Abstract
The sessile lifestyle of plants constrains their ability to acquire mobile nutrients such as nitrate. Whereas proliferation of roots might help in the longer term, nitrate-rich patches can shift rapidly with mass flow of water in the soil. A mechanism that allows roots to follow and capture this source of mobile nitrogen would be highly desirable. Here, we report that variation in nitrate concentration around roots induces an immediate alteration of root hydraulic properties such that water is preferentially absorbed from the nitrate-rich patch. Further, we show that this coupling between nitrate availability and water acquisition results from changes in cell membrane hydraulic properties and is directly related to intracellular nitrate concentrations. Split-root experiments in which nitrate was applied to a portion of the root system showed that the response is both localized and reversible, resulting in rapid changes in water uptake to the portions of the roots exposed to the nitrate-rich patch. At the same time, water uptake by roots not supplied with nitrate was reduced. We believe that the increase in root hydraulic conductance in one part causes a decline of water uptake in the other part due to a collapse in the water potential gradient driving uptake. The translation of local information, in this case nitrate concentration, into a hydraulic signal that can be transmitted rapidly throughout the plant and thus coordinate responses at the whole plant level, represents an unexpected, higher level physiological interaction that precedes the level of gene expression.
Nitrogen is the mineral nutrient that plants require in greatest quantities. Despite its abundance, nitrogen availability in soils is limited by mineralization rates and immobilization (Tinker and Nye, 2000). Many plants respond to low nitrate availability by induction of root extension growth with suppressed branching or to regions of high nitrate availability by root proliferation (Zhang and Forde, 1998; Robinson et al., 1999; Forde and Lorenzo, 2001; Remans et al., 2006). However, often growth cannot match the temporal and spatial variation of nitrate patches (Robinson, 2001; Cardon and Gage, 2006). Furthermore, in field conditions, root densities can reach as high as 40 cm/cm3 (De Baets et al., 2006), leaving little room for additional proliferation. An optimal solution to this problem must accomplish two things: (1) increase nitrate uptake in the event of identifying a nitrate-rich patch; and (2) increase nitrate delivery to the root surface to avoid local depletion resulting from increased uptake rates. Recent advances in our understanding of nitrate uptake and assimilation using genomics and proteomics presents a complex picture with multiple pathways controlled by exogenous and endogenous nitrate levels (Stitt, 1999; Sakakibara et al., 2006). Multiple lines of evidence indicate that nitrate transporters and proteins involved in nitrate assimilation are up-regulated in response to exogenous nitrate concentrations, fulfilling the first desideratum (Forde and Clarkson, 1999; Forde, 2002). The second possibility, that plants might control the flow of nitrate ions toward the root, thus maintaining high concentrations at the root surface, has not been demonstrated. A significant drop in nitrate concentration at the root surface during active uptake (Taylor and Bloom, 1998) suggests that a coordinated increase in mass flow rates may be necessary to assure nitrate delivery and maintenance of higher concentrations of nitrate at the root surface. The most powerful way to achieve such increases in mass flow would be through induction of local changes in root hydraulic properties.
A number of studies have linked root hydraulic properties with nitrate availability. Initial information comes from analysis of root hydraulic properties of cotton (Gossypium hirsutum) plants exposed to different levels of nitrogen availability (Radin and Matthews, 1988; Radin, 1990). These studies suggest that exposure of nitrate-deprived plants to elevated nitrate levels for several days results in an increase in root hydraulic conductance. Further research suggests that this up-regulation is associated with water channel function (Carvajal et al., 1996; Hoarau et al., 1996; Clarkson et al., 2000) and involves the downstream products of nitrate assimilation (Hoarau et al., 1996; Clarkson et al., 2000). Here, we provide detailed analysis of nitrate-induced changes in root hydraulic properties within minutes from application of nitrate and provide a physiologically based analysis suggesting that cellular nitrate levels, not the products of nitrate assimilation, are directly involved in changes of hydraulic membrane properties. We then demonstrate that the interaction between nitrate availability and root hydraulic properties leads to system-level adjustments in patterns of water uptake that increases the net uptake of nitrogen at the whole plant level. The rapidity of this response allows plants to optimize resource acquisition in the face of environmental heterogeneity. Linking resource availability with hydraulic adjustments represents a novel mechanism by which sessile organisms can exhibit dynamic responses and provides a means for translating local information into appropriate response at the whole plant level.
RESULTS
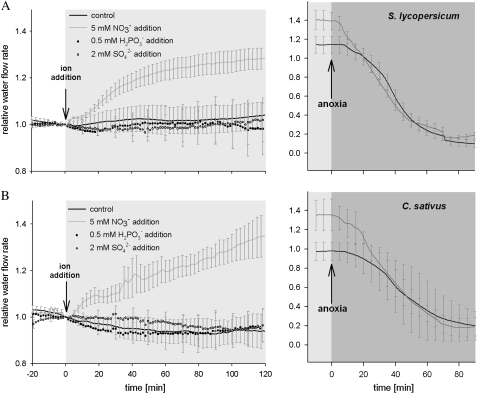
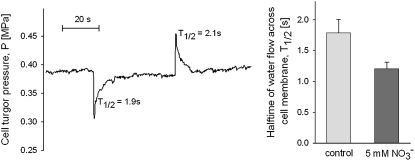
We have observed that hydrostatic pressure generated flux rates across roots of tomato (Solanum lycopersicum) and cucumber (Cucumis sativus) increased upon addition of nitrate to the hydroponic medium, although details of their response dynamics varied (Fig. 1). The increase in root water uptake was associated with nitrate addition, but not with the addition of other anions, namely, SO42− and H2PO3− (Fig. 1). The increased flux can be explained only by changes in root hydraulic conductance because measurements were conducted in the linear portion of the pressure-flow relation and analysis of root exudates excluded the possibility that the observed flux increases resulted from changes in the osmotic driving force across the root (Table I). Temporal analysis of the response suggests that the initial increase in root hydraulic conductance is almost simultaneous with the application of nitrate to the root medium, with the maximum conductance, which persists as long as nitrate levels are maintained, being achieved in approximately 2 h (Fig. 1), although a further slow increase of conductance could be noticed. Cell pressure probe measurements showed that the half-time (T1/2) for pressure relaxation of cucumber root cells was around 1.8 s, but decreased to 1.2 s upon treatment with 5 mm NO3− solution for 2 h (Fig. 2). Because cell hydraulic conductance is proportional to 1/T1/2, this translates into an increase in plasma membrane hydraulic conductance of approximately 30%, a value similar to the increase observed at the whole root level. This finding demonstrates that the increase in root hydraulic conductance is the result of changes accruing at the cell membrane level. We used anoxia to assay the contribution of aquaporins to the observed changes in root hydraulic conductance (Tournaire-Roux et al., 2003), noting that application of anoxia collapsed the hydraulic conductance of both tomato and cucumber roots exposed to high nitrate levels to the same level, approximately 10% of initial conductance (Fig. 1).
Figure 1.
Pressure-driven exudation from whole root systems of tomato (A) and cucumber (B). Application of nitrate is indicated by downward-pointing arrows and blue shading. Each line is the average of four plants; vertical bars = ses and are shown for every 10th time point. Circular points are average of three points and error bars are presented for every 20th point for clarity. Upward-pointing arrows and dark-gray shading denote application of anoxia, 4 to 5 h after the nitrate addition (note change in vertical scale).
Table I.
Xylem sap osmotic pressure (MPa) following addition of nitrate at time 0
Data are means (MPa) ± se from three independent experiments.
| Time | Tomato | Cucumber | ||
|---|---|---|---|---|
| min | ||||
| −30 | 0.034 | ±0.004 | 0.034 | ±0.001 |
| 0 | 0.032 | ±0.005 | 0.034 | ±0.003 |
| 30 | 0.032 | ±0.002 | 0.039 | ±0.006 |
| 60 | 0.038 | ±0.005 | 0.041 | ±0.004 |
| 90 | 0.042 | ±0.003 | 0.044 | ±0.004 |
| 120 | 0.044 | ±0.001 | 0.044 | ±0.004 |
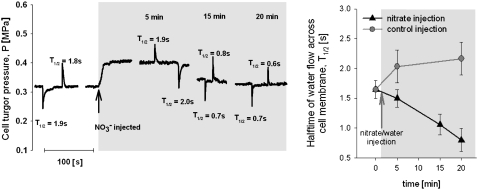
Figure 2.
Left, Typical measurements of turgor pressure and hydraulic properties of cortical cells in cucumber roots. Measured cells were located three to four cells beneath the epidermis. The volume clamp method (Steudle, 1993; Ye and Steudle, 2006) was used to measure the half-time for water flow through the cell membrane. Right, Average half-time before addition of nitrate and 2 h after addition of nitrate was significantly different (T value = 2.32, df = 25, P = 0.029).
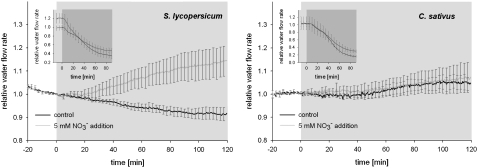
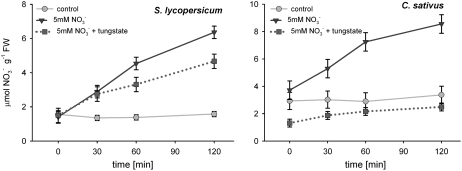
To elucidate the signaling path responsible for the observed changes in root hydraulic conductance, we pretreated tomato and cucumber plants with a molybdenum-free medium containing tungstate, a well-known inhibitor of nitrate assimilation (Deng et al., 1989). The treatment resulted in strong inhibition of nitrate reductase (NR), effectively removing any downstream products of nitrate assimilation (Table II). The hydraulic response of cucumber roots to nitrate addition was eliminated by this treatment; however, tungstate-treated tomato roots continued to show an increase in hydraulic conductance in response to nitrate addition (Fig. 3). Further analysis of the differential response to nitrate addition between cucumber and tomato showed that the effect of tungstate application on nitrate accumulation in root tissue differed between the two species. In tungstate-treated tomato roots, nitrate concentration increased, whereas in cucumber, levels remained flat (Fig. 4). This finding carries important methodological implications given that tungsten is frequently used to block NR, but very rarely are such studies accompanied by an analysis of nitrate uptake/accumulation (Klobus et al., 1991). The differential nitrate accumulation in tungstate-treated roots of tomato and cucumber suggests that an increase in intracellular nitrate concentration, rather than the products of nitrate assimilation, is responsible for the signal initiation that leads to changes in membrane hydraulic properties. To confirm the above assessment, we blocked nitrate accumulation in cucumber roots with tungstate and bypassed this block by direct injection of nitrate into root cells. The half-time for pressure relaxations of cells injected with nitrate decreased from 1.60 s to 0.83 s over 20 min following injection (Fig. 5). Thus, an increase in intracellular nitrate concentration is sufficient to trigger changes in plasma membrane hydraulic conductance.
Table II.
Actual activity of NR in tomato and cucumber roots
Data are means (μmol NO2− g−1 fresh weight h−1) ± se from three independent experiments.
| Time | Control | 5 mm NO3− | 5 mm NO3− and Tungstate | ||||
|---|---|---|---|---|---|---|---|
| min | |||||||
| Tomato | 0 | 0.087 | ±0.017 | 0.087 | ±0.017 | 0.0105 | ±0.0017 |
| 30 | 0.086 | ±0.035 | 0.102 | ±0.022 | 0.0082 | ±0.0026 | |
| 60 | 0.077 | ±0.036 | 0.103 | ±0.028 | 0.0061 | ±0.0024 | |
| 120 | 0.099 | ±0.032 | 0.101 | ±0.042 | 0.0063 | ±0.0023 | |
| Cucumber | 0 | 0.040 | ±0.021 | 0.040 | ±0.022 | 0.0046 | ±0.0015 |
| 30 | 0.067 | ±0.015 | 0.019 | ±0.014 | 0.0026 | ±0.0026 | |
| 60 | 0.032 | ±0.012 | 0.051 | ±0.013 | 0.0010 | ±0.0034 | |
| 120 | 0.032 | ±0.008 | 0.047 | ±0.012 | 0.0019 | ±0.0019 | |
Figure 3.
Pressure-driven exudation from whole root systems of tomato and cucumber after 48-h exposure to tungstate and anoxia treatment (insets). Tungstate blocked the effect of nitrate on water uptake in cucumber, but not in tomato. Each line is the average of four plants; ses are shown for every 10th time point.
Figure 4.
Increases in nitrate concentrations in the roots of tomato and cucumber following nitrate addition was significant (tomato ANOVA F(3,8) = 25.26, P = 0.0002; cucumber ANOVA F(3,8) = 6.58, P = 0.0149). Following tungstate treatment, a significant increase in nitrate accumulation was observed in tomato [ANOVA F(3,8) = 10.80, P = 0.00347], but not in cucumber [ANOVA F(3,8) = 0.439, P = 0.7310]. Accumulation of nitrate in the presence of tungstate was not affected in tomato, i.e. difference between treated and nontreated plants exposed to nitrate [ANOVA F(3,8) = 1.76, P = 0.2306], but was significantly reduced in cucumber [ANOVA F(3,8) = 11.11, P = 0.00317]. Error bars = se.
Figure 5.
Left, Half-time of water flow across root cortical cell plasma membranes prior to and after injection with water (control) or nitrate solution (60 mm) that increased internal nitrate concentrations by approximately 2 mm. Right, A significant drop in half-time was observed over a 20-min period in nitrate-injected cells [ANOVA F(3, 22) = 4.68, P = 0.0111], whereas no significant change was observed in water-injected cells [ANOVA F(2,13) = 1.19, P = 0.33]. Error bars = se.
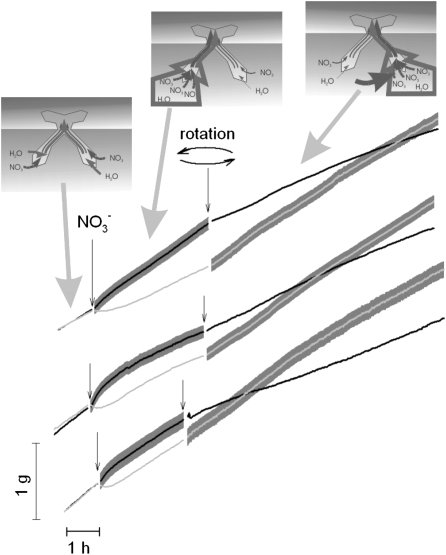
We conducted a split-root experiment to determine whether the application of nitrate to one-half of the root system results in preferential uptake from the nitrate-treated roots. Within minutes, water uptake increased in the nitrate-treated portion of the root system, whereas the volume of water flowing into the nontreated portion of the root system was reduced (Fig. 6). Switching exposure to nitrate from one-half of the root system to the other was accompanied by a reversal in the relative magnitude of water uptake by the two portions of the root system (Fig. 6). The fact that this response was both localized and reversible supports the idea that plants can use this mechanism to chase mobile patches of nitrate around the soil using only its ability to fast change root hydraulic properties.
Figure 6.
Cumulative water uptake by transpiring plants with split-root system (cucumber only). The results of three independent experiments are shown. Addition of nitrate to one of the two halves of the root system (shaded area) resulted in a rapid change in the rate of water uptake. Approximately 3 h into the experiment, roots were washed and the treatments rotated such that the nitrate-exposed roots were returned to low nitrate conditions, whereas the low nitrate half was exposed to 5 mm NO3−.
DISCUSSION
Our results clearly show that roots react to sudden changes in nitrate concentrations via adjustments in their hydraulic properties. Although such a hydraulic response was suggested earlier, it was either a result of long-term observation (Radin and Matthews, 1988; Radin, 1990) or demonstrated based on the analysis of flux generated by root pressure (Hoarau et al., 1996; Clarkson et al., 2000). More recent work indicates that this response can be very fast (Gloser et al., 2007). In this study, real-time analysis of the flow shows that response to sudden change in nitrate availability is almost simultaneously translated to hydraulic changes in the roots of tomato and cucumber. Such a dramatic response is likely to reflect changes in membrane permeability, a hypothesis that we confirmed by direct determination of root cell hydraulic properties.
Root hydraulic conductance is markedly dependent on both the presence and activity of plasma membrane aquaporins (Chrispeels and Maurel, 1994; Maurel and Chrispeels, 2001; Tournaire-Roux et al., 2003), making them prime suspects for the underlying cause of this response. The speed with which nitrate application alters root hydraulic conductivity, however, suggests that the initial response is independent of both gene expression and de novo protein synthesis (Sakakibara et al., 2006). This statement is further supported by a recent microarray analysis of Arabidopsis (Arabidopsis thaliana) treated with increased nitrate levels. Despite massive multigene changes in expression levels, no significant increases in the expression of genes encoding aquaporins were observed even several hours following nitrate application (Scheible et al., 2004). In contrast, aquaporin activity is known to be rapidly altered in response to a number of physiological parameters and it can be altered by a number of experimental treatments, including the application of heavy metals and the imposition of anoxia (Chaumont et al., 2005), the latter operating via an effect on cellular pH (Tournaire-Roux et al., 2003). Because the observed collapse in hydraulic conductance was so dramatic, we believe that the nitrate-associated increase in hydraulic conductance was related to aquaporin activity, consistent with earlier suggestions (Carvajal et al., 1996; Hoarau et al., 1996).
Tungstate was used experimentally to block NR, allowing us to test the hypothesis that the products of nitrate assimilation are involved in the signal transduction pathway linking nitrate availability with changes in root hydraulic conductance. However, despite the fact that tungstate blocked NR in both species, only in cucumber did tungstate eliminate the effect of nitrate application on root hydraulic conductance. Tomato plants treated with tungstate continued to exhibit a significant increase in root hydraulic conductance in response to nitrate. This conundrum was resolved after analysis of root tissue nitrate concentrations in the tungstate-treated plants showed that root tissue nitrate concentrations increased in tomato following nitrate application, but remained at control levels in cucumber. Because tungstate did not eliminate the stimulatory effect of nitrate on root hydraulic conductance in tomato, this suggests that nitrate concentration within cells, rather than the products of nitrate assimilation, are responsible for the triggering hydraulic response. This was confirmed by injecting nitrate directly into cucumber cells. Thus, bypassing on the inhibition of nitrate uptake caused by tungstate restored the hydraulic response to nitrate at the membrane level in roots of cucumber. It is worth noting that the response is nitrate specific because treatment with different salts (Fig. 1) or other forms of nitrogen (Gorska et al., 2008) did not elicit a hydraulic response.
The ability of roots to modify their hydraulic properties in response to nitrate availability represents a new paradigm for how sessile organisms such as plants can acquire mobile resources without the aid of moving parts. This mechanism, however, will only work if the induction of root hydraulic conductance is localized; a systemic response across redundant (parallel) organs would not alter system level patterns of water utilization.
The work presented here illustrates how organismal motility, as the major means of scavenging the environment for resources, can be replaced by functional motility, in which complex interactions enable efficient utilization of a dynamic environment by the transference of physiological activities among parallel organs. Specifically, localized changes in membrane hydraulic conductivity in response to local stimuli can lead to adjustments in water uptake at the whole plant level. The speed with which information on nutrient availability is translated into a hydraulic response is much faster than any chemically based information system known in plants. We propose that physiological coupling between local information on resource availability and hydraulic properties forms a general mechanism for integrating system level responses, allowing these sessile organisms to exhibit dynamic behavioral responses.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of tomato [Solanum lycopersicum ‘Paragon (F1)’] and cucumber (Cucumis sativus ‘Marketmore 76’) were planted on wet filter paper (Whatman Quantitative Circles, 90 mm Ø; catalog no. 1001 090; Whatman, Schleicher & Schuell) in covered petri dishes and left to germinate in darkness at room temperature. Three days following germination, the seedlings moved to aerated hydroponics (6.5-L containers) filled with modified Hoagland solution [pH approximately 6.1; 795 μm KNO3, 603 μm Ca(NO3)2, 270 μm MgSO4, and 109 μm KH2PO4; micronutrients, 40.5 μm Fe(III)-EDTA, 20 μm H3BO4, 2 μm MnSO4, 0.085 μm ZnSO4, 0.15 μm CuSO4, and 0.25 μm Na2MoO4] and located in a growth chamber (PAR 600 mm, temperature 25°C day/21°C night, humidity 65%). After 1 week, the young plants were transferred to 42-L boxes (12 plants/box) and cultivated for 2 weeks (the medium was renewed biweekly). After 2 weeks, medium was replaced with a low nitrate solution [pH approximately 6.1; 79.5 μm KNO3, 60.3 μm Ca(NO3)2, 270 μm MgSO4, and 109 μm KH2PO4, 795 μm K2SO4, 603 μm CaCl2; micronutrients, 40.5 μm Fe(III)-EDTA, 20 μm H3BO4, 2 μm MnSO4, 0.085 μm ZnSO4, 0.15 μm CuSO4, and 0.25 μm Na2MoO4] for a minimum of 1 week, but no more than 2 weeks, before plants were used in experiments. Acidity of the medium was adjusted daily to its initial pH value (approximately 6.1).
Harvesting Plant Material for Nitrate Tissue Content and NR Activity
Two days prior to harvest, four plants were transferred to a 4-L box filled with low nitrate solution in which 0.25 μm Na2MoO4 was replaced with sodium tungstate (1 mm concentration), an NR inhibitor. On the evening prior to the harvest, eight additional plants were transferred into two 4-L boxes (four plants/box) filled with the low nitrate hydroponic solution from the larger growth reservoir. One box served as a low nitrogen control, whereas the other two (one with the tungstate pretreatment, the other freshly transferred) received aliquots of 3.2 m KNO3 and 2.4 m Ca(NO3)2 solution to achieve a final concentration of 5 mm NO3−. Whole plants were collected from each container immediately prior to the imposition of the treatments and then 30, 60, and 120 min after nitrate addition. Harvested root systems were washed in deionized (DI) water, dried with paper towels, wrapped in aluminum foil, and put into liquid N2. The roots were subsequently ground to a fine powder in liquid nitrogen and stored at −80°C for further analysis. This experiment was repeated three times for a total of 36 plants per species (12 plants/treatment).
Forced Root Exudation
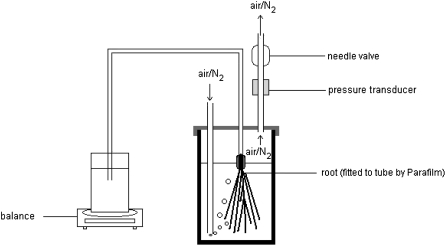
The hydraulic conductance of cucumber and tomato root systems was determined by measuring the flow induced in response to an applied pressure gradient. Detopped root systems were fitted with a plastic tube filled with DI water and connected to a beaker located on a balance (±0.00001 g). The root system was sealed in a chamber containing the low nitrate hydroponic solution in which the plants had been grown. The pH was kept at approximately 6.1 using MES buffer (1 g/L). The pressure in the chamber was regulated using a needle valve, which was adjusted so as to allow a small leak through the chamber such that air used to pressurize the chamber also served to aerate the medium (Fig. 7). Water flow through the root system was automatically recorded by computer at 30-s intervals. Flow stabilization occurred within 10 to 20 min after the plant was exposed to pressure. Plants were allowed an additional 2-h acclimation before an aliquot of 3.2 m KNO3 and 2.4 m Ca(NO3)2 solution was added to each chamber such that a final concentration of 5 mm NO3− was achieved. This solution was injected through the aeration system, such that the pressure in the chamber remained unchanged. Flow data were then collected for an additional 3- to 4-h period. Two plants, only one of which received the nitrate addition, were always measured in parallel to control for diurnal changes in root hydraulic parameters. A total of eight plants were measured per species in this experiment. We also examined whether other anions might elicit the same response. Specifically, we examined the response of three plants of each species to the addition of either 2 mm K2SO4 or 0.5 mm KH2PO3.
Figure 7.
Schematic of system for measuring root exudation generated by hydrostatic pressure.
Tests of the Pressure System
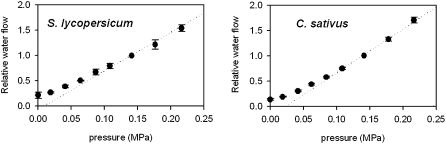
Relation between Applied Pressure and Root Water Flow
At the beginning of our experiments, we quantified the relation between applied pressure and water flow rate to determine the optimal pressure to be used in all experiments. Our goal was to use an applied pressure that is above the plant's ability to influence water uptake by changes in the osmotic activity of the root (Fig. 8). We determined, for both species, that the linear phase of the pressure:flow curve starts around 0.1 MPa. In all of the measurements reported here, we used 0.2 MPa pressure to minimize any possibility of root activity affecting our measurements. Four plants were tested per species.
Figure 8.
Relation between applied pressure and water flow through roots of tomato (left) and cucumber (right) plants.
Anoxia Treatment
Anoxia treatment was applied at the end of each measurement by switching the intake from air to nitrogen gas without affecting the pressure in the root chamber. A significant response to anoxia provided a further check that there was no leak either through the root (i.e. significant damage) or around the seal.
Xylem Sap Osmotic Pressure
We determined the osmotic pressure of xylem sap exudate to confirm that, despite the use of a high pressure to generate flow (linear phase of the pressure flow relation), changes in the osmotic driving force were not responsible for the observed increases in flow rate. Detopped root systems of cucumber or tomato were treated in the same way as for the flow measurements, but instead of directing the outflow to the balance, exudates were collected and measured using a vapor pressure osmometer (5520 Varro; Wescor). Samples were collected every 30 min starting one-half hour before nitrate addition. Observed changes in xylem sap osmotic pressure were minor, within 0.01 MPa over the period of the experiment, and smaller in magnitude than the decrease in osmotic potential in the medium caused by nitrate addition (Table I). Thus, changes in root osmotic activity cannot account for changes of the observed increases in flow rate. Three plants per species were used in this experiment.
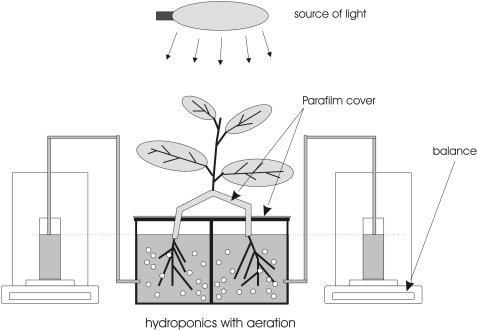
Split-Root System
The root system of intact cucumber plants was split into two equal halves by gentle root separation in water 1 d before the experiment to allow any minor damage inflicted during handling to heal. After 24 h, each of the two halves was placed in a 0.5-L container filled with low nitrate medium and fitted with an aeration system. Each container was independently connected to a beaker situated on a balance (Fig. 9). The water level between containers and beakers was equilibrated, thus allowing for determination of root water uptake generated by transpiration. This system was calibrated to allow for determination of the true mass taken from the containers, taking into account both uptake and evaporation, of which the latter accounted for only approximately 1% of the flow rate generated by a transpiring plant. Plants were illuminated (500 μmol photons m−2 s−1) during the entire experiment; air temperature was maintained at approximately 23°C and humidity was approximately 60%. Following the initial phase of the experiment (approximately 1.5 h), which was designed to allow the plant to acclimate and achieve steady-state transpiration rates, an aliquot of 3.2 m KNO3 and 2.4 m Ca(NO3)2 solution was added to one-half of the root system (final concentration of 5 mm NO3−). After approximately 2.5 h, both containers were flushed with DI water and the solutions rotated such that the half treated with high nitrate concentration was now located in the container with low nitrate medium, whereas the other half of the root system, which had been in low nitrate, was placed in the container filled with the 5 mm NO3− medium. The measurements were then continued for a total of 5 h. Three plants were used in this experiment.
Figure 9.
Schematic of system used to determine water uptake in split-root system experiments.
Cell Pressure Probe Measurement
Root segments, 80 to 120 mm in length, were fixed to a metal sledge positioned at an angle of approximately 75°. The root was covered by one layer of paper tissue such that only 5 mm of the root, at a distance of approximately 50 mm from the root tip, was exposed. An aerated nutrient solution was circulated along the root to maintain hydration. Cortical cells from the second to the fourth layer were punctured using a cell pressure probe. As cells were punctured, cell sap entered the microcapillary forming a meniscus between cell sap and oil. Cell turgor was restored by gently pushing the meniscus to a position close to the surface of the root. The hydrostatic half-time (T1/2) of water flow across the cell membrane was measured from pressure relaxation curves (Fig. 2) with the aid of the probe (Steudle, 1993; Ye and Steudle, 2006). Two additional experiments were conducted to test the effect of nitrate on cell membrane hydraulic conductivity (Lp ∼ 1/T1/2).
The goal of the first experiment was to determine the effect of externally applied nitrate. Hydraulic properties of cells were first measured on low nitrate-grown roots. Then a solution of 3.2 m KNO3 and 2.4 m Ca(NO3)2 was added to the root medium flowing over the root such that a concentration of 5 mm NO3− was achieved. Root cell hydraulic properties were then measured 2 h after nitrate addition. A total of 26 cells were measured in this experiment, including control and treated roots.
In the second experiment, a high concentration of KNO3 (60 mm) solution was introduced into the oil-filled microcapillary of the probe before puncturing the cell (in the control measurements, DI water was used). An oil/solution meniscus was clearly visible under the microscope. Following successful impalement, cell turgor pressure was restored by moving the oil/solution meniscus back to its original position (prior to the puncture) and the initial hydraulic properties of the cell were determined. Following this, the nitrate solution from the capillary was injected into the cell (in control measurements, water was injected). The average increase of the nitrate concentration in the cell was calculated using the injected volume (based on the meniscus movement and the size of the capillary tip) and average size of the cortical cells. Following the initial injection, a series of T1/2 measurements were performed after 5, 15, and 20 min. In water-injected cells, a total of seven cells were successfully measured over a period of 5 to 20 min, whereas in the case of nitrate-injected cells, a total of seven cells were successfully measured over a period of 10 to 20 min following injection. Details of pressure probe methods can be found on the following Web site: http://zatoichi.huh.harvard.edu/∼zwieniecki/methods/PressureProbe/pressureprobe.html.
Nitrate Tissue Concentration
For determination of tissue nitrate content, hot water extracts from frozen, pulverized tomato and cucumber roots were prepared in a ratio of 1:2 (100°C, 20 min). After centrifugation (18,000g, 10 min), the nitrate concentration of the supernatant was determined using the salicylic acid nitration method (Cataldo et al., 1975).
NR Activity
Frozen powder of root tissue was added to an extraction buffer (50 mm HEPES-KOH [pH 7.6], 1 mm dithiothreitol, 10 μm fatty acid desaturase, 10 mm MgCl2, and 50 μm cantharidine) in a 1:2 ratio and ground until thawed using a glass tissue grinder. After centrifugation (18,000g, 10 min, 4°C) a part of the supernatant was removed for nitrite determination (to establish baseline nitrite levels). The remaining aliquot was desalted at 4°C on home-made Sephadex G-25 columns (1-mL gel volume, 600-μL extract). Enzyme activity was assayed in the presence of Mg2+ ions (actual activity; Kaiser et al., 2000). Aliquots (500 μL) were added to the reaction mix (500 μL, 50 mm HEPES-KOH [pH 7.6], 20 μm fatty acid desaturase, 2 mm dithiothreitol, 20 mm MgCl2, 10 mm KNO3, 0.4 mm NADH) and incubated for 4 min at 23°C. The reaction was stopped by the addition of 30 μL of 2 m zinc acetate. After centrifugation, the supernatant was used for colorimetric determination of nitrite production (Wray and Filner, 1970).
Acknowledgments
We thank A.J. Bloom and H. BassiriRad for comments on the manuscript.
This work was supported by National Research Initiative of the U.S. Department of Agriculture-Cooperative State Research, Education, and Extension Service (grant no. 2005–35100–16057).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Maciej A. Zwieniecki (mzwienie@oeb.harvard.edu).
Open Access articles can be viewed online without a subscription.
References
- Cardon Z, Gage DJ (2006) Resource exchange in the rhizosphere: molecular tools and the microbial perspective. Annu Rev Ecol Evol Syst 37 459–488 [Google Scholar]
- Carvajal M, Cooke D, Clarkson D (1996) Response of wheat plants to nutrient depravation may involve the regulation of water channel function. Planta 199 372–381 [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Commun Soil Sci Plant Anal 6 71–80 [Google Scholar]
- Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97 749–764 [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C (1994) Aquaporins: the molecular basis of facilitated water movement through living plant cells? Plant Physiol 105 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000) Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J Exp Bot 51 61–70 [PubMed] [Google Scholar]
- De Baets S, Poesen J, Gyssels G, Knapen A (2006) Effects of grass roots on the erodibility of topsoils during concentrated flow. Geomorphology 76 64–67 [Google Scholar]
- Deng MD, Moureaux T, Caboche M (1989) Tungstate, a molybdate analog inactivating nitrate reductase, deregulates the expression of the nitrate reductase structural gene. Plant Physiol 91 304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232 51–68 [Google Scholar]
- Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol 53 203–224 [DOI] [PubMed] [Google Scholar]
- Forde BG, Clarkson DT (1999) Nitrate and ammonium nutrition of plants: physiological and molecular perspectives. Adv Bot Res 30 1–90 [Google Scholar]
- Gloser V, Zwieniecki MA, Orians C, Holbrook NM (2007) Dynamic changes in root hydraulic properties in response to nitrate availability. J Exp Bot 58 2409–2415 [DOI] [PubMed] [Google Scholar]
- Gorska A, Zwieniecka A, Holbrook NM, Zwieniecki MA (2008) Nitrate induction of root hydraulic conductivity in maize is not correlated with aquaporin expression. Planta (in press) [DOI] [PubMed]
- Hoarau J, Barthes L, Bousser A, Deleens E, Prioul JL (1996) Effect of nitrate on water transfer across roots of nitrogen pre-starved maize seedlings. Planta 200 405–415 [Google Scholar]
- Kaiser WM, Kandlbinder A, Stoimenova M, Glaab J (2000) Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase activity in leaf extracts: What limits nitrate reduction in situ? Planta 210 801–807 [DOI] [PubMed] [Google Scholar]
- Klobus G, Lobocka J, Buczek J (1991) Effect of sodium tungstate on NO3− uptake by cucumber seedlings. Acta Physiol Plant 13 227–233 [Google Scholar]
- Maurel C, Chrispeels MJ (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW (1990) Responses of transpiration and hydraulic conductance to root temperature in nitrogen and phosphorus-deficient cotton seedlings. Plant Physiol 92 855–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW, Matthews MA (1988) Water transport properties of cortical cells in roots of nitrogen and phosphorus-deficient cotton seedlings. Plant Physiol 89 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A (2006) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA 103 19206–19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D (2001) Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant Soil 232 41–50 [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH (1999) Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc R Soc Lond B Biol Sci 266 431–435 [Google Scholar]
- Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11 440–448 [DOI] [PubMed] [Google Scholar]
- Scheible W, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi M, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth, processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136 2483–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue, and organ level. In J Smith, H Griffith, eds, Water Deficits: Plant Responses from Cell to Community. BIOS Scientific Publishers, Oxford, pp 5–36
- Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2 178–186 [DOI] [PubMed] [Google Scholar]
- Taylor A, Bloom A (1998) Ammonium, nitrate, and proton fluxes along the maize root. Plant Cell Environ 21 1255–1263 [Google Scholar]
- Tinker PB, Nye PH (2000) Solute Movement in the Rhizosphere. Oxford University Press, New York
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425 393–397 [DOI] [PubMed] [Google Scholar]
- Wray JL, Filner P (1970) Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J 119 715–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Steudle E (2006) Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ 29 459–470 [DOI] [PubMed] [Google Scholar]
- Zhang HM, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279 407–409 [DOI] [PubMed] [Google Scholar]