1.
The involvement of ethylene in the modification of AQP channel activity has been suggested in a few reports. A general inhibitor of AQP activities, HgCl2, decreased ethylene-enhanced water flow in aspen (Populus spp.) roots (Kamaluddin and Zwiazek, 2002). A protein kinase inhibitor, okadaic acid, could inhibit the ethylene-induced phosphorylation in mung bean (Vigna radiata) hypocotyls (Kim et al., 1997), orchid petals (Wang et al., 2001), and pea (Berry et al., 1996; Kwak and Lee, 1997), and the inhibitor also inhibited the phosphorylation of AQP PM28A in spinach leaves (Johansson et al., 1996). Since the phosphorylation of AQPs is considered to be an important regulatory mechanism controlling channel activity (Tornroth-Horsefield et al., 2006), it is reasonably speculated that ethylene may be involved in the regulation of water channel activities via the protein phosphorylation/dephosphorylation pathway.
However, little direct evidence has been obtained at either the transcriptional or posttranscriptional level to support the role of AQPs in ethylene-regulated water transport.
In this work, we found that ethylene substantially decreased the transcript levels of Rh-PIP2;1 (Fig. 6, C and D). The suppressing effect of ethylene on Rh-PIP2;1 expression was observed within only 0.5 to 1 h of the treatment (Fig. 6D), suggesting that Rh-PIP2;1 expression is likely regulated directly by ethylene. Additionally, the Rh-PIP2;1-silenced flowers exhibited similar morphological and anatomical phenotypes to flowers treated by ethylene (Figs. 7 and 8), further supporting the notion that ethylene inhibits petal expansion of rose by suppressing Rh-PIP2;1 expression. Taken together, we argue that Rh-PIP2;1 is able to respond to ethylene promptly at the transcriptional level and is involved in ethylene-regulated petal expansion. However, we cannot rule out the possibility that ethylene also contributes to regulating petal water uptake and petal expansion by influencing the preexisting Rh-PIP2;1 protein activity. However, further work is required to clarify this possibility.
In this study, we also found that although the Rh-PIP2;1-silenced and ethylene-treated flowers exhibited similar morphological and anatomical phenotypes, overall the ethylene-treated flowers showed a more severe phenotype regarding the inhibited petal expansion. This indicates that some other ethylene-regulated genes might also be involved in ethylene-inhibited petal expansion.
On the other hand, it is possible that the cross talk between different hormones is involved in the regulation of plant organ expansion. In maintaining leaf expansion of Arabidopsis and tomato, and primary root elongation of Arabidopsis, endogenous abscisic acid was thought to play an important role by restricting ethylene production (Sharp et al., 2000; Spollen et al., 2000; LeNoble et al., 2004).
In Arabidopsis, the inhibitory effect of ethylene on GA-induced root elongation was accomplished through enhancing the stability of the conserved repressors of GA signaling, GAI (GA insensitive) and RGA (repressor of ga1-3; Achard et al., 2003), suggesting that there may be cross talk in influencing root elongation between ethylene and GA.
Taken together, ethylene, abscisic acid, and GA may synergistically influence petal expansion by regulating relevant genes, like AQP, at the transcriptional and/or the posttranscriptional level. However, further studies are required to understand the possible mechanisms.
MATERIALS AND METHODS
Plant Materials
Cut roses (Rosa hybrida ‘Samantha’) were harvested at stage 2 of flower opening from a local commercial grower and placed in water immediately. Flower-opening stages were as defined by Wang et al. (2004) and Ma et al. (2005): stage 0, unopened bud; stage 1, partially opened bud; stage 2, completely opened bud; stages 3 and 4, partially opened flower; stage 5, fully opened flower with anther appearance (yellow); stage 6, fully opened flower with anther appearance (black). The flowers were delivered to our laboratory within 1 h of harvest. Their stems were then cut to a length of 25 cm under water and placed in deionized water, which was refreshed daily. For all analyses in this study, samples were collected from outermost ray petals of the flowers.
Treatment of Flowers with Ethylene and 1-MCP
Based on our previous work (Ma et al., 2006), we used 10 μL L−1 ethylene or 2 μL L−1 1-MCP for treatments. The flowers were sealed in a 64-L chamber with ethylene, 1-MCP, or regular air as the control at 25°C for different periods of time. NaOH (1 mol L−1) was put into the chamber to prevent the accumulation of CO2. After treatment, flowers were placed in a vase with deionized water under the following controlled conditions: approximately 23°C to 25°C room temperature, approximately 30% to 40% relative humidity, and 12/12-h light/dark photoperiod with an illumination of approximately 40 μmol m−2 s−1.
Microscopic Examination and Cell Counting
Petal samples were taken as 0.5-cm × 0.4-cm slices from regions at 25%, 50%, and 75% of the petal length from the top. For scanning electron microscopy, slices of the petal middle region (50% of the length) of fully opened flowers were selected and then fixed and processed according to a standard protocol (Bowman et al., 1989). The slices were flat mounted on either their adaxial or abaxial surface, or fractured to reveal internal anatomy transverse to the petal longitudinal axis. Scanning electron microscopy was performed using a Philip S3400N apparatus.
AbsE cell photography and cell counting were performed as described by Dewitte et al. (2007). In brief, rose flowers were treated at the 1st d (day 0) of opening for 24 h, and petal samples were taken on the 2nd d after the respective treatments. Petal samples were taken as 0.5-cm × 0.4-cm slices at 25%, 50%, and 75% of the petal length from the petal top. The slices were fixed in formaldehyde and then cleared in ethanol. AbsE cells of the slices were photographed using a Nikon IX-71 camera. The traces were drawn using Photoshop 7.0 software. Fifteen flowers were used in each treatment.
Numbers of AbsE cells were counted using ImageJ software in a visual field of 1,360 × 1,024 μm2.
Rh-PIP2;1 Subcellular Localization
Rh-PIP2;1 cDNA was amplified with primers Rh-PIP2;1_up (5′-GGACGAGCTGTACAAGATGACGAAGGAAGTG-3′) and Rh-PIP2;1_down (5′-GCGTGGATCCTTAGTTGGAGGGGTTGCTCCG-3′). gfp cDNA was amplified with primers gfp_up (5′-ATCCCATGGATGGTGAGCAAGGGCGAGGAGCT-3′) and gfp_down (5′-CACTTCCTTCGTCATCTTGTACAGCTCGTCC-3′). The resulting fragments were used to generate the GFP-Rh-PIP2;1 fusion gene by overlapping PCR. The fused fragment was inserted into pGEM T-Easy vector (Promega) and subjected to sequencing for verification. After NcoI and BamHI digestion, the cassette was inserted into pRTL2 to construct the pRTL-GFP-Rh-PIP2;1 transient expression vector. Transient expression was conducted according to Kovtun et al. (2000). Arabidopsis (Arabidopsis thaliana) leaf mesophyll protoplasts were isolated from well expanded leaves of 3- to 4-week-old plants. Leaf sections of roughly 1 g were cut into 0.5- to 1-mm strips with a razor blade. The strips were placed into 15 mL of preheated enzyme solution (1% cellulase R10, 0.25% macerozyme R10, 0.4 m mannitol, 20 mm KCl, and 20 mm MES, pH 5.7) and gently shaken at 23°C in darkness for 3 h. Then, the protoplasts were filtered and spun at 1,000 rpm for 1 min. The resulting pellets were washed once by cold W5 solution (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, 5 mm Glc, and 2 mm MES, pH 5.7), suspended in the same solution, kept on ice for 30 min, centrifuged at 1,000 rpm for 1 min, and then resuspended in MMg solution (0.4 m mannitol, 15 mm MgCl2, and 4 mm MES, pH 5.7). Around 15 μg of plasmid DNA was mixed with 5 × 104 protoplasts in 0.1 mL of MMg solution. An equal volume of 40% (w/v) PEG4000 (Fluka) in 0.1 m Ca(NO3)2 and 0.4 m mannitol solution of pH 10 was added and gently mixed, and then the mixture was incubated at 23°C for 25 min. After incubation, a 4-fold volume W5 solution was added and mixed gently, and the mixture was centrifuged at 1,000 rpm for 2 min. The pellets were resuspended gently in 1 mL of W5 solution and were incubated at 23°C for 20 to 22 h in darkness. All transformation was performed three times, and assays were repeated on at least three separate occasions.
pRTL-GFP-Rh-PIP2;1 was digested by HindIII, and the 35S∷GFP-Rh-PIP2;1-Nos cassette was inserted into the binary vector pGreen 0229 and transformed into Agrobacterium tumefaciens GV3101. Arabidopsis plants were infiltrated with Agrobacterium using the floral dip method (Clough and Bent, 1998). The GFP florescence was visualized using a confocal microscopy (Nikon).
Colocalization experiments were performed using the mCherry-labeled plasma membrane marker (PM-rk; CD3-1007) and the endoplasmic reticulum marker (ER-rk; CD3-959) purchased from the Arabidopsis Biological Resource Center (Nelson et al., 2007). The plasmids of CD3-1007 and CD3-959 were separately transformed into protoplasts of Arabidopsis transgenic plants overexpressing GFP-PIP2;1. Imaging of protoplasts was conducted with a laser scanning confocal microscope (Nikon C1). The excitation wavelengths for GFP and mCherry were 488 and 543 nm, respectively, and the emission filter wavelengths were 505 to 530 nm for GFP and 560 to 615 nm for mCherry.
In Situ Hybridization
In situ hybridization was performed as described by Xu et al. (2005) with minor modifications. A 216-bp fragment at the 3′ end of Rh-PIP2;1 was amplified using the following primer pairs: 5′-CCTCCAACTAAATGAGAAGAAGC-3′ and 5′-GCTAATACGACTCACTATAGGGCAAAACACCACTACTCATTGAC-3′ for the antisense probe and 5′-GCTAATACGACTCACTATAGGGCCTCCAACTAAATGAGAAGAAGC-3′ and 5′-CAAAACACCACTACTCATTGAC-3′ for the sense probe. The PCR products were purified (Axyprep DNA gel extraction kit; Axygen) and used as the template to generate the antisense or sense probe via in vitro transcription using the DIG RNA labeling mix (Roche).
Petal samples were fixed in cold phosphate-buffered saline (pH 7.0) containing 4% (w/v) paraformaldehyde overnight at 4°C. After being washed twice with cold phosphate-buffered saline, the samples were dehydrated with an ethanol series and then embedded in Paraplast plus (Sigma). Subsequently, the samples were cut into 7-μm sections and mounted on slides pretreated with 0.01% poly-l-Lys (Sigma). The hybridization was performed at 43°C overnight with a 0.1 to 0.2 ng μL−1 RNA probe concentration. After washing and digestion by RNase A, the slides were incubated with Anti-Digoxigenin AP-Conjugate (Roche). Hybridizations were visualized using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate stock solution (Roche) and photographed with an Olympus BX51 microscope.
Measurement of Pf on Protoplasts
The Pf of Rh-PIP2;1 in leaf protoplasts of Arabidopsis wild-type and transgenic plants was measured according to Ramahaleo et al. (1999), Moshelion et al. (2004), and Cabañero et al. (2006).
In brief, leaves of 20- to 22-d-old plants were harvested, and three different preparations of protoplasts were generated. Fifteen-microliter protoplast suspensions were dispersed on a microscope slide, and the same amount of water was added to decrease the osmotic potential of the medium to half. Cell volumes were captured with an inverted Nikon microscope (IX-71), and images were acquired at 3-s intervals for a total of 90 s using a CCD camera (DP 70; Nikon). Protoplast swelling was analyzed using the ImageJ program (http://rsb.info.nih.gov/ij/). The Pf values were calculated according to the equation from Zhang and Verkman (1991): Pf = (V0/S0)[k/Vw(Osmolin − Osmolout)], where V0 is the initial cell volume, S0 is the initial cell surface area, Vw is the molar volume of water (18 cm3 mol−1), k is the fitted exponential rate constant of the initial phase of the swelling, and osmolin and osmolout are the internal and external osmolarities, respectively. Measurements of osmolarity (mosmol kg−1) were carried out using an osmometer (Fiske ONE-TENTM Osmometer).
For each line, the Pf of 75 protoplasts was measured, and the initial diameter of the protoplasts was about 60 μm.
RNA Extraction, Northern-Blot Analysis, and Reverse Transcription PCR
The full-length cDNA of Rh-PIP2;1 was obtained by RACE (SMART RACE cDNA Amplification; Clontech) and deposited into GenBank under accession number EU572717. Procedures for total RNA extraction and RNA gel-blot analysis were reported by Ma et al. (2006). Petals were ground with liquid nitrogen and homogenized with the preheated extraction buffer (200 mm sodium tetraborate decahydrate, 30 mm EGTA, 1% deoxycholic acid sodium salt, 10 mm dithiothreitol, 2% polyvinylpyrrolidone 40, and 1% Nonidet P-40). RNA was first precipitated overnight with 2 m LiCl, then washed by 2 m LiCl and dissolved in 1 m Tris-Cl (pH 7.5). Subsequently, the RNA was precipitated with 100% ethanol at −80°C for 2 h. For RNA gel-blot analysis, 10 μg of total RNA was fractionated on 1.2% agarose gels containing 2.5% (v/v) formaldehyde. The 3′-UTR region of Rh-PIP2;1 was used as the probe. DIG-labeled probes were generated by PCR using a PCR DIG probe synthesis kit (Boehringer Mannheim). Hybridization was carried out overnight at 46°C. The membranes were washed twice in 2× SSC at 37°C and twice in 0.1× SSC at 59°C, with 30 min for each wash, and then were incubated with Anti-Digoxigenin AP-Conjugate (Roche). The membranes were then subjected to a chemiluminescent reaction with CDP-Star according to the manufacturer's protocol (Roche) and exposed to Fuji Medical X-Ray Film (Fuji Photo Film). All of the hybridizations were performed with at least three biological replicates.
For reverse transcription (RT)-PCR, first-strand cDNA was synthesized from 1 μg of total RNA using SuperScript Moloney murine leukemia virus reverse transcriptase (Promega). Semiquantitative RT-PCR was performed as described by Fu et al. (2005). The primers used for RT-PCR were 5′-TTGAAAATGACGAAGGAAGTGAGCGA-3′ and 5′-CCGGATCAGAGAGACCTTACGAG-3′, which were outside the region used for VIGS. This ensured that only the endogenous Rh-PIP2;1 transcripts were examined in VIGS plants. The 18S rRNA gene was used as an internal control for RNA quantity. The PCR product amplified from a reaction without reverse transcriptase was used as the negative control. The PCR products were analyzed on 1.0% agarose gels and imaged by AlphaImager 2200 (Alpha Innotech).
Silencing of Rh-PIP2;1 in Rose Flowers by VIGS
The pTRV1 and pTRV2 VIGS vectors (described by Liu et al., 2002) were kindly provided by Dr. S.P. Dinesh-Kumar at Yale University. The Rh-PIP2;1 silencing was performed as described by Fu et al. (2005). A 302-bp fragment at the 3′ end of Rh-PIP2;1 was amplified using primers 5′-CGTTCTAGAGTGCAGCCGCGTACCACCAG-3′ with an XbaI restriction site and 5′-CATCTCGAGGATTGGCAAAACACCACTA-3′ with an XhoI restriction site. The resulting product was cloned into pTRV2 to form the pTRV2-Rh-PIP2;1 construct. pTRV2-Rh-PIP2;1, as well as the pTRV1 and pTRV2 vectors, were transformed into Agrobacterium GV3101 by electroporation. The Agrobacterium strain GV3101 containing pTRV1, pTRV2, or pTRV2-Rh-PIP2;1 was grown at 28°C in Luria-Bertani medium supplemented with 10 mm MES, 20 mm acetosyringone, and 50 mg L−1 kanamycin for about 24 h. Agrobacterium cells were harvested and suspended in the infiltration buffer (10 mm MgCl2, 150 mm acetosyringone, and 10 mm MES, pH 5.6) to a final optical density at 600 nm of around 1.0. A mixture of Agrobacterium cultures containing pTRV1 and pTRV2-Rh-PIP2;1 in a ratio of 1:1 (v/v), as well as a mixture containing pTRV1 and pTRV2 in a 1:1 ratio (which served as the negative control), were shaken at room temperature for 4 h before vacuum infiltration.
For vacuum infiltration, rose flower branches at flower stage 1 were placed into the bacterial suspension solution and infiltrated by vacuum at 30 mmHg for 30 s. After release of the vacuum, the flower branch base was washed by deionized water and kept in deionized water for about 10 d at 18°C. The petals were collected when flowers fully opened, approximately at the 7th d after the infiltration.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EU572717.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Anatomic structure of a petal by scanning electron microscopy.
Supplemental Figure S2. Petal AbsE cell expansion during flower opening.
Supplemental Figure S3. The quantification of the in situ signals of Figure 4C.
Supplemental Figure S4. The quantification of the northern hybridization signals in Figure 6, C (left) and D (right).
Supplementary Material
Acknowledgments
We are grateful to the laboratory of Dr. Shu-Nong Bai for their kind help with in situ hybridization. We also thank Dr. Zhangjun Fei for critical review of the manuscript and Ms. Sara Zimmer and Ryan McQuinn for proofreading.
This work was supported by the National Natural Science Foundation of China (grant no. 30671480) and the National High Technology Research and Development Program (863 Program) of China (grant no. 2006AA100109).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Junping Gao (gaojp@cau.edu.cn).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology. Academic Press, San Diego
- Achard P, Vriezen WH, van der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Ferrante A, Vernieri P, Chrispeels MJ (2006) Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann Bot (Lond) 98 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AK, Sawa Y, Ishikawa T, Shibata H (2004) Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol 45 608–617 [DOI] [PubMed] [Google Scholar]
- Berry AW, Cowan DSC, Harpham NVJ, Hemsley RJ, Novikova GV, Smith AR, Hall MA (1996) Studies on the possible role of protein phosphorylation in the transduction of the ethylene signal. Plant Growth Regul 18 135–141 [Google Scholar]
- Binder BM, O'Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005. a) PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J Exp Bot 56 113–121 [DOI] [PubMed] [Google Scholar]
- Bots M, Vergeldt F, Wolters-Arts M, Weterings K, van As H, Mariani C (2005. b) Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiol 137 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C (2005) Early effects of salinity on water transport in Arabidopsis roots: molecular and cellular features of aquaporin expression. Plant Physiol 139 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui AQ, O'Neill SD (1998) Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol 116 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko MA, Stenvik GE, Alm V, Sæther B, Patterson SE, Aalen RB (2006) Ethylene-dependent and -independent pathways controlling floral abscission are revealed to converge using promoter:reporter gene constructs in the ida abscission mutant. J Exp Bot 57 3627–3637 [DOI] [PubMed] [Google Scholar]
- Cabañero FJ, Martínez-Ballesta MC, Terue JA, Carvajal M (2006) New evidence about the relationship between water channel activity and calcium in salinity-stressed pepper plants. Plant Cell Physiol 47 224–233 [DOI] [PubMed] [Google Scholar]
- Cai L, Zhang XH, Shen HX, Gao JP (2002) Effects of ethylene and its inhibitor on flower opening and senescence of cut roses. Acta Hortic Sinica 29 467–472 (in Chinese with English abstract) [Google Scholar]
- Chadwick AV, Burg SP (1967) An explanation of the inhibition of root growth caused by indole-3-acetic acid. Plant Physiol 42 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143 122–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafny-Yelin M, Guterman I, Menda N, Ovadis M, Shalit M, Pichersky E, Zamir D, Lewinsohn E, Adam Z, Weiss D, et al (2005) Flower proteome: changes in protein spectrum during the advanced stages of rose petal development. Planta 222 37–46 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reaction. New Phytol 168 541–550 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V, et al (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci USA 104 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews GN, Beals TP, Bui AQ, Goldberg RB (1992) Regional and cell-specific gene expression patterns during petal development. Plant Cell 4 1383–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268 667–675 [DOI] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Bögemann GM, Visser EJW, Lambers H, Voesenek LACJ (2002) Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiol 129 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruits. Plant J 31 299–308 [DOI] [PubMed] [Google Scholar]
- Galmés J, Pou A, Alsina MM, Tomàs M, Medrano H, Flexas J (2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226 671–681 [DOI] [PubMed] [Google Scholar]
- Guenther JF, Chanmanivone N, Galetovic MP, Wallace IS, Cobb JA, Roberts DM (2003) Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 15 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman I, Shalit M, Menda M, Piestun D, Dafny-Yelin M, Shalev G, Bar E, Davydov O, Ovadis M, Emanuel M, et al (2002) Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell 14 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Zelazny E, Chaumont F (2006) Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions? Biochim Biophys Acta 1758 1142–1156 [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269 1712–1714 [DOI] [PubMed] [Google Scholar]
- Ingram GC, Waites R (2006) Keeping it together: co-ordinating plant growth. Curr Opin Plant Biol 9 12–20 [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y (2006) Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 142 1202–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H (2004) An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol Biol 54 713–725 [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Frank KI, Schäffner AR, Bouchez D, et al (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding Major Intrinsic Proteins in Arabidopsis provides a framework for a new nomenclature for Major Intrinsic Proteins in plants. Plant Physiol 126 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000) The role of aquaporin in cellular and whole plant water balance. Biochim Biophys Acta 1465 324–342 [DOI] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjellbom P (1996) The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Woodson WR (1997) Pollination-induced ethylene in carnation: role of stylar ethylene in corolla senescence. Plant Physiol 115 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Fischer M (2006) Functional aquaporin diversity in plants. Biochim Biophys Acta 1758 1134–1141 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998) Significance of plasmalemma aquaporins for water transport in Arabidopsis thaliana. Plant J 14 121–128 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Kolling A, Richter G (1993) A novel blue light- and abscisic acid-inducible gene of Arabidopsis thaliana encoding an intrinsic membrane protein. Plant Mol Biol 23 1187–1198 [DOI] [PubMed] [Google Scholar]
- Kamaluddin M, Zwiazek JJ (2002) Ethylene enhances water transport in hypoxic aspen. Plant Physiol 128 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72 427–441 [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim WT, Kang BG, Yang SF (1997) Induction of 1-aminocyclopropane-1-carboxylate oxidase mRNA by ethylene in mung bean hypocotyls: involvement of both protein phosphorylation and dephosphorylation in ethylene signaling. Plant J 11 399–405 [Google Scholar]
- Kjellbom P, Larsson C, Johansson I, Karlsson M, Johanson U (1999) Aquaporins and water homeostasis in plants. Trends Plant Sci 25 308–314 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S, Lee SH (1997) The requirements of Ca2+, protein phosphorylation and dephosphorylation for ethylene signal transduction in Pisum sativum L. Plant Cell Physiol 38 1142–1149 [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2001) In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol 125 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85 183–194 [DOI] [PubMed] [Google Scholar]
- LeNoble ME, Spollen WG, Sharp RE (2004) Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot 55 237–245 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31 777–786 [DOI] [PubMed] [Google Scholar]
- Luu DT, Maurel C (2005) Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ 28 85–96 [Google Scholar]
- Ma N, Cai L, Lu WJ, Tan H, Gao JP (2005) Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci China C Life Sci 48 434–444 [DOI] [PubMed] [Google Scholar]
- Ma N, Tan H, Liu XH, Xue JQ, Li YH, Gao JP (2006) Transcriptional regulation of ethylene receptor and CTR genes involved in ethylene-induced flower opening in cut rose (Rosa hybrida) cv. Samantha. J Exp Bot 57 2763–2773 [DOI] [PubMed] [Google Scholar]
- Martin C, Gerats T (1993) Control of pigment biosynthesis genes during petal development. Plant Cell 5 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Kado RT, Guern J, Chrispeels MJ (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin alpha-TIP. EMBO J 14 3028–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y (2001) A matter of size: developmental control of organ size in plants. Curr Opin Plant Biol 4 533–539 [DOI] [PubMed] [Google Scholar]
- Morillon R, Catterou M, Sangwan RS, Sangwan BS, Lassalles JP (2001) Brassinolide may control aquaporin activities in Arabidopsis thaliana. Planta 25 199–204 [DOI] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Moran N, Chaumont F (2004) Dynamic changes in the osmotic water permeability of protoplast plasma membrane. Plant Physiol 135 2301–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51 1126–1136 [DOI] [PubMed] [Google Scholar]
- Patterson SE, Bleecker AB (2004) Ethylene dependent and independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol 134 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC, Pawlowski K, Kende H (1998) Asymmetric responsiveness to ethylene mediates cell elongation in the apical hook of peas. Plant Cell 10 713–720 [Google Scholar]
- Phillips AL, Huttly AK (1994) Cloning of two gibberellin-regulated cDNAs from Arabidopsis thaliana by subtractive hybridization: expression of the tonoplast water channel, γ-TIP, is increased by GA3. Plant Mol Biol 24 603–615 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11 176–183 [DOI] [PubMed] [Google Scholar]
- Pierik R, Verkerke W, Voesenek RLACJ, Blom KCWPM, Visser EJW (1999) Thick root syndrome in cucumber (Cucumis sativus L.): a description of the phenomenon and an investigation of the role of ethylene. Ann Bot (Lond) 84 755–762 [Google Scholar]
- Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ (2002). From genome to function: the Arabidopsis aquaporins. Genome Biol 3 0001.1–0001.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramahaleo T, Morillon R, Alexandre J, Lassalles JP (1999) Osmotic water permeability of isolated protoplasts: modifications during development. Plant Physiol 119 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R (2004) Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. J Exp Bot 55 433–447 [DOI] [PubMed] [Google Scholar]
- Reid MS, Dodge LL, Mor Y, Evans RY (1989. a) Effects of ethylene on rose opening. Acta Hortic 261 215–220 [Google Scholar]
- Reid MS, Evans RY, Dodge LL, Mor Y (1989. b) Ethylene and silver thiosulfate influence opening of cut rose flowers. J Am Soc Hortic Sci 114 436–440 [Google Scholar]
- Saab IN, Sharp RE, Pritchard J (1992) Effect of inhibition of abscisic acid accumulation on the spatial distribution of elongation in the primary root and mesocotyl of maize at low water potentials. Plant Physiol 99 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serek M, Tamari G, Sisler EC, Borochov A (1995) Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropene, a new inhibitor of ethylene action. Physiol Plant 94 229–232 [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 1567–1572 [DOI] [PubMed] [Google Scholar]
- Sharp RS, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51 1575–1584 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Biela A, Eckert M, Otto B, Uhlein N, Kaldenhoff R (2001) The tobacco plasma membrane aquaporin NtAQP1. J Exp Bot 52 1953–1957 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R (2004) The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J 37 147–155 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Dupille E, Serek M (1996) Effect of 1-methylcyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations. Plant Growth Regul 18 79–86 [Google Scholar]
- Sisler EC, Serek M (1997) Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 100 577–582 [Google Scholar]
- Smalle J, Van der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100 593–605 [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Komatsu S, Maeshima M (2002) Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol 43 1229–1237 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Woodson WR (1996) Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol 112 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439 688–694 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25 173–194 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, van Meeteren U (2003) Flower opening and closure: a review. J Exp Bot 54 1801–1812 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2008) Physiology and molecular biology of petal senescence. J Exp Bot 356 1–28 [DOI] [PubMed] [Google Scholar]
- Visser EJW, Nabben RHM, Blom CWPM, Voesenek LACJ (1997) Elongation by primary lateral roots and adventitious roots during conditions of hypoxia and high ethylene concentrations. Plant Cell Environ 20 647–653 [Google Scholar]
- Vreeburg RA, Benschop JJ, Peeters AJ, Colmer TD, Ammerlaan AH, Staal M, Elzenga TM, Staals RH, Darley CP, McQueen-Mason SJ, et al (2005) Ethylene regulates fast apoplastic acidification and expansin: a transcription during submergence-induced petiole elongation in Rumex palustris. Plant J 43 597–610 [DOI] [PubMed] [Google Scholar]
- Wang D, Fan J, Ranu RS (2004) Cloning and expression of 1-aminocyclopropane-1-carboxylate synthase cDNA from rosa (Rosa x hybrida). Plant Cell Rep 22 422–429 [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14 131–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NN, Yang SF, Charng Y (2001) Differential expression of 1-aminocyclopropane-1-carboxylate synthase genes during orchid flower senescence induced by the protein phosphatase inhibitor okadaic acid. Plant Physiol 126 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering EJ, van Doorn WG (1988) Role of ethylene and senescence of petals: morphological and taxonomical relationships. J Exp Bot 39 1605–1616 [Google Scholar]
- Xu CR, Liu C, Wang YL, Li LC, Chen WQ, Xu ZH, Bai SN (2005) Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proc Natl Acad Sci USA 102 14469–14474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JQ, Li YH, Tan H, Yang F, Ma N, Gao JP (2008) Expression of ethylene biosynthetic and receptor genes in rose floral tissues during ethylene-enhanced flower opening. J Exp Bot 59 2161–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Katsuhara M, Kelly WB, Michalowski C, Bohnert H (1995) A family of transcripts encoding the water channel proteins: tissue-specific expression in the common ice plant. Plant Cell 7 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Fujii N, Takahashi H (2003) Characterization of ethylene effects on sex determination in cucumber plants. Sex Plant Reprod 16 103–111 [Google Scholar]
- Zelazny E, Borst W, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007) FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci USA 104 12359–12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Verkman AS (1991) Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am J Physiol 260 C26–C34 [DOI] [PubMed] [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schäffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56 2971–2981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.