Abstract
Peroxisomes are important for recycling carbon and nitrogen that would otherwise be lost during photorespiration. The reduction of hydroxypyruvate to glycerate catalyzed by hydroxypyruvate reductase (HPR) in the peroxisomes is thought to be facilitated by the production of NADH by peroxisomal malate dehydrogenase (PMDH). PMDH, which is encoded by two genes in Arabidopsis (Arabidopsis thaliana), reduces NAD+ to NADH via the oxidation of malate supplied from the cytoplasm to oxaloacetate. A double mutant lacking the expression of both PMDH genes was viable in air and had rates of photosynthesis only slightly lower than in the wild type. This is in contrast to other photorespiratory mutants, which have severely reduced rates of photosynthesis and require high CO2 to grow. The pmdh mutant had a higher O2-dependent CO2 compensation point than the wild type, implying that either Rubisco specificity had changed or that the rate of CO2 released per Rubisco oxygenation was increased in the pmdh plants. Rates of gross O2 evolution and uptake were similar in the pmdh and wild-type plants, indicating that chloroplast linear electron transport and photorespiratory O2 uptake were similar between genotypes. The CO2 postillumination burst and the rate of CO2 released during photorespiration were both greater in the pmdh mutant compared with the wild type, suggesting that the ratio of photorespiratory CO2 release to Rubisco oxygenation was altered in the pmdh mutant. Without PMDH in the peroxisome, the CO2 released per Rubisco oxygenation reaction can be increased by over 50%. In summary, PMDH is essential for maintaining optimal rates of photorespiration in air; however, in its absence, significant rates of photorespiration are still possible, indicating that there are additional mechanisms for supplying reductant to the peroxisomal HPR reaction or that the HPR reaction is altogether circumvented.
The oxygenation of ribulose-1,5-bisphosphate by Rubisco initiates the photorespiratory metabolic pathway (Badger, 1985). This reaction competes with photosynthetic carbon assimilation, producing CO2 and NH3 while consuming ATP and reducing equivalents, thus minimizing the efficiency of net CO2 assimilation (Tolbert, 1997; Wingler et al., 2000; Reumann and Weber, 2006). Rates of photorespiration are about 20% of the rate of net CO2 assimilation in C3 plants under current ambient CO2 concentrations at 25°C and thus consume a large portion of the solar energy absorbed by a leaf (Ort and Baker, 2002). The oxygenation reaction yields 3-phosphoglycerate (3-PGA) and 2-phosphoglycolate (2-PG). The 3-PGA is assimilated into the Calvin-Benson cycle, whereas 2-PG cannot enter this cycle directly, nor can it be converted to carbohydrates. To minimize carbon loss and to prevent the depletion of intermediates of the Calvin-Benson cycle, three-quarters of the carbon in 2-PG is recycled by the photorespiratory pathway into 3-PGA, typically releasing one CO2 per two 2-PG molecules.
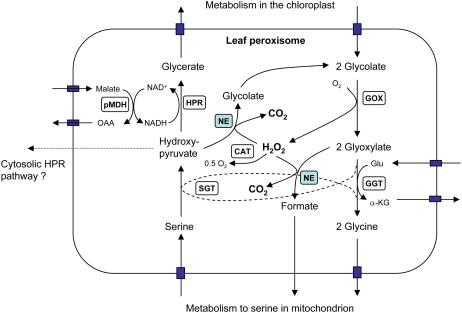
The recycling of carbon by the photorespiratory pathway involves at least 11 enzymatic steps that occur in three leaf organelles (chloroplast, mitochondria, and peroxisomes; Reumann and Weber, 2006). The peroxisomes oxidize glycolate, produced in the chloroplast, to glyoxylate by transferring electrons to O2, thus consuming O2 and forming hydrogen peroxide (H2O2; Reumann, 2000; Mano and Nishimura, 2005). The glyoxylate is further metabolized by two aminotransferases in the peroxisomes, producing Gly, which is subsequently processed by the mitochondrion, releasing CO2 and NH3 while producing NADH and Ser. It is generally accepted that the Ser returns to the leaf peroxisomes, where the amino group is transferred to glyoxylate, forming Gly and hydroxypyruvate (Reumann and Weber, 2006). The hydroxypyruvate is thought to be reduced to glycerate by hydroxypyruvate reductase (HPR), with NADH being provided by the peroxisomal malate dehydrogenase (PMDH; Reumann and Weber, 2006). The glycerate is phosphorylated within the chloroplast and enters into the Calvin-Benson cycle as 3-PGA.
Mutants of the photorespiratory cycle have provided much to the understanding of this biochemical pathway (Somerville and Ogren, 1982; Leegood et al., 1995; Somerville, 2001; Reumann and Weber, 2006). These mutations generally show chlorotic phenotypes when grown under ambient CO2 concentrations but are not affected under growth conditions that suppress photorespiration, such as elevated CO2 (Somerville and Ogren, 1982; Reumann and Weber, 2006). The observed phenotypes have been assumed to be due to the depletion of photosynthetic carbon and nitrogen cycle intermediates and perhaps to the accumulation of toxic photorespiratory intermediates. However, recently, it has become apparent that photorespiratory mutants exposed to air show severe photodamage to PSII due to an inhibition of protein repair processes, linked perhaps to increased oxidative damage to stromal protein synthesis machinery (Takahashi et al., 2007). The original mutant screens searching for chlorosis under ambient CO2 concentrations primarily identified conditional lethal mutations (plants not viable under long-term exposure to ambient CO2 concentrations), and more subtle mutations in the photorespiratory pathway were not easily resolved.
However, some subtle mutations in photorespiration do exist; an example of this is peroxisomal HPR, in which a mutant in barley (Hordeum vulgare) was selected by screening plants that grew poorly in air. The HPR mutant showed reduced levels of the NADH-dependent peroxisomal HPR activity, but rates of photosynthesis were only reduced by 25% compared with wild-type plants when measured under ambient CO2 concentrations (Murray et al., 1989). Related mutants lacking PMDH have also been reported in Arabidopsis (Arabidopsis thaliana) as growing slower in air, but surviving (Pracharoenwattana et al., 2007). It was proposed that an alternative pathway may metabolize hydroxypyruvate during photorespiration in these mutants, such as a cytosolic HPR or being linked to simultaneous β-oxidation in the peroxisome (Murray et al., 1989; Kleczkowski et al., 1990; Pracharoenwattana et al., 2007). This alternative pathway may provide an overflow metabolic route of hydroxypyruvate reduction if the commonly recognized reaction becomes limiting when rates of photorespiration are high (Murray et al., 1989).
Perturbing the flow of carbon through an alternative photorespiratory pathway has important implications for CO2 efflux following the Rubisco oxygenation reaction. Evidence strongly indicates that the photorespiratory pathway releases 1 mol of CO2 for every 2 mol of 2-PG entering the cycle during the conversion of Gly to Ser (Farquhar et al., 1980). However, disruption of the photorespiratory pathway may lead to the accumulation of intermediate compounds, allowing for alternative enzymatic and spontaneous reactions to occur. For example, in barley Gly decarboxylase (GDC) mutants lacking the ability to decarboxylate Gly, the levels of glyoxylate and formate under photorespiratory conditions increased in comparison with those in wild-type plants (Wingler et al., 1999). The production of formate can come from glyoxylate reacting with H2O2 nonenzymatically, and the formate can be further incorporated into Ser via the C1-tetrahydrofolate synthase/Ser hydroxymethyl transferase pathway, partially compensating for the lack of GDC activity in these plants (Wingler et al., 1999).
The role of PMDH and the HPR reaction are unclear in photorespiration. In Arabidopsis, two PMDH genes have been identified and inactivated either individually or together (Pracharoenwattana et al., 2007). While the PMDH1 gene is preferentially expressed in seedlings and the PMDH2 gene is preferentially expressed in leaves, they do appear to be partially redundant (Pracharoenwattana et al., 2007). Mutants containing T-DNA insertions in both PMDH genes demonstrate that this enzyme plays an important role in NADH oxidation during fatty acid β-oxidation in germinating seeds (Pracharoenwattana et al., 2007). However, as these plants appeared to grow well in air, this study cast doubt on the role of PMDH during photorespiration and challenged the notion that PMDH provides the NADH required for the reduction of hydroxypyruvate by HPR (Reumann and Weber, 2006). Here, we reanalyzed the Arabidopsis single and double pmdh mutants and discovered that these mutations cause subtle changes in the rates of photosynthesis under photorespiratory conditions (ambient CO2 and O2 concentrations) and significantly influence the flow of carbon during photorespiration, leading to increased CO2 released relative to the rates of Rubisco oxygenation reactions.
RESULTS
CO2 Assimilation Rate, Growth Analysis, and Leaf Characteristics
Rates of net CO2 assimilation measured at approximately air CO2 concentrations (373 μbar) in both the wild-type and pmdh1 plants were significantly higher than in the pmdh2 and double pmdh1pmdh2 lines (Table I). However, when rates of net CO2 assimilation were measured at elevated CO2 (1,515 μbar), there was no significant difference between the lines (Table I). Stomatal conductance was similar in all genotypes except for the pmdh2 lines, which had lower stomatal conductance values then the other lines under both measurement conditions (Table I). The CO2 compensation point (Γ), inferred from the initial slope of a CO2 response curve at lowest CO2 concentrations, was significantly higher in the pmdh1pmdh2 plants compared with the other three genotypes (Table I). These data were consistent with variation in compensation points measured for leaf discs in a mass spectrometer closed cuvette compensation point measurement system (data not shown).
Table I.
Leaf gas-exchange measurements
The rates of net CO2 assimilation and stomatal conductance in air (373 μbar) and elevated (1,515 μbar) CO2 concentrations and the Γ in wild-type plants, peroxisomal NAD+-malate dehydrogenase 1 and 2 plants (pmdh1 and pmdh2, respectively), and double pmdh1pmdh2 T-DNA mutants of Arabidopsis. Gas-exchange measurements were made at a pO2 of 200 mbar, irradiance of 500 μmol quanta m−2 s−1, and leaf temperature of 25°C. Values of Γ were inferred from the initial slope of a CO2 response curve at lowest CO2 concentrations. Shown are means ± se of measurements made on three to five leaves from separate plants. Statistical analysis was conducted using ANOVA, and different letters indicate significant differences between plants at P < 0.05.
| Variable | Wild Type | pmdh1 | pmdh2 | pmdh1pmdh2 |
|---|---|---|---|---|
| Net CO2 assimilation rate (μmol CO2 m−2 s−1) | ||||
| Air | 10.6 ± 1.1 a | 9.9 ± 1.3 a | 8.2 ± 1.3 a,b | 6.9 ± 0.4 b |
| Elevated CO2 | 17.5 ± 1.4 a | 17.2 ± 2.6 a | 14.5 ± 2.9 a | 15.2 ± 1.0 a |
| Stomatal conductance (mmol m−2 s−1) | ||||
| Air | 0.20 ± 0.02 a | 0.20 ± 0.02 a | 0.15 ± 0.02 b | 0.20 ± 0.01 a |
| Elevated CO2 | 0.21 ± 0.03 a | 0.17 ± 0.03 a | 0.13 ± 0.01 b | 0.19 ± 0.02 a |
| Γ (μbar) | 59 ± 5 a | 65 ± 3 a | 81 ± 1 a | 109 ± 9 b |
The pmdh1pmdh2 lines grew more slowly in air (as would be expected), as indicated by plant size, and growth analyses showed that growth rate on a leaf area basis was 30% to 40% less than in the wild type (Table II). However, under elevated atmospheric CO2 concentrations (2.8 mbar), the growth rates of the wild-type and pmdh1pmdh2 lines were not significantly different (Table II). The growth analysis measurements were conducted during the first 31 d after germination, before the plants were too big for the imaging system. Additionally, there were no significant differences in Rubisco content (μmol site m−2), total leaf nitrogen per area (mmol m−2), and leaf mass per area (g m−2) in the wild-type and pmdh1pmdh2 lines grown under elevated atmospheric CO2 (Table II).
Table II.
Growth analysis and leaf characteristics
The growth rate under ambient CO2 and elevated atmospheric CO2 (2.8 mbar) in wild-type and pmdh1pmdh2 plants. Rubisco content, total leaf nitrogen per area, and leaf mass per area in both genotypes were determined in plants grown under elevated atmospheric CO2 only. Values shown are means ± se of measurements made from three to five leaves from separate plants. Statistical analysis was conducted using Student's t test, and different letters indicate significant differences between plants at P < 0.05.
| Variable | Wild Type | pmdh1pmdh2 |
|---|---|---|
| Growth rate in air CO2 (cm2 cm−2 d−1) | 0.200 ± 0.045 a | 0.125 ± 0.035 b |
| Growth rate at elevated CO2 (cm2 cm−2 d−1) | 0.155 ± 0.014 a | 0.137 ± 0.016 a |
| Rubisco content (μmol site m−2) | 5.8 ± 1.6 a | 6.3 ± 1.0 a |
| Total leaf nitrogen (mmol m−2) | 92.2 ± 5.0 a | 89.8 ± 6.2 a |
| Leaf mass per area (g m−2) | 23.0 ± 1.5 a | 21.1 ± 1.7 a |
The Γ in Response to Oxygen Concentrations
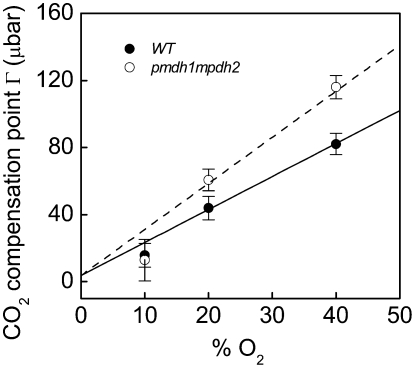
The Γ, as determined from measurements made with the closed leaf chamber coupled to the mass spectrometer (Fig. 1), had a positive relationship with increased oxygen concentration, as predicted from Equation 3 (Fig. 2). Values of Γ were similar in the wild-type and pmdh1pmdh2 lines at low oxygen (10%), when the rates of photorespiration are expected to be low (see below and Fig. 2); however, Γ was greater in the pmdh1pmdh2 plants compared with the wild-type plants when measured at the higher oxygen levels (Fig. 2).
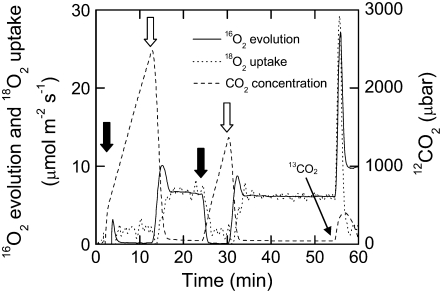
Figure 1.
Typical trace of 16O2 evolution (solid line), 18O2 uptake (dotted line), and CO2 concentration (dashed line) of a leaf disc in a closed chamber coupled to a mass spectrometer. A leaf disc was placed in the chamber and flushed with nitrogen gas, and a volume of pure oxygen highly enriched in 18O was injected to obtain an O2 concentration of 10%, 20%, or 40%. After a 10-min dark period, the leaf disc was illuminated (300 μmol quanta m−2 s−1) for 10 min, then darkened for 5 min and again illuminated for 10 min. Black arrows indicate lights off, and white arrows indicate lights on. The PIB was determined after the initial 10-min light period, and the parameters relating to the Γ were determined at the end of the final light period. CO2 enriched in 13C was injected into the leaf chamber to saturate photosynthesis in the light, and the rates of photorespiratory CO2 release were estimated from the rate of 12CO2 released during this time period.
Figure 2.
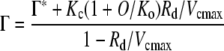 |
(3) |
O2 Exchange and Rubisco Reactions at the Γ
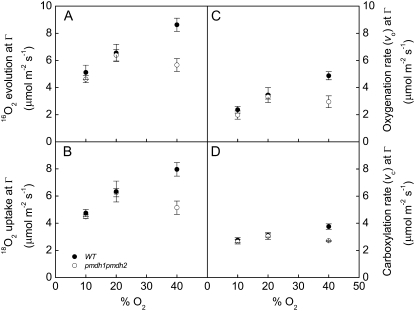
The rates of 16O2 evolution, 18O2 uptake, and net CO2 exchange were also measured with the leaf discs in a closed system attached to the mass spectrometer (Fig. 1). The rates of CO2 exchange and 18O2 uptake in the dark were similar between the wild-type and pmdh1pmdh2 plants in response to changing oxygen levels (data not shown). Additionally, measurements of 16O2 evolution and 18O2 uptake at the compensation point, where net CO2 exchange is zero, were similar in the wild-type and pmdh1pmdh2 plants at both 10% and 20% oxygen (Fig. 3, A and B). However, 16O2 evolution and 18O2 uptake were significantly lower in the pmdh1pmdh2 plants compared with the wild-type plants at the highest oxygen levels (40%; Fig. 3, A and B). The rates of Rubisco oxygenation (vo) and carboxylation (vc) reactions at the Γ were determined using Equations 1 and 2, respectively (see “Materials and Methods”). The values of vo and vc were similar between the wild-type and pmdh1pmdh2 plants at 10% and 20% oxygen (Fig. 3, C and D). However, similar to the rates of 16O2 evolution and 18O2 uptake, the rates of vo and vc were lower in the pmdh1pmdh2 plants compared with the wild-type plants at 40% oxygen (Fig. 3, C and D).
Figure 3.
Oxygen exchange. 16O2 evolution (A), 18O2 uptake (B), vo (C), and vc (D) rates at the Γ at different O2 partial pressures in wild-type (black symbols) and pmdh1pmdh2 (white symbols) plants. Data are means of measurements of three to five different plants, and error bars represent se. Calculation of vo [2/3(18O2 uptake in light − 18O2 uptake in dark)] and vc [16O2 evolution − vo] were determined from mass spectrometer measurements of O2 as presented in Figure 2.
Postillumination Burst and Photorespiratory Release of CO2
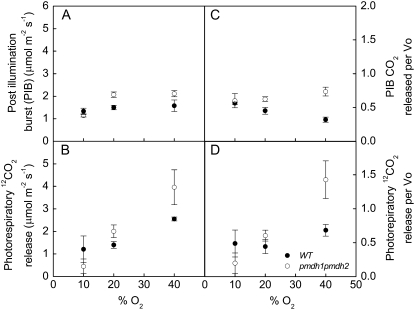
The postillumination burst (PIB), determined by turning off the light and monitoring the rapid efflux of 12CO2 in the dark after an initial 10-min period in the light, was significantly higher in the pmdh1pmdh2 plants compared with the wild-type plants at 20% and higher oxygen levels (Fig. 4A). The values of PIB measured at low oxygen (10%) were similar in the wild-type and pmdh1pmdh2 plants (Fig. 4A). The photorespiratory release of CO2 was determined by injection of CO2 enriched in 13C into the leaf chamber to saturate the rates of the Rubisco carboxylation reaction in the light and subsequently monitoring the rates of photorespiratory 12CO2 release. The release was greater in the pmdh1pmdh2 plants compared with the wild-type plants at 20% and higher oxygen levels but not at 10% oxygen (Fig. 4B). The ratio of PIB to the rate of vo as well as the ratio of photorespiratory CO2 release to vo were higher in the pmdh1pmdh2 plants compared with the wild-type plants at 20% and 40% oxygen but not at 10% oxygen (Fig. 4, C and D).
Figure 4.
Changes in photorespiratory CO2 release per Rubisco oxygenation. The PIB (A), the photorespiratory release of CO2 (B), the PIB relative to the rate of vo (C), and the photorespiratory release of CO2 relative to vo (D) at different O2 partial pressures in wild-type (black symbols) and pmdh1pmdh2 (white symbols) plants. Data are means of measurements of three to five different plants, and error bars represent se.
Metabolite Analysis
Leaf discs were harvested after 10 min at the Γ and extracts subjected to gas chromatography-mass spectrometry (GC-MS) analysis. Relative amounts of numerous primary metabolites that were identified reliably were compared (Supplemental Table S1). Several photorespiratory intermediates were detected by this method, but differences in relative amounts between pmdh1pmdh2 and the wild type were no more than 1.6-fold. Glycolate, glyoxylate, Glu, and glycerate were unchanged in amount, while Gln and Asp were marginally lower and malate, Gly, and Ser were marginally higher. The method employed did not detect hydroxypyruvate or oxaloacetate.
DISCUSSION
The Role of PMDH
It is apparent that PMDH activity is not essential for photosynthesis in air in Arabidopsis. However, it is necessary for optimal rates of photosynthesis and growth in air (Tables I and II). The explanation for how the photorespiratory pathway functions without PMDH activity in air may lie in two areas: first, the potential sources of reductant for the HPR reaction; second, the ability of hydroxypyruvate to be processed by alternative reactions either in the peroxisome or the cytosol.
It is widely accepted that the PMDH provides reductant in the form of NADH to facilitate the reduction of hydroxypyruvate by HPR (Reumann and Weber, 2006). The peroxisomal membrane is assumed to be impermeable to NADH (Donaldson, 1982; Vanroermund et al., 1995; Reumann and Weber, 2006), and the NADH required within the peroxisomes must be synthesized in situ. Traditionally, the malate/oxaloacetate shuttle across the peroxisomal membrane is believed to supply malate derived from either the chloroplast or the mitochondrion to facilitate the reduction of NAD+ to NADH via PMDH. Additionally, β-oxidation of fatty acids results in the reduction of NAD+ to NADH within the peroxisomes (Pracharoenwattana et al., 2007). However, under ambient air conditions, the NADH produced by β-oxidation would presumably be insufficient to meet the requirement of photorespiratory NADH consumption in a typical C3 plant. Here, we show that of the two genes known to encode PMDHs, it appears that PMDH2 has a more dominant role in photorespiration (Table I), as predicted by RNA coexpression analysis (Reumann and Weber, 2006). However, in the double mutant pmdh1pmdh2 lacking the expression of both PMDH genes, the rate of net CO2 assimilation was still 70% of the wild-type rate under ambient CO2 and O2 concentrations during the measurement conditions.
Previous studies of a barley HPR mutant (Murray et al., 1989) also found that although the peroxisomal HPR step was apparently blocked, photosynthesis at air CO2 concentrations was only reduced by 20% to 30%. This study concluded that an alternative NADPH-dependent HPR pathway based in the cytosol may metabolize a portion of the hydroxypyruvate (Fig. 5). However, preliminary studies indicate that extracts prepared from leaves of an Arabidopsis hpr knockout mutant have negligible rates of HP reduction with either NADH or NADPH (I. Pracharoenwattana, unpublished data). In addition to this possibility, the oxidation of hydroxypyruvate to glycolate by nonenzymatic reaction with H2O2 (see below and Fig. 5) may also play a role.
Figure 5.
The path of carbon and CO2 release in the peroxisome. Reactions in the central part of the scheme highlight the potential for H2O2 to nonenzymatically (NE) interact with glyoxylate and hydroxypyruvate. Enzymatic steps shown are as follows: CAT, catalase; GGT, Gln:glyoxylate aminotransferase; GOX, glycolate oxidase; SGT, Ser:glyoxylate aminotransferase. A potential pathway for hydroxypyruvate metabolism in the cytosol is indicated. OAA, Oxaloacetate; α-KG, α-ketoglutarate.
Growth and Leaf Analysis
As in other photorespiratory mutants, the growth rates at high CO2 were similar between the wild-type and pmdh1pmdh2 plants (Table II). Additionally, the amount of Rubisco, the leaf mass per area, and the total leaf nitrogen were similar in wild-type and pmdh1pmdh2 plants grown under elevated CO2 (Table II). These similarities between wild-type and pmdh1pmdh2 plants under high CO2 growth conditions are important because they indicate that the differences in photosynthetic and photorespiratory parameters in air between the genotypes are based on metabolic differences during the measurement conditions and are not due to prior growth conditions. This is particularly important with regard to Rubisco content, as will be further discussed below.
Γ and the Response to Oxygen Availability
The Γ, in the presence of mitochondrial respiration, increased with oxygen concentration in both wild-type and pmdh1pmdh2 plants (Fig. 2). However, Γ was higher in the pmdh1pmdh2 plants compared with the wild-type plants at ambient and higher oxygen concentrations, where the rates of photorespiration would be high (Table I; Fig. 2). There are several factors that can potentially influence Γ, as shown in Equation 3 in Figure 2. For example, Γ is influenced by the Rubisco Michaelis-Menten constants for CO2 and O2, Kc and Ko, respectively, and the maximal rates of Rubisco carboxylation (Vcmax) and oxygenation (Vomax) reactions, as shown in Equation 3. These parameters determine the CO2/O2 specificity of Rubisco (Sc/o), the ratio of the carboxylase to oxygenase rate at equal CO2 and O2 concentrations, as described by Sc/o= (Vcmax/Kc)(Ko/Vomax) (von Caemmerer, 2000). These kinetic values, and subsequently Sc/o, vary between species but are generally considered constant within an individual organism even when grown under different environmental conditions (Jordan and Ogren, 1981). The ratio of respiration (Rd) in the light to Vcmax (Rd/Vcmax) can also influence values of Γ, as demonstrated in tobacco (Nicotiana tabacum) plants, which had reduced amounts of Rubisco but similar rates of Rd (Ruuska et al., 2000). Here, the wild-type and pmdh1pmdh2 plants had similar rates of respiration in the dark (data not shown) and similar amounts of total Rubisco (Table II). Additionally, the rates of vo and vc were similar at 20% and lower oxygen concentrations, as estimated from the leaf O2 exchange at the Γ (Fig. 3, C and D). Assuming that respiration in the dark is similar to Rd, these data indicate that the increased values of Γ in pmdh1pmdh2 at 20% O2 were not related to changes in Sc/o or alterations in the ratio Rd/Vcmax.
In addition to the above parameters, values of Γ are also influenced by Γ*, the Γ in the absence of respiration (Fig. 2, Eq. 3). Values of Γ* are estimated as Γ* = α(VomaxKcO)/(VcmaxKo), where α is the ratio of CO2 released per oxygenation reaction and O is the oxygen concentration. As described in the following section and in Figure 2, changes in α can have a significant effect on Γ, especially when rates of photorespiration are high.
Photorespiratory CO2 Release per Oxygenation Reaction
During photorespiration, 1 mol of 2-PG is assumed to release 0.5 mol of CO2 during the conversion of Gly to Ser; therefore, the value of α = 0.5 (Farquhar et al., 1980; von Caemmerer and Farquhar, 1981). As shown in Figure 2, changes in α can have a large effect on the predicted Γ values. For example, at 20% O2, changing α from 0.5 to 0.8 increases the predicted value of Γ from 38 to 62 μbar, and the effect is greater at higher O2 concentrations (Fig. 2). The predicted Γ values in Figure 2 when α = 0.5 (solid line) describes the response of Γ to increasing O2 concentration in the wild-type plants but underestimates Γ in the pmdh1pmdh2 plants. However, when changing α to 0.8 in Equation 3 (Figure 2, dashed line), the theoretical estimates of Γ in response to O2 fit the measured values of Γ in the pmdh1pmdh2 plants (Fig. 2).
The rates of O2 uptake and evolution, measured at the Γ with the mass spectrometer, were similar in wild-type and pmdh1pmdh2 plants at 20% and lower O2 concentrations (Fig. 3, A and B). At 40% O2, at which photorespiration would be higher, the rates of O2 uptake and evolution were significantly lower in the pmdh1pmdh2 plants compared with the wild-type plants (Fig. 3, A and B). This is likely because at 40% O2, the unidentified alternative photorespiratory pathway utilized by the pmdh1pmdh2 plants is insufficient to keep up with the recycling of photorespiratory intermediates of the Calvin-Benson cycle, and Rubisco activity either becomes ribulose-1,5-bisphosphate limited or inhibited by the increased levels of photorespiratory intermediates (Wingler et al., 2000). The lower rates of vo and vc in the pmdh1pmdh2 plants compared with wild-type plants at 40% O2 further support the idea that Rubisco activity becomes inhibited at the highest O2 concentration in the pmdh1pmdh2 plants (Fig. 3, C and D).
We used two techniques to investigate the relationship of the ratio of photorespiratory CO2 released per oxygenation reaction. In the first method, we monitored the PIB of CO2 in the dark from the leaf tissue after measuring O2 and CO2 exchange at the Γ for 10 min (Fig. 1). These experiments were conducted on the mass spectrometer, where net CO2 exchange, O2 evolution, O2 uptake, and O2 concentrations were simultaneously determined (Figs. 1 and 4). The PIB represents the CO2 released by the photorespiratory cycle after the oxygenation and carboxylation reactions have ceased after turning off the light. Figure 4A shows that the PIB was higher in the pmdh1pmdh2 plants compared with the wild-type plants at the higher O2 concentrations, indicating that there is a greater release of photorespiratory CO2 per glycolate molecule in the pmdh1pmdh2 plants compared with the wild-type plants. The photorespiratory pathway will continue to function after the light has been turned off until the pools of photorespiratory intermediates produced in the light period have been consumed (Laisk and Edwards, 1997; Hoefnagel et al., 1998; Atkin et al., 2000). Therefore, the peak rate of CO2 released during the PIB is an indicator of the rate of CO2 produced during photorespiration prior to darkening. In the traditional view of photorespiration, the CO2 released is primarily produced from the conversion of Gly to Ser by GDC within the mitochondria, but it is not a specific measure of α. However, in the pmdh1pmdh2 plants and other photorespiratory mutants, such as the GDC barley mutant, the PIB will also measure other decarboxylating reactions associated with the altered photorespiratory pathways.
Additionally, the export of Ser and/or Gly via the vascular system (Madore and Grodzinski, 1984; Wingler et al., 2000) or the involvement of Ser/Gly in one-carbon metabolism and protein synthesis (Hanson and Roje, 2001) would alter the flow of carbon through the photorespiratory cycle. If large quantities of Gly were lost from the photorespiratory cycle, due to alternative metabolism or export to vascular tissue, this would decrease α and thus Γ, since the amount of CO2 released per Rubisco oxygenation reaction would approach zero. Alternatively, the catabolism of Gly through C1 metabolism could potentially increase α if the stoichiometry was greater than 0.5 CO2 released per Gly molecule. However, Gly does not appear to be used directly for C1 metabolism but is first converted to Ser through GDC and Ser hydroxymethyltransferase reactions (Mouillon et al., 1999). Alternatively, if large quantities of Ser were diverted out of the photorespiratory cycle, there would be no effect on α, as the release of CO2 due to the conversion of Gly to Ser would be conserved. Although the potential for Ser and/or Gly loss from the photorespiratory cycle cannot be excluded, it does not fit with the increase in Γ seen in the pmdh1pmdh2 mutant.
The second method of estimating the release of CO2 during photorespiration was to take advantage of the mass spectrometer's ability to measure CO2 isotopologues, 12CO2 and 13CO2, simultaneously. After the rates of O2 and 12CO2 exchange were measured at the Γ for 10 min (Fig. 1), a volume of 13CO2 was injected into the leaf chamber to saturate the rates of vc and eliminate the vo reaction. By monitoring the release of 12CO2 directly after the injection of 13CO2, the rates of CO2 released by photorespiration prior to the 13CO2 injection can be estimated in the light without darkening. The rates of CO2 released during photorespiration determined from this method were also greater in the pmdh1pmdh2 plants compared with the wild-type plants (Fig. 4B).
Both the PIB and 13CO2 techniques show that the rates of photorespiratory release of CO2 were greater in the pmdh1pmdh2 plants compared with the wild-type plants (Fig. 4, A and B). This indicates that either the pmdh1pmdh2 plants had greater rates of photorespiration or that the amount of CO2 released per oxygenation reaction was greater compared with that in the wild-type plants. At 20% oxygen, the measured rates of vo were similar in both genotypes (Fig. 3C), and the ratio of photorespiratory CO2 release per Rubisco oxygenation reaction in the pmdh1pmdh2 plants was higher compared with the wild-type plants using both methods of determining photorespiratory CO2 (Fig. 4, C and D). This supports the predicted higher α values in the pmdh1pmdh2 plants compared with the wild-type plants, as presented in Figure 2.
Photorespiratory Metabolites
Overall, the metabolite analysis did not provide any evidence for major perturbation of photorespiratory metabolism (Supplemental Table S1). The small increases in Gly and Ser are consistent with a block downstream at the PMDH step, while decreases in Gln and Asp are consistent with less efficient ammonia reassimilation. It is intriguing 2-ketoglutaric acid and isocitrate increased, by 3.6- and 7.5-fold, respectively, in the pmdh1pmdh2 plants compared with the wild-type plants (Supplemental Table S1). This may suggest that the altered consumption of reductant (NADH) in the peroxisomes due to the lack of PMDH caused the NADH/NAD ratio in the mitochondria to increase. An increase in mitochondrial NADH/NAD has been demonstrated to inhibit the NAD-dependent isocitrate dehydrogenase (Igamberdiev and Gardestrom, 2003) and may explain the relatively large increase in isocitrate in the pmdh1pmdh2 plants. The increase in 2-oxoglutarate could potentially be attributed to an alteration in the Glu requirement for recycling nitrogen through the GS/GOGAT-catalyzed reactions. However, a full evaluation of the metabolic basis for the greater CO2 release in the absence of PMDH will require more detailed analysis in the future.
How Is the Stoichiometry of CO2 Release Altered?
The data reported here with the pmdh1pmdh2 mutant clearly show that some restrictions to the photorespiratory pathway can lead to changes in the pathway of carbon and photorespiratory CO2 release. Indeed, the results here represent the most dramatic demonstration of this reported in the literature. Studies on various photorespiratory mutants have not consistently agreed on the way that potential CO2 release steps may be altered. Studies with a barley mutant deficient in Ser:glyoxylate aminotransferase demonstrated significantly elevated release of labeled CO2 from exogenously supplied glyoxylate (Murray et al., 1987). In contrast, a HPR barley mutant (Murray et al., 1987) showed little evidence for an increased CO2 release, even though there was considerable photosynthesis in air. A barley mutant deficient in catalase (Kendall et al., 1983) showed no evidence for increased CO2 release, while an Arabidopsis transgenic plant with antisense reduction in the levels of the 2-oxoglutarate/malate translocator (Schneidereit et al., 2006) showed a significant increase in Γ.
Based on previous studies, the most obvious explanation for an increased CO2 release stoichiometry would be an alternative oxidative decarboxylation of accumulating pools of glyxoylate and hydroxypyruvate through their nonenzymatic interactions with H2O2 to produce formate and glycolate, respectively, plus CO2 (Halliwel, 1974; Halliwel and Butt, 1974; Grodzinski, 1979; Walton and Butt, 1981; Wingler et al., 1999). In addition, the resulting formate can also be decarboxylated in the peroxisomes by its oxidation via catalase and H2O2 (Halliwel and Butt, 1974) or can be oxidized to CO2 by formate dehydrogenase in the mitochondrion (Hourton-Cabassa et al., 1998). These potential reactions are shown in Figure 5. The potential for these alternative decarboxylations to occur would increase under conditions in which both glyoxylate and hydroxypyruvate accumulate, as would be expected for the pmdh1pmdh2 mutant described here. Only mutations inactivating PMDH, HPR, or glycerate processing steps would be expected to elevate hydroxypyruvate. Most of the previously studied photorespiratory mutants are blocked in earlier steps, and it is possible that the large increased in CO2 release stoichiometry observed here may be due to the greater potential for hydroxypyruvate decarboxylation and glycolate recycling in the peroxisome (Walton and Butt, 1981).
CONCLUSION
The peroxisomal NAD+-malate dehydrogenase 2 (pmdh2) appears to play a more prominent role than the peroxisomal NAD+-malate dehydrogenase 1 (pmdh1) during photorespiration. Rates of photosynthesis in air were reduced in the double pmdh1pmdh2 mutant, but by only 30%. The rates of gross O2 evolution and uptake were similar in pmdh1pmdh2 and wild-type plants, indicating that chloroplast linear electron transport and photorespiratory O2 uptake by Rubisco were similar between genotypes. The CO2 PIB and the rate of CO2 released during photorespiration were both greater in the pmdh1pmdh2 mutant compared with the wild-type plants, suggesting that the ratio of photorespiratory CO2 release to Rubisco oxygenation was altered in the mutant. In summary, PMDH is essential for maintaining optimal rates of photorespiration in air; however, in its absence, significant rates of photorespiration are still possible, indicating that there are additional mechanisms for supplying reductant to the peroxisomal HPR reaction or that the HPR reaction is altogether circumvented.
MATERIALS AND METHODS
Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized, stratified, and allowed to imbibe on wet filter paper. Seeds were germinated on half-strength Murashige and Skoog medium with 1% (w/v) Suc and then transferred to potting soil (Debco seedling raising mix). For the gas-exchange measurements and the high-CO2 growth analysis, plants were grown under 2.8 mbar of CO2 in a controlled-environment growth cabinet at an irradiance of 200 μmol quanta m−2 s−1 at plant height, and air temperature of 25°C during the day and 15°C at night, with a daylength of 14 h. Wild-type and pmdh1pmdh2 plants used for the growth analysis under ambient CO2 concentrations were grown during the southern hemisphere's autumn month of May in a glasshouse under natural light conditions (25°C day and 15°C night temperatures). For all plants, 0.9-L pots were used, and the potting soil was mixed with 2.4 to 4 g Osmocote/L soil (15:4.8:10.8:1.2 nitrogen:phosphorus:potassium:magnesium + trace elements: boron, copper, iron, manganese, molybdenum, and zinc; Scotts Australia) and watered daily.
Gas-Exchange Measurements
Measurements of Net CO2 Assimilation Rates
Individual plants were brought from the growth chambers into the laboratory, and a single leaf was dark adapted for 30 min in the LI6400 (LiCor Biosciences) leaf chamber (LI6400-40). Gas-exchange measurements were made at pO2 of 200 mbar, irradiance of 500 μmol quanta m−2 s−1, and leaf temperature of 25°C. The Γ measurements shown in Table I were inferred from the initial slope of a CO2 response curve at lowest CO2 concentrations. All other values of Γ were measured on the mass spectrometer as described below.
Mass Spectrometric Measurements
The exchange of O2 and CO2 was measured from leaf discs placed into a closed leaf chamber attached to a mass spectrometer (Isoprime Micromass) as described previously (Maxwell et al., 1998; Ruuska et al., 2000). After the leaf disc was placed in the chamber, the chamber was flushed with nitrogen gas and a volume of pure oxygen enriched in 18O was injected to obtain an O2 concentration of 10%, 20%, or 40%. After a 10-min dark period, the leaf disc was illuminated (300 μmol quanta m−2 s−1) for 10 min, then darkened for 5 min, and again illuminated for 10 min (Fig. 1). The PIB was determined after the initial 10-min light period, and the parameters relating to the Γ were determined at the end of the final light period. CO2 enriched in 13C (13CO2; 99% enrichment) was injected into the leaf chamber to saturate the rates of the Rubisco carboxylation reaction in the light. The PIB and the photorespiratory CO2 release in the light were estimated from the rate of 12CO2 released. The rate of CO2 release was calculated from the 12CO2 concentration signal by measuring the slope of a moving five-point window (10-s width) over the data during this time period, and the peak values for rates were determined. To correct for the contamination of 12CO2 during the injection of 13CO2, several injections were conducted with an empty leaf chamber throughout each day that measurements were conducted. The background 12CO2 increase during the blank injection was subtracted from the data collected during the measurement with the leaf disc enclosed in the chamber.
Growth Analysis
Growth analyses were done on a leaf area expansion basis by nondestructively measuring the displayed leaf area of individual plants at time intervals. Total leaf area (including petioles) was measured by imaging the chlorophyll fluorescence (CF Imager; Technologica) and using the leaf area function available for total fluorescent pixels of each plant. The area was calibrated against a known image standard. Leaf area expansion rates are calculated from the slope of ln(area) versus time in units of cm2 cm−2 d−1, and means and se values are reported for four to five separate plants imaged over a 7- to 10-d time course during the exponential growth phase of each seedling. Imaging commenced at approximately 22 d after sowing.
Metabolite Analysis
Plant tissues (10–30 mg) were snap frozen and pulverized in liquid nitrogen. The enzymes in the tissue were inactivated by heating at 70°C for 15 min in 500 μL of extraction solution (methanol:water = 20:3, with adipic acid as internal standard). Cells debris was removed by centrifugation. For each sample, 60 μL of supernatant was transferred to a new tube and dried using a SpeedVac. The residue was dissolved in 20 μL of pyridine with methoxylamine hydrochloride (20 mg mL−1) and incubated at 30°C for 90 min with shaking. Metabolites were converted to tert-butyldimethylsilyl derivatives by reacting at 70°C for 30 min with 30 μL of N-methyl-N-[tert-butyldimethylsilyl]trifluoroacetimide plus 1% tert-butyldimetheylchlorosilane. The metabolite derivatives were analyzed by GC-MS (Agilent 6890 gas chromatograph coupled with a 5973N mass selective detector). GC-MS data were processed by AMDIS. Metabolite derivatives were identified by comparison of the retention time and mass spectra of authentic standards, and the amount of metabolites was calculated by total ion current signal of each metabolite peak normalized to the internal standard and tissue weight.
Rubisco Content
The content of Rubisco catalytic sites was measured by stoichiometric binding of [14C]carboxy-arabinitol-P2, and its carbamylation status was measured by exchanging [14C]carboxy-arabinitol-P2 loosely bound at uncarbamylated sites with an excess of unlabeled C-carboxy-arabinitol-P2 (Ruuska et al., 1998). Leaf tissue was ground on ice in 600 mL of extraction buffer (50 mm EPPS [4-2(2-hydroxyethyl)-1-piperazine propane sulfonic acid], pH 8.1, 1% polyvinylpolypyrrolidone, 1 mm EDTA, 10 mm dithiothreitol, and 0.1% Triton) with 20 μL of protease inhibitor cocktail (Sigma) and briefly centrifuged.
Calculations of vo and vc from MS Measurements
The calculations used were described previously (Ruuska et al., 2000). For each oxygenation reaction of Rubisco, 1 mol of O2 is consumed, and a further 0.5 mol of O2 is consumed during photorespiration by glycolate oxidase in the peroxisomes. We assume that O2 uptake by PSI via the Mehler reaction is negligible and that O2 uptake by mitochondria was the same in the light and dark. Therefore, vo was calculated as:
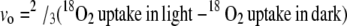 |
(1) |
If the whole chain electron transport results in NADPH production for photosynthesis and photorespiration, then vc can be calculated as:
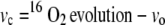 |
(2) |
Statistical Analysis
ANOVA was conducted using Student's t test in Statistica (version 6.0; StatSoft). Tukey's lsd test was used for post hoc comparisons.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Relative amounts of numerous primary metabolites that were identified reliably with GC-MS. Leaf material was harvested after 10 min at the Γ.
Supplementary Material
Acknowledgments
We thank Dave Zeelenberg for running the metabolite analysis and Steve Clayton for total nitrogen analysis. Additionally, A.B.C. thanks Susanne von Caemmerer for many helpful discussions.
This work was supported by the Australian Research Council (grant nos. FF0457721 and CE0561495) and the Centres of Excellence Program of the Government of Western Australia.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Steven M. Smith (ssmith@cyllene.uwa.edu.au).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Atkin OK, Millar AH, Gardestrom P, Day DA (2000) Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism, Vol 9. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 153–185
- Badger M (1985) Photosynthetic oxygen exchange. Annu Rev Plant Physiol 36 27–53 [Google Scholar]
- Donaldson RP (1982) Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys 215 274–279 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149 78–90 [DOI] [PubMed] [Google Scholar]
- Grodzinski B (1979) Study of formate production and oxidation in leaf peroxisomes during photo-respiration. Plant Physiol 63 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwel B (1974) Oxidation of formate by peroxisomes and mitochondria from spinach leaves. Biochem J 138 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwel B, Butt VS (1974) Oxidative decarboxylation of glycollate and glyoxylate by leaf peroxisomes. Biochem J 138 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Roje S (2001) One-carbon metabolism in higher plants. Annu Rev Plant Physiol Plant Mol Biol 52 119–137 [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta 1366 235–255 [Google Scholar]
- Hourton-Cabassa C, Ambard-Bretteville F, Moreau F, de Virville JD, Remy R, des Francs-Small CC (1998) Stress induction of mitochondrial formate dehydrogenase in potato leaves. Plant Physiol 116 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igamberdiev AU, Gardestrom P (2003) Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in mitochondria and cytosol of pea leaves. Biochim Biophys Acta 1606 117–125 [DOI] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL (1981) Species variation in the specificity of ribulose-biphosphate carboxylase-oxygenase. Nature 291 513–515 [Google Scholar]
- Kendall AC, Keys AJ, Turner JC, Lea PJ, Miflin BJ (1983) The isolation and characterization of a catalase-deficient mutant of barley (Hordeum vulgare L). Planta 159 505–511 [DOI] [PubMed] [Google Scholar]
- Kleczkowski LA, Randall DD, Zahler WL (1990) Adenylate kinase from maize leaves: true substrates, inhibition by P-1,P-5-di(adenosine-5′)pentaphosphate and kinetic mechanism. Z Naturforsch [C] 45 607–613 [Google Scholar]
- Laisk A, Edwards GE (1997) Post-illumination CO2 exchange and light-induced CO2 bursts during C-4 photosynthesis. Aust J Plant Physiol 24 517–528 [Google Scholar]
- Leegood R, Lea P, Adcock M, Hausler R (1995) The regulation and control of photorespiration. J Exp Bot 46 1397–1414 [Google Scholar]
- Madore M, Grodzinski B (1984) Effect of oxygen concentration on C-14-photoassimilate transport from leaves of Salvia splendens L. Plant Physiol 76 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Nishimura M (2005) Plant peroxisomes. Vitam Horm 72 111–154 [DOI] [PubMed] [Google Scholar]
- Maxwell K, Badger MR, Osmond CB (1998) A comparison of CO2 and O2 exchange patterns and the relationship with chlorophyll fluorescence during photosynthesis in C3 and CAM plants. Aust J Plant Physiol 25 45–52 [Google Scholar]
- Mouillon JM, Aubert S, Bourguignon J, Gout E, Douce R, Rebeille F (1999) Glycine and serine catabolism in non-photosynthetic higher plant cells: their role in C1 metabolism. Plant J 20 197–205 [DOI] [PubMed] [Google Scholar]
- Murray AJS, Blackwell RD, Joy KW, Lea PJ (1987) Photorespiratory-N donors, aminotransferase specificity and photosynthesis in a mutant of barley deficient in serine-glyoxylate aminotransferase activity. Planta 172 106–113 [DOI] [PubMed] [Google Scholar]
- Murray AJS, Blackwell RD, Lea PJ (1989) Metabolism of hydroxypyruvate in a mutant of barley lacking NADH-dependent hydroxypyruvate reductase, an important photorespiratory enzyme activity. Plant Physiol 91 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5 193–198 [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Cornah JE, Smith SM (2007) Arabidopsis peroxisomal malate dehydrogenase functions in beta-oxidation but not in the glyoxylate cycle. Plant J 50 381–390 [DOI] [PubMed] [Google Scholar]
- Reumann S (2000) The structural properties of plant peroxisomes and their metabolic significance. Biol Chem 381 639–648 [DOI] [PubMed] [Google Scholar]
- Reumann S, Weber APM (2006) Plant peroxisomes respire in the light: some gaps of the photorespiratory C2 cycle have become filled—others remain. Biochim Biophys Acta 1763 1496–1510 [DOI] [PubMed] [Google Scholar]
- Ruuska S, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S (1998) The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol 25 859–870 [Google Scholar]
- Ruuska S, Badger MR, Andrews TJ, von Caemmerer S (2000) Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. J Exp Bot 51 357–368 [DOI] [PubMed] [Google Scholar]
- Schneidereit J, Hausler RE, Fiene G, Kaiser WM, Weber APM (2006) Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J 45 206–224 [DOI] [PubMed] [Google Scholar]
- Somerville CR (2001) An early Arabidopsis demonstration: resolving a few issues concerning photorespiration. Plant Physiol 125 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL (1982) Genetic modification of photo-respiration. Trends Biochem Sci 7 171–174 [Google Scholar]
- Takahashi S, Bauwe H, Badger M (2007) Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol 144 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert NE (1997) The C2 oxidative photosynthetic carbon cycle. Annu Rev Plant Physiol Plant Mol Biol 48 1–25 [DOI] [PubMed] [Google Scholar]
- Vanroermund CWT, Elgersma Y, Singh N, Wanders RJA, Tabak HF (1995) The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J 14 3480–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153 376–387 [DOI] [PubMed] [Google Scholar]
- Walton NJ, Butt VS (1981) Metabolism and decarboxylation of glycollate and serine in leaf peroxisomes. Planta 153 225–231 [DOI] [PubMed] [Google Scholar]
- Wingler A, Lea P, Quick W, Leegood R (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Leegood RC (1999) Photorespiratory metabolism of glyoxylate and formate in glycine-accumulating mutants of barley and Amaranthus edulis. Planta 207 518–526 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.