Abstract
Indole acetic acid (IAA) is an important regulator of adventitious rooting via the activation of complex signaling cascades. In animals, carbon monoxide (CO), mainly generated by heme oxygenases (HOs), is a significant modulator of inflammatory reactions, affecting cell proliferation and the production of growth factors. In this report, we show that treatment with the auxin transport inhibitor naphthylphthalamic acid prevented auxin-mediated induction of adventitious rooting and also decreased the activity of HO and its by-product CO content. The application of IAA, HO-1 activator/CO donor hematin, or CO aqueous solution was able to alleviate the IAA depletion-induced inhibition of adventitious root formation. Meanwhile, IAA or hematin treatment rapidly activated HO activity or HO-1 protein expression, and CO content was also enhanced. The application of the HO-1-specific inhibitor zinc protoporphyrin IX (ZnPPIX) could inhibit the above IAA and hematin responses. CO aqueous solution treatment was able to ameliorate the ZnPPIX-induced inhibition of adventitious rooting. Molecular evidence further showed that ZnPPIX mimicked the effects of naphthylphthalamic acid on the inhibition of adventitious rooting, the down-regulation of one DnaJ-like gene (CSDNAJ-1), and two calcium-dependent protein kinase genes (CSCDPK1 and CSCDPK5). Application of CO aqueous solution not only dose-dependently blocked IAA depletion-induced inhibition of adventitious rooting but also enhanced endogenous CO content and up-regulated CSDNAJ-1 and CSCDPK1/5 transcripts. Together, we provided pharmacological, physiological, and molecular evidence that auxin rapidly activates HO activity and that the product of HO action, CO, then triggers the signal transduction events that lead to the auxin responses of adventitious root formation in cucumber (Cucumis sativus).
Adventitious root formation involves the development of a meristematic tissue after removal of the primary root system. It is well known that adventitious root formation is a complex process that is affected by multiple endogenous factors, including different phytohormones, and various environmental factors, such as light and wounding. Among phytohormones, auxin is particularly important because it can initiate cell division and primordium formation (Doerner, 2000; Berleth and Sachs, 2001; Casimiro et al., 2001).
Normally, auxin can induce various distinct developmental responses through direct effects on membrane or cytoskeletal functions or by regulating the expression of many genes or altering related protein activities. For example, DnaJ-like genes have been identified in organisms ranging from yeast to plants and humans. All DnaJ-like proteins are characterized by a J domain, which mediates interactions with Hsp70 in protein folding and the assembly and disassembly of protein complexes. When alternative in vitro morphogenesis processes were induced by auxins, a preferential accumulation of MsJ1, known as an alfalfa (Medicago sativa) DnaJ-like gene, message occurred during root initiation and formation, illustrating a phase-specific modulation during the cell cycle in G2/M (Frugis et al., 1999). Analysis of gene expression showed that CSDNAJ-1 is expressed in cucumber (Cucumis sativus) seedlings in all tissues but is exceedingly high in hypocotyledons and roots. The level of CSDNAJ-1 transcript was also transiently increased by a factor of 1.5 to 2.0 when heat shock was applied to the seedlings (Preisig-Muller and Kindl, 1993). Additionally, calcium-dependent protein kinases (CDPKs) constitute a unique family of enzymes that is characterized by a C-terminal calmodulin-like domain. Treatment of phytohormones, including indole acetic acid (IAA), 6-benzyladenine, abscisic acid, and GA, enhanced the accumulation of CSCPK5 transcript in etiolated cucumber cotyledons (Kumar et al., 2004). Recently, it was suggested that, together with nitric oxide (NO), which also increases the activity of CDPK and phospholipase D, IAA plays a role in a signaling pathway that regulates CDPK and phospholipase D activity, leading to cucumber adventitious root formation (Lanteri et al., 2006, 2008).
Carbon monoxide (CO) is one of the most important reactive trace gases in the troposphere. Since the 17th century, CO has been known as a poisonous gas, also termed the silent killer. However, in the last few years, evidence has accumulated showing that CO is a significant modulator of inflammatory reactions, influencing underlying processes such as cell proliferation and the production of cytokines and growth factors (Verma et al., 1993; Coceani, 2000; Piantadosi, 2002; Ryter et al., 2002). It was proven that upon the application of hydrogen peroxide or ascorbic acid, heme methylene bridges can be broken and CO released (Dulak and Józkowicz, 2003). Besides the nonenzymatic heme metabolism, which as mentioned above occurs in vivo, the majority of CO in mammalian cells is generated by heme oxygenases (HOs; EC 1.14.99.3). It was well known that HO in animals can catalyze the conversion of heme to biliverdin IXα (BV), CO, and free iron (Fe2+) through successive reduction and oxygenation reactions in the presence of molecular oxygen and electrons supplied by NADPH (Ryter et al., 2002; Gohya et al., 2006). There are at least three isoforms of HO. HO-1 is the inducible isoform. An increased cellular stress level is one common denominator for most of the stimuli to up-regulate de novo transcription of HO-1. For example, in addition to oxidants, the induction of the HO-1 gene also follows cellular exposure to agents such as growth factor, proinflammatory cytokines, bacterial endotoxins, NO, and tumor promoters. Interestingly, the rat HO-1 protein classifies as a heat shock protein (HSP32) since it responds to transcriptional regulation by heat (42°C), and the 5′ regulatory region of its gene contains heat shock elements resembling those described in the promoter regions of heat shock genes (i.e. HSP70; Ewing and Maines, 1991). HO-2 is constitutively expressed in many mammalian cells, and HO-3 is also a constitutive isoform of HO (Dulak and Józkowicz, 2003).
As in the animal kingdom, the presence of CO biosynthesis in plants was first reported by Wilks (1959). Since then, there have been several reports on the photoproduction of CO from living plants, which increases linearly with solar actinic irradiance and CO2-O2 ratio in the ambient atmosphere (Lüttge and Fischer, 1980; Tarr et al., 1995; Schade et al., 1999). Interestingly, germinating seeds of rye (Secale cereale), pea (Pisum sativum), cucumber, and lettuce (Lactuca sativa) also produced CO at levels of 10 to 25 μL L−1, and neither light nor chlorophyll was necessary for the above CO formation (Siegel et al., 1962), suggesting that there was an existing novel route for CO formation in higher plants. Muramoto et al. (2002) also found that a plastid HO (AtHO1) recombinant protein exhibits the ability to catalyze the formation of CO from heme with the concomitant production of BV in vitro. Until now, HO-1 has been claimed as the sole enzymatic source of CO as well as being responsible for the biosynthetic pathway leading to phytochrome metabolism and exhibiting the antioxidant behavior in plants (Davis et al., 2001; Noriega et al., 2004; Han et al., 2007, 2008).
In comparison with animal studies, it is generally agreed that CO at high concentrations inhibits plant growth and development (Wilks, 1959). On the other hand, Zimmerman et al. (1933) found that exogenous CO induced the initiation and stimulation of adventitious roots in plants, but little was known about the underlying mechanism of the above role of endogenous CO or even its signal transduction pathway. Recently, our results proved that exogenous CO induces adventitious rooting of hypocotyl cuttings (primary roots removed) from mung bean (Vigna radiata) seedlings (Xu et al., 2006). More recently, we also found that CO produced by HO might mediate the induction of growth elongation of wheat (Triticum aestivum) root segments by IAA, which might be related to NO/cGMP-dependent pathways (Xuan et al., 2007, 2008). However, whether the endogenous HO/CO signal system is involved in the auxin-induced adventitious rooting process remains to be identified.
Moreover, it was well known that the depletion of endogenous auxin appears to be a useful tool to investigate the auxin signal transduction pathway (Reed et al., 1998). Using this experimental approach, we provide pharmacological, physiological, and molecular evidence here that HO/CO, besides exhibiting antioxidative activity, also acts as a novel downstream signal system in the auxin-induced pathway that leads to cucumber adventitious root formation.
RESULTS
Hematin- or Hemin-Induced Adventitious Rooting in a Dose-Dependent Manner
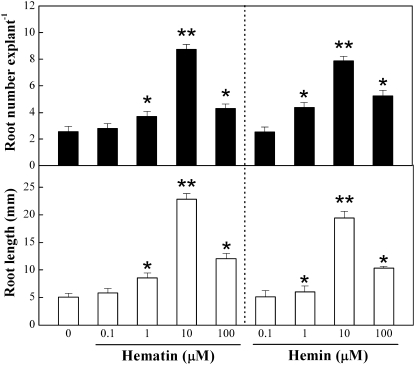
We discovered that exogenous hematin or hemin, two HO-1 activators (also termed CO artificial donor) applied in animal and plant research (Lamar et al., 1996; Han et al., 2007; Xuan et al., 2007), induced adventitious rooting in cucumber explants (without any pretreatment). Figure 1 shows two parameters of root growth, adventitious root number and length, in cucumber explants. As expected (Xu et al., 2006), in comparison with water treatment, concentrations between 0.1 and 100 μm hematin and hemin significantly induced adventitious root numbers and root length in a dose-dependent manner (P < 0.05 or P < 0.01), with a maximal response at 10 μm hematin and hemin.
Figure 1.
Effects of hematin or hemin on the induction of adventitious root formation. The primary root system was removed from hypocotyls of 5-d-old germinated cucumbers. Explants without any pretreatment were incubated with water, hematin, or hemin at the indicated concentrations. Adventitious root number and length were determined after 5 d of treatment. Mean and se values were calculated from at least three independent experiments (n = 20). Bars with asterisks were significantly different in comparison with water treatment at P < 0.05 and P < 0.01 (t test).
The IAA Depletion Treatment Not Only Prevents Adventitious Root Formation, But Also Decreases Endogenous HO Activity and CO Content
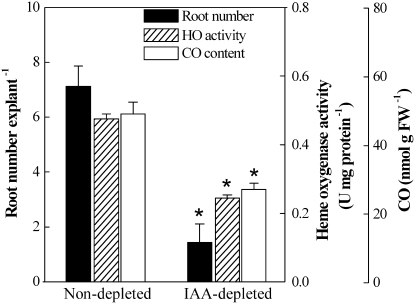
We further applied an inhibitor of basipetal auxin efflux, naphthylphthalamic acid (NPA; 10 μm, auxin depleted), to determine if inhibiting auxin transport would influence the adventitious rooting process, HO activity, and CO content. As expected, NPA applied to decapitated cucumber explants for 2 d before primary root removal, which was reported to reduce basal IAA concentration and inhibit adventitious rooting (Nordström and Eliasson, 1991), prevented adventitious root formation (P < 0.05; Fig. 2) with respect to that of nondepleted treatment. Figure 2 also shows that after the IAA depletion treatment for 2 d, HO activity and CO content in cucumber hypocotyls were significantly lower than those of nondepletion-treated samples. Taken together, these results clearly indicate a possible interrelationship among IAA, HO, and CO during adventitious root formation.
Figure 2.
Effects of IAA depletion treatment on adventitious root formation, HO activity, and CO content in cucumber explants. Explants treated with water (nondepleted) or with IAA depletion pretreatment (10 μm NPA; IAA depleted) for 2 d were further incubated in water for another 5 d, then root number per explant was recorded. HO activity and CO content were determined after 2 d of water or IAA depletion treatment. Mean and se values were calculated from at least three independent experiments (n=20). Bars with asterisks were significantly different in comparison with water treatment at P < 0.05 (t test). FW, Fresh weight.
The IAA Depletion-Induced Inhibition of Adventitious Root Formation Is Alleviated by Sodium Nitroprusside, IAA, and Hematin Treatments
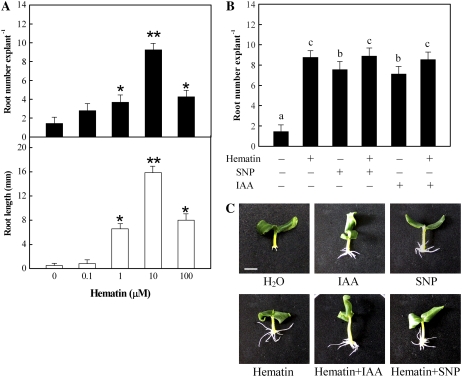
As was reported previously (Pagnussat et al., 2003), the NO donor sodium nitroprusside (SNP; 10 μm) was able to mimic the effect of IAA (10 μm) in restoring adventitious root formation in comparison with the inhibition effect conferred by IAA-depletion treatment alone (Fig. 3, B and C). To test the possible role of HO/CO in auxin signaling leading to adventitious root formation, we added hematin to auxin-depleted cucumber explants and monitored the effect on the NPA-induced process. As shown in Figure 3A, hematin at concentrations lower than 1 μm had no apparent effect on adventitious root formation, while a concentration-dependent restoration of adventitious root formation was observed when hematin concentration was ≥1 μm. The promotion of adventitious root development in auxin-depleted cucumber explants was maximal at 10 μm hematin and at 10 μm SNP or 10 μm IAA (data not shown). We also noted that hematin response was greater in comparison with the IAA or SNP effect (Fig. 3B). Thus, a hematin concentration of 10 μm was used to investigate the role of the HO/CO system throughout this study. Meanwhile, no additive effects of hematin with IAA or SNP were discovered (Fig. 3, B and C). In contrast, previous results reported by Pagnussat et al. (2002) showed that treatment with SNP or S-nitroso,N-acetyl penicillamine, another NO donor, plus IAA resulted in an increase in the induction of adventitious root development.
Figure 3.
Hematin, IAA, and SNP alleviate the IAA depletion-induced inhibition of adventitious root development in cucumber. Explants with auxin depletion pretreatment were further incubated as indicated for 5 d. A, Hematin-induced adventitious rooting in cucumber. B, No additive effects of hematin (10 μm) with IAA (10 μm) or SNP (10 μm) were discovered. C, Photographs were taken after 5 d of treatment. Bar=1 cm. Mean and se values were calculated from at least three independent experiments (n=20). Bars with asterisks were significantly different in comparison with water treatment (0 μm hematin) at P < 0.05 and P < 0.01 according to t test (A). Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan's multiple test (B).
HO Activity and CO Content Are Increased in Response to IAA, Hematin, and SNP Application
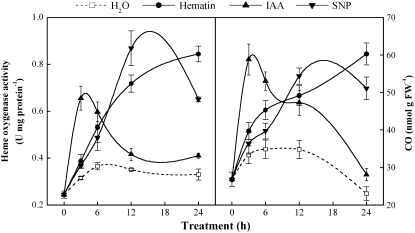
The IAA- and NO-like effect of applying the HO-1 activator hematin to auxin-depleted cucumber explants first led us to investigate whether changes in the endogenous levels of HO activity and CO content occurred in response to IAA, hematin, and SNP. In our experiment, auxin-depleted explants were treated with 10 μm IAA, hematin, or SNP, and CO synthesis in cucumber hypocotyls was observed (Fig. 4). The IAA or SNP treatment induced rapid production of CO and induction of HO activity. The time course experiments showed that, in comparison with water-treated control samples, a rapidly maximum response was discovered as early as 3 h (IAA) or 12 h (SNP) after treatment, followed by a gradual decrease until 24 h. It was interesting that the course of the changes of CO level seemed to parallel the time course of the HO activities. A weaker increased level of CO content and HO activities around 12 h after treatment in the control sample was also observed.
Figure 4.
IAA, hematin, and SNP treatments induce endogenous HO activity and CO content rapidly in auxin-depleted cucumber explants. Explants were incubated in water, IAA (10 μm), hematin (10 μm), or SNP (10 μm), and then HO activity and CO content were measured at the indicated times. Mean and se values were calculated from at least three independent experiments (n = 20). FW, Fresh weight.
Hematin-induced HO activity and CO content are different from those with IAA or SNP treatment. Figure 4 shows that HO activity and CO content rapidly increased within 6 h after hematin treatment and continued to increase relatively slowly up to 24 h. In fact, the effects of hematin are approximately in a time-dependent manner during 24 h of treatment. We also noted that the enhancement of CO synthesis and HO activity induced by IAA, SNP, or hematin apparently preceded adventitious root formation.
The Application of Zinc Protoporphyrin IX to Auxin-Depleted Cucumber Explants Inhibits the IAA and Hematin Responses
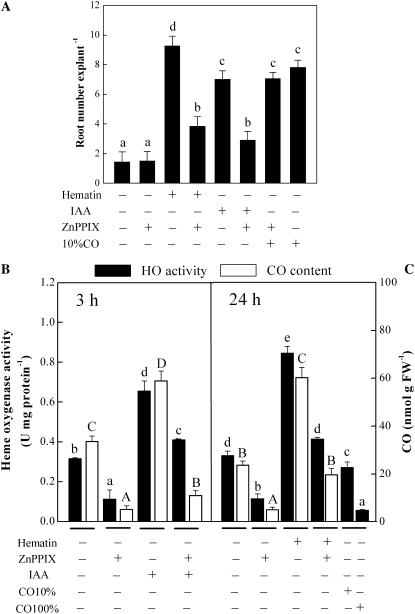
Our further goal was to confirm that the nature of the hematin- or IAA-induced restoration of the inhibition of adventitious root formation induced by IAA depletion is related to HO activity and CO content. Various compounds have been used to inhibit CO synthesis derived from HO in animals and plants. The potent HO-1 inhibitor zinc protoporphyrin IX (ZnPPIX) was first found to inhibit HO activity in both animals and plants (Lamar et al., 1996; Iyer et al., 2003; Lang et al., 2005; Liu et al., 2007; Xuan et al., 2007; Song et al., 2008). In our test, treating auxin-depleted cucumber explants with this compound prevents the induction action of IAA or hematin on adventitious root formation (Fig. 5A). However, when exogenous 10% saturation of CO aqueous solution was added together with ZnPPIX, the root number inhibited by ZnPPIX treatment was relieved and returned to a similar extent to that displayed in explants treated with 10% CO aqueous solution alone, which confirmed that changes in endogenous CO are likely to be involved in adventitious rooting and that HO-mediated CO production plays an important role in this process. Meanwhile, the application of ZnPPIX alone did not produce a different effect from that of the water-treated control sample.
Figure 5.
Application of the HO-1 potent inhibitor ZnPPIX to auxin-depleted cucumber explants inhibits the IAA and hematin response. Auxin-depleted cucumber explants were incubated with hematin (10 μm), IAA (10 μm), ZnPPIX (200 μm), 10% or 100% saturation of CO aqueous solutions alone, or the combination solutions, then root number per explant was recorded after 5 d of treatment (A). HO activity and CO content were assayed after the indicated times (B, 3 h; C, 24 h). Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan's multiple test. FW, Fresh weight.
In the absence of added hematin or IAA, ZnPPIX-inhibited HO activity and CO content in auxin-depleted cucumber explants were significantly lower than those of control samples at 3 and 24 h (Fig. 5, B and C). When the above samples were treated simultaneously with hematin or IAA, the IAA- or hematin-induced HO activity and CO content were partially blocked. Interestingly, 100% saturation of CO aqueous solution applied for 24 h severely inhibited HO activity in cucumber explants, while 10% aqueous solution exhibited a weaker effect. This was consistent with our previous result (Han et al., 2008), which showed that in comparison with cadmium-treated sample, pretreatment with 50% CO saturation aqueous solution was able to decrease the level of HO-1 transcript or HO activity after further cadmium treatment at 12 h in alfalfa seedling roots.
Induction of HO in Response to IAA
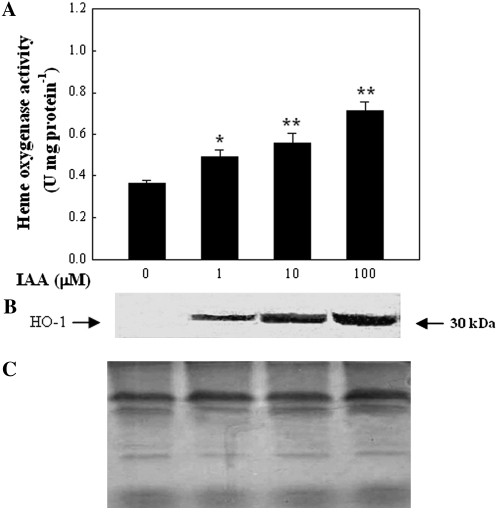
To assess if HO is associated with the auxin response leading to adventitious rooting, we undertook a detailed study on the auxin-induced expression of this enzyme. Hypocotyls of auxin-depleted cucumber explants were tested for HO accumulation, through enzymatic activity determination and immunoblot analysis, at 6 h after the further application of IAA at 0 to 100 μm. The results in Figure 6A demonstrate that IAA induced HO activity in a dose-dependent manner. Western-blot analysis for HO-1 showed only a single band with a molecular mass of 30 kD, as determined using molecular mass markers (data not shown), a similar value to that reported for the alfalfa root nodules (Baudouin et al., 2004) and soybean leaves (Yannarelli et al., 2006). Additionally, western-blot analysis also revealed an approximate correlation between inducible HO-1 protein level and the enhancement of HO activity. Recent results have confirmed that changes in the relative abundance of the HO-1 transcript or its protein expression were similar to those for CO content or HO activity under cadmium stress or UV-B radiation (Yannarelli et al., 2006; Han et al., 2008).
Figure 6.
IAA dose-dependently induces HO activity (A) and HO-1 protein expression (B) in auxin-depleted cucumber explants. Explants were incubated with IAA at the indicated concentrations for 6 h. Coomassie Brilliant Blue-stained gels (C) are present to show that equal amounts of proteins were loaded. Bars with asterisks were significantly different in comparison with water treatment (0 μm IAA) at P < 0.05 and P < 0.01 according to t test (A). Three independent experiments were performed, and the immunoblotting results show similar trends of protein expression (B).
Effects of NPA, ZnPPIX, and IAA on Adventitious Rooting, and the Expression Profiles of CSDNAJ-1 and CSCDPK1/5
In our experiments, NPA was shown not only to prevent adventitious root formation but also to decrease endogenous HO activity and CO content (Fig. 2). Thus, we first tested whether NPA and ZnPPIX could affect cucumber adventitious root formation and CSDNAJ-1 and CSCDPK1/5 transcripts.
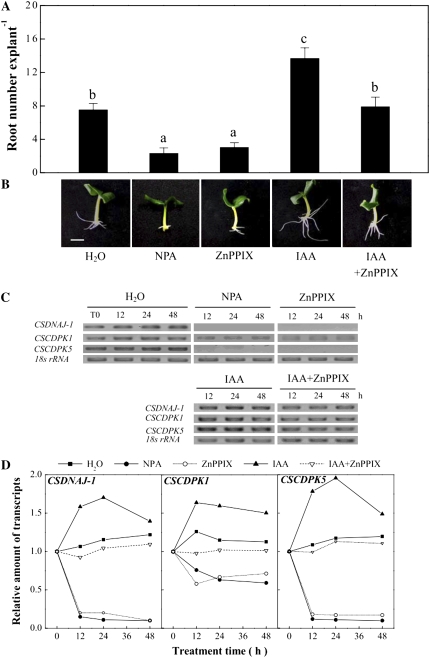
In the following experiment, IAA-nondepleted cucumber explants, after removing the primary root, were incubated in water, NPA (10 μm), or ZnPPIX (200 μm) for 2 d. Figure 7, A and B, shows that both NPA and ZnPPIX pretreatment resulted in a significant reduction of adventitious root formation (P < 0.05), with respect to the effect of water pretreatment for another 5 d. Semiquantitative RT-PCR results (Fig. 7, C and D) illustrated that CSDNAJ-1 and CSCDPK1/5 transcripts were slightly enhanced in water treatment within 48 h, but CSDNAJ-1 and CSCDPK5 were totally blocked since the beginning of NPA or ZnPPIX pretreatment. Meanwhile, CSCDPK1 transcript decreased abruptly at 12 h of pretreatment and kept the steady-state amount during the resting time. These results clearly show that ZnPPIX exhibited a similar effect of NPA on the above parameters.
Figure 7.
Effects of NPA, ZnPPIX, and IAA on adventitious rooting, and the expression profiles of CSDNAJ-1 and CSCDPK1/5 in auxin-nondepleted cucumber explants. A, After the primary root system was removed from hypocotyls of 5-d-old germinated cucumbers, explants were preincubated with water, NPA (10 μm), ZnPPIX (200 μm), IAA (10 μm) alone, or IAA plus ZnPPIX for 2 d. Then, adventitious root number was quantified after another 5 d of treatment. Mean and se values were calculated from at least three independent experiments (n=20). B, Photographs were taken. Bar=1 cm. C, CSDNAJ-1 and CSCDPK1/5 expression was analyzed by semiquantitative RT-PCR. D, Quantitative time course analysis of CSDNAJ-1 and CSCDPK1/5 transcript levels under different treatment conditions. The data were obtained by densitometric analysis. The values represent relative transcript levels with respect to the data at time zero. Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan's multiple test.
Based on the results described above, the influence of IAA and ZnPPIX applied alone or in combination on adventitious rooting and the expression of CSDNAJ-1 and CSCDPK1/5 were analyzed. IAA induced the expression of CSDNAJ-1 and CSCDPK1/5 during 48 h of treatment (Fig. 7, C and D). Interestingly, the IAA-induced adventitious rooting and expression of CSDNAJ-1 and CSCDPK1/5 were prevented or delayed when ZnPPIX was added simultaneously (Fig. 7). These findings provided preliminary evidence and suggested that endogenous HO and its releasing products could modulate the expression of these genes, which are also involved in the IAA-induced effects, including adventitious rooting and the up-regulation of CSDNAJ-1 and CSCDPK1/5.
CO Aqueous Solution Blocks the IAA Depletion-Induced Inhibition of Adventitious Root Formation and Up-Regulates CSDNAJ-1 and CSCDPK1/5 Transcripts
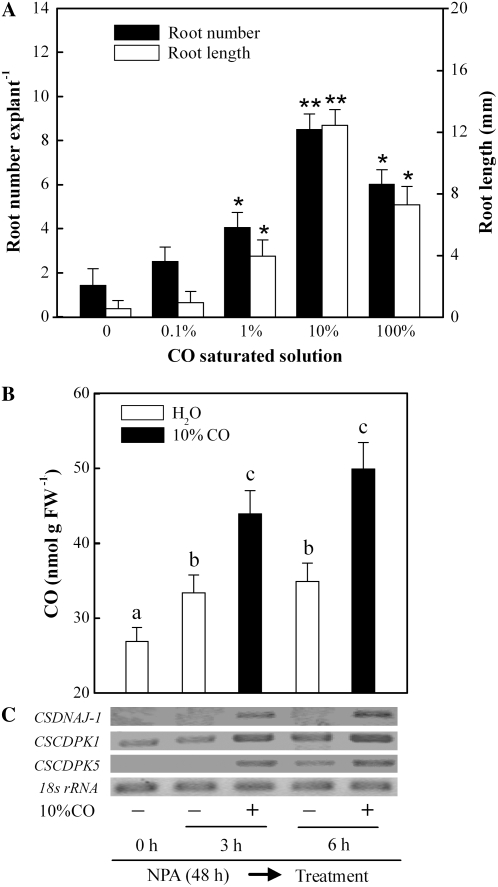
Further results also showed that the effect of CO aqueous solution on the restoration of the IAA depletion-induced inhibition of adventitious root formation was dose dependent (Fig. 8A), as in hematin-treated samples (Fig. 3A), further strengthening the hypothesis that CO produced by HO is responsible for inducing adventitious rooting. The promotion of adventitious root development was maximal with 10% CO saturated solution (approximately equivalent to 18.7 μm; P < 0.01), which was higher than the concentration of hematin applied (10 μm; Fig. 3A).
Figure 8.
Application of CO aqueous solution not only dose-dependently blocked the IAA depletion-induced inhibition of adventitious root formation but also enhanced CO content and up-regulated CSDNAJ-1 and CSCDPK1/5 transcripts in auxin-depleted explants. A, Adventitious root number and lengths were quantified after 5 d of treatment. B, CO level was determined following further water or 10% CO aqueous solution treatment during a 6-h period. C, CSDNAJ-1 and CSCDPK1/5 expression was analyzed by semiquantitative RT-PCR. Mean and se values were calculated from at least three independent experiments (n=20). Bars with asterisks were significantly different in comparison with water treatment at P < 0.05 and P < 0.01 according to t test (A). Bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan's multiple test (B).
We next investigated the molecular mechanism underlying adventitious root formation induced by 10% saturated CO aqueous solution in auxin-depleted cucumber explants. Interestingly, we observed that CO content in cucumber hypocotyls was elevated rapidly during the 6-h period when further treated with 10% CO aqueous solution, in comparison with water-treated samples (Fig. 8B), then declined rapidly (data not shown). Furthermore, to compare the mRNA changes of CSDNAJ-1 and CSCDPK, semiquantitative RT-PCR analysis was used. We discovered that the slightly enhanced expression pattern of CSCDPK1 following water treatment for 6 h was approximately similar to that of CSCDPK5, in comparison with the expression of CSDNAJ-1. After the addition of 10% CO aqueous solution, there were apparent increases in the levels of CSDNAJ-1 and CSCDPK1/5 mRNA in auxin-depleted explants (Fig. 8C), all of which were consistent with the restoration of the IAA depletion-induced inhibition of adventitious root formation (Fig. 8A).
DISCUSSION
Our recent work (Xuan et al., 2007) has shown that treatment with exogenous hematin or IAA can result in the strong induction of HO-1 transcript or CO content in wheat root segments, both of which were reversed by the addition of ZnPPIX, a potent inhibitor of HO-1 in both animals and plants. However, whether induction of HO-mediated CO synthesis by auxin is a general response of all plant cells still remains to be determined. In this report, we further demonstrate a previously uncharacterized signaling role for endogenous CO produced by HO, one of which is responsible for mediating IAA-induced adventitious root formation in auxin-depleted cucumber explants.
A Critical Window of CO Hormonal Function during the Adventitious Rooting Process, Which Is Similar to NO
Akin to NO in animals, CO was recently found to play various important roles in mediating neuronal transmission (Verma et al., 1993; Zhuo et al. 1993; Watts et al., 2003) and in the regulation of vasomotor tone (Morita et al., 1995). On the other hand, mounting evidence has demonstrated the presence of CO in plants (Wilks, 1959; Siegel et al., 1962; Lüttge and Fischer, 1980; Tarr et al., 1995; Schade et al., 1999), and its involvement in different biological processes was also found. For instance, it acts as a compound with hormonal effects, including affecting seed dormancy and germination (Dekker and Hargrove, 2002), inducing lateral root formation, and being involved in abscisic acid-induced stomatal closure (Cao et al., 2007a, 2007b), most of which were similar to some hormone-like behaviors reported for NO in plants (Beligni and Lamattina, 2000; Pagnussat et al., 2002; Delledonne, 2005; Hu et al., 2005).
Our previous report found that exogenous CO aqueous solution or its artificial donor hematin was able to induce adventitious rooting of hypocotyl cuttings (primary root removed) from mung bean seedlings (Xu et al., 2006); a similar phenomenon was first discovered using CO gas (Zimmerman et al., 1933). In this report, we further show that hematin and hemin, or CO aqueous solution, applied to auxin-nondepleted or auxin-depleted cucumber explants was able to dose-dependently induce adventitious root formation (Figs. 1, 3A, and 8A), mimicking the effects of the NO donor SNP and IAA (Pagnussat et al., 2002, 2003). Interestingly, we noticed that the effect of hematin on the length of adventitious roots (Fig. 3A) was more obvious than that of CO aqueous solution-treated samples (Fig. 8A) in auxin-depleted explants. Because Landaw et al. (1970) reported that after intravenous infusion of hematin-14C, rats produced equimolar amounts of labeled bilirubin and CO, the above varying effect might be derived from hematin- or hemin-induced HO's enzymatic reaction products, including BV and Fe2+. In our recent study, administration of CO aqueous solution could substitute for HO-1 or BV with respect to its cytoprotective role against oxidative damage in plant tissues (Han et al., 2008), further suggesting a role for CO as a key mediator of HO-1 function, as well as BV, as recently proven in plants (Noriega et al., 2004). Overall, our results further support the idea that exogenous CO, whether dissolved in water and applied in aqueous solution or used as a gas, or even its artificial synthesis activators (hematin and hemin, etc.), exhibits hormone-like properties in the induction of the adventitious rooting process, which is similar to NO recently proven in plants.
Endogenous CO Produced by HO Is a New Component of the Auxin Signaling Pathway Leading to Adventitious Rooting
Although a role for CO in root development was proposed, the cross talk between auxin and endogenous CO still remains to be examined. In the following experiments, we provided further pharmacological, physiological, and molecular evidence that endogenous CO and its synthesizing enzyme HO is a new system in the auxin signaling pathway leading to adventitious rooting.
Previous results showed that apical IAA production could be disrupted by decapitation of the explants, and basipetal transport of auxins was inhibited by NPA (Pagnussat et al., 2003). In our experiment, we discovered that IAA depletion not only prevented adventitious root formation but also decreased endogenous HO activity and CO content after IAA depletion for 2 d in cucumber explants (Fig. 2). Application of the HO-1 activator hematin (Fig. 3A) and CO aqueous solution (Fig. 8A) was able to dose-dependently restore adventitious rooting in auxin-depleted cucumber explants. Meanwhile, data obtained from treating cucumber explants with ZnPPIX indicate that it provoked a significant reduction in both IAA- and hematin-induced adventitious rooting, while no similar response was found in the treatment with 10% CO aqueous solution plus ZnPPIX (Fig. 5A). Thus, these results preliminarily suggested that endogenous CO produced by HO might play a key role in auxin-mediated adventitious root development.
In human monocytes, the application of hemin was able to induce HO-1 in a dose- and time-dependent fashion, as measured by semiquantitative RT-PCR and flow cytometry (Lang et al., 2005). Similar results were confirmed in plants using hematin (Han et al., 2007; Xuan et al., 2007). Furthermore, we demonstrated that HO is activated in vivo during IAA and hematin treatment, thus resulting in the enhancement of CO production. For example, Figure 4 shows that IAA and the HO-1 activator hematin enhanced HO activity and endogenous CO synthesis rapidly in auxin-depleted cucumber explants. Furthermore, application of the specific HO-1 inhibitor ZnPPIX could block the IAA- or hematin-induced restoration of adventitious root formation in auxin-depleted cucumber explants, both of which were consistent with the changes of HO activity and CO production (Fig. 5). Using enzyme activity analysis and western blotting, we also found that IAA induces HO activity and HO-1 protein expression in a dose-dependent manner in auxin-depleted cucumber explants (Fig. 6). We also deduced that the difference between the basal level of HO activity and the weaker HO-1 protein expression in control samples (Fig. 6, A and B) might be derived from the constitutively expressed HO-2/3 or from IAA depletion. Interestingly, ZnPPIX prevented the activation of HO activity and endogenous CO synthesis in both IAA- and hematin-treated explants (Fig. 5, B and C), suggesting that CO production requires the presence of HO in vivo. However, we do not yet know whether the activation of 30-kD HO-1 is accompanied by a parallel increase in cucumber HO-1 gene transcripts because it (gene or cDNA) has not been isolated yet. Taken together, these results suggest that the auxin signal may be transduced, at least in our experimental model system, through the activation of endogenous CO production by HO.
In a subsequent experiment, molecular evidence also illustrated that IAA treatment induced higher expression of the CSDNAJ-1 and CSCDPK1/5 genes during a 48-h treatment, and these were consistent with the number of adventitious roots observed after another 5-d treatment (Fig. 7). It was previously reported by Frugis et al. (1999) that the transcript of MsJ1, an alfalfa DnaJ-like gene, exhibited a phase-specific modulation during the cell cycle, with a 2-fold induction in G2/M of synchronized alfalfa suspension-cultured cells. An increase in MsJ1 transcript in naphthylacetic acid-treated callus was also detected in correspondence with adventitious root induction and formation. It is well known that protein synthesis occurs during all phases of the cell cycle but peaks in G2. Thus, a high expression of CSDNAJ-1 transcript (Fig. 7, C and D) at early stages of adventitious root formation might be consistent with a higher need for molecular chaperones to fold newly synthesized polypeptides requested for the cell division microtubule apparatus. Genetic results also showed that one yeast mutant defective in the synthesis of Ydj1, the dnaJ yeast homolog, has been found to be impaired in the formation of a mitotic spindle necessary for yeast cell division (Zarzov et al., 1997). Meanwhile, auxins have been proven to influence the expression and activity of CDPKs. For example, when tobacco (Nicotiana tabacum) leaves were treated with IAA, a slight induction of NtCDPK1 transcript was observed (Yoon et al., 1999). Some interesting results previously reported by Kobayashi and Fukuda (1994) and by Komatsu et al. (1996) showed that Ca2+-binding proteins and protein kinases were involved in plant differentiation processes. Recently, Lanteri et al. (2006) discovered that the activity of the 50-kD cucumber CDPK was induced by IAA and NO signal and was also required for cucumber adventitious root formation, suggesting that CDPK could be associated with cell dedifferentiation, division, and/or differentiation. Taking the above evidence into consideration along with our results, it can be suggested that CSDNAJ-1 and CSCDPK1/5 expression at the earlier stage might be required for auxin-induced adventitious root development.
Further molecular evidence showed that besides the inhibition of adventitious root formation, the potent HO-1 inhibitor ZnPPIX as well as NPA decreased the transcription of CSDNAJ-1 and CSCDPK1/5 in cucumber explants, in comparison with the water treatment. Additionally, ZnPPIX treatment could block the IAA responses of these parameters (Fig. 7). Moreover, the application of CO aqueous solution was able to up-regulate the expression of CSDNAJ-1 and CSCDPK1/5 inhibited by IAA-depleted treatment and dose-dependently led to the recovery of adventitious root formation (Fig. 8). Thus, we deduced that DnaJ-1 and CDPK might receive auxin signal or surrounding stimuli to regulate cell division and differentiation in the plant adventitious rooting process. Probably, CO produced by HO acts in a signaling cascade downstream of auxin to induce CSDNAJ-1 and CSCDPK1/5 gene expression and, consequently, cell division and differentiation are activated. Thus, future analysis of HO/CO involvement in the regulation of DnaJ-like genes, CDPK genes, other auxin-induced genes, cell division, and root primordia formation during the adventitious root formation process will surely contribute to our understanding of the molecular mechanisms that regulate root morphogenesis.
CONCLUDING REMARKS
The evidence provided here (Figs. 1–8) further confirmed that, at least in our experimental conditions, there exists a serial linkage IAA → HO/CO → adventitious rooting. Certainly, cloning of the cucumber HO genes and screening of the corresponding mutants will provide greater insights into the mechanisms that regulate adventitious root development and the role of HOs in this process.
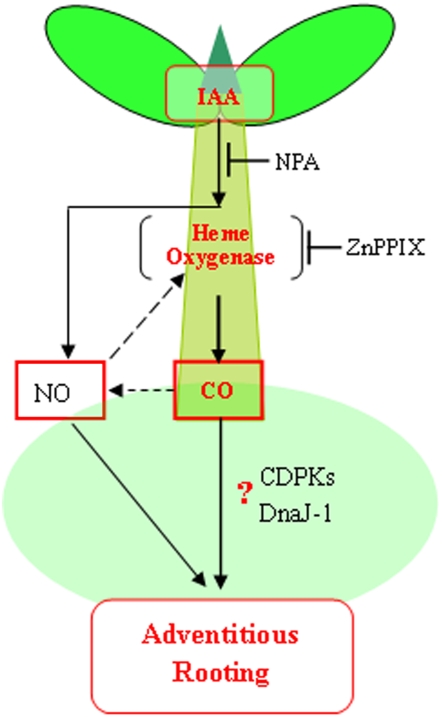
Figure 9 shows a representative scheme integrating the different molecules involved in adventitious root formation in cucumber, which were partially described here. Basipetal transport of auxins induces a CO burst synthesized by HO in the basal region of the cucumber hypocotyls. CO then triggers the signal transduction events that lead to the auxin responses of adventitious root formation. Additionally, this pathway might be mediated by the expression of DnaJ-1 and CDPK genes. Meanwhile, previous results have confirmed that IAA triggers a local and transient generation of NO. This NO production might activate a CDPK-dependent transduction pathway, thus leading to adventitious rooting (Pagnussat et al., 2002, 2003; Lanteri et al., 2006). On the other hand, we also discovered that the NO donor SNP used at 10 μm not only promoted adventitious rooting (Fig. 3) but also induced endogenous HO activity and CO synthesis in auxin-depleted cucumber explants (Fig. 4). Our recent results showed that exogenous application of 50% CO-saturated aqueous solution enhanced salt tolerance, and NO might be part of the downstream signal molecules of the above CO action by the maintenance of ion homeostasis and up-regulation of antioxidant defense in wheat seedling root tissues (Xie et al., 2008). Thus, the cross talk between endogenous HO/CO and NO during adventitious rooting still remains to be examined.
Figure 9.
Schematic representation of the signaling pathway involving auxin, the HO/CO system, and NO during the adventitious rooting process in cucumber. Basipetal transport of auxin induces a CO burst synthesized by HO in the basal region of hypocotyls, thus leading to adventitious root formation. This pathway might be mediated by the expression of DnaJ-1 and CDPK genes (question mark). Meanwhile, IAA triggers a local and transient generation of NO. This NO production might activate a CDPK-dependent transduction pathway, thus leading to adventitious rooting. Additionally, the possibility of cross talk between endogenous HO/CO and NO during adventitious rooting could not be ruled out. Dashed lines denote indirect or still undescribed pathways. T bars, Inhibition.
In conclusion, the results presented in this report are significant for both fundamental and applied plant biology. Our results suggest that HO/CO, besides exhibiting the antioxidative machinery recently discovered in the plant kingdom (Noriega et al., 2004, 2007; Liu et al., 2007; Han et al., 2008), also represent a new signal system with significant impact on auxin-induced adventitious root development. Thus, our results open the possibility of using either CO donor or HO activator to improve plant production. As a matter of fact, substantial CO gas, the most common cause of fatal poisoning in the world, is also emitted from plants into the atmosphere. Conversely, the toxic CO gas in the atmosphere, which is usually released by the combustion of fossil fuels, can influence plants. Therefore, it is likely that plant HO/CO, like those in animals, are part of macromolecular complexes in which CO functions within highly localized environments.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Cucumber seeds (Cucumis sativus ‘Lufeng’) were kindly supplied by Jiangsu Agricultural Institutes. Selected identical seeds were germinated in petri dishes on filter papers imbibed in distilled water, then transferred to an illuminating incubator and maintained at 25°C ± 1°C for 5 d with a 14-h photoperiod at 200 μmol m−2 s−1 intensity. Cucumber seedlings were used either intact (auxin nondepleted, 0 h) or decapitated by excising the apical bud immediately above the cotyledons and incubated in the presence of 10 μm NPA (auxin depleted) for 48 h, before removing the primary root. Cucumber explants were then maintained under the same conditions of temperature and photoperiod for another 5 d in the presence of different media as indicated.
Chemicals
All chemicals were obtained from Sigma unless stated otherwise. Hematin and hemin were used at concentrations of 0.1, 1.0, 10.0, and 100.0 μm. ZnPPIX, a specific inhibitor of HO-1, was used at 200 μm. SNP was used at 10 μm as NO donor. IAA was also purchased from Sigma. NPA, from Chem Service, was used as the auxin transport inhibitor at 10 μm.
CO Aqueous Solution Treatment and CO Content Determination
The preparation of CO aqueous solution and the determination of CO content in cucumber hypocotyls by gas chromatography and mass spectrometry were carried out according to the method described in our previous reports (Liu et al., 2007; Xuan et al., 2007; Han et al., 2008). The saturated stock solution (100% saturation) was diluted immediately with distilled water to the concentrations required (0.1%, 1%, and 10% saturation [v/v]). In our experimental conditions, the concentration of CO in the saturated stock solution was about 187 μm. Meanwhile, the half-life of CO loss from the stock solution at 30°C was about 210 min. Thus, CO aqueous solutions with or without other chemicals were supplied to plants by renewing the solution every 2 h over an early 6-h period, then applying daily to ensure that exogenous CO supply is continuous and sufficient, which could increase endogenous CO content approximately to the level triggered by IAA.
Explant Treatments
After primary roots were removed, eight cucumber explants were put into a petri dish containing 8 mL of distilled water, varying concentrations of IAA, hematin, hemin, and CO aqueous solution, 10 μm NPA or SNP, 200 μm ZnPPIX, or combination treatments and kept at 25°C ± 1°C for different periods according to the experiment. The concentrations of the above chemicals used for our experiments were supported by other published results (Pagnussat et al., 2002, 2003, 2004; Cao et al., 2007b; Xuan et al., 2007). Excised cucumber hypocotyls (5-mm-long segments of the hypocotyl base, where adventitious roots develops; Lanteri et al., 2008) were used for the following determination.
HO Activity Determination
HO activity from excised cucumber hypocotyls was analyzed using the method described in our previous reports (Liu et al., 2007; Xuan et al., 2007; Han et al., 2008). Excised cucumber hypocotyls (0.4 g) were homogenized using 30 mL of ice-cold isolation medium containing 250 mm mannitol, 25 mm HEPES-Tris (pH 7.4), 1 mm EDTA, 1% (w/v) polyvinylpyrrolidone, 10% (v/v) glycerol, and 1 mm dithiothreitol. The whole isolation procedure was carried out at 4°C. The homogenate was filtered through four layers of cheesecloth and centrifuged at 1,300g for 30 min. The supernatant was centrifuged at 60,000g for 30 min to yield a crude membrane fraction for further resuspension, which was used for HO activity determination. The assays (final volume, 80 μL) contained 20 μL of enzyme resuspension solution, 10 μm hemin, 0.15 mg mL−1 bovine serum albumin, 50 μg mL−1 (4.2 μm) spinach (Spinacia oleracea) ferredoxin (Sigma), 0.025 units mL−1 spinach ferredoxin-NADP+ reductase (Sigma), 5 mm ascorbate, and 2 mm desferrioxamine in 100 mm HEPES-NaOH (pH 7.2). The reaction was started by adding NADPH to a final concentration of 100 μm, samples were incubated at 37°C for 30 min, and BV formation was calculated by measuring the absorbance change at 650 nm. The concentration of BV was estimated using a molar absorption coefficient at 650 nm of 6.25 mm−1 cm−1 in 100 mm HEPES-NaOH (pH 7.2). One unit of activity was calculated as the quantity of enzyme needed to produce 1 nm BV per 30 min. Protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as the standard.
Western-Blot Analysis for HO-1
Homogenates obtained for HO activity assays were also analyzed by western blotting. Sixty micrograms of protein from homogenates were subjected to SDS-PAGE using a 12.5% acrylamide resolving gel (Mini Protean II System; Bio-Rad) according to Laemmli (1970). Separated proteins were then transferred to polyvinylidene difluoride membranes, and nonspecific binding of antibodies was blocked with 5% nonfat dried milk in PBS (pH 7.4) for 2 h at room temperature. Membranes were then incubated overnight at 4°C with primary antibodies raised against rice (Oryza sativa) HO-1 (OsHO1) diluted 1:200 in phosphate-buffered saline plus 1% nonfat milk. Immune complexes were detected using horseradish peroxidase-conjugated goat anti-rabbit IgG. The color was developed with a solution containing 3,3′-diaminobenzidine tetrahydrochloride as the horseradish peroxidase substrate.
Semiquantitative RT-PCR Analysis
Total RNA was isolated from 100 mg (fresh weight) of excised cucumber hypocotyls by grinding with mortar and pestle in liquid nitrogen until a fine powder appeared and using Trizol reagent (Invitrogen) according to the manufacturer's instructions. DNA-free total RNA (5 μg) from different treatments was used for first-strand cDNA synthesis in a 20-μL reaction volume containing 2.5 units of avian myeloblastosis virus reverse transcriptase XL (Takara) and 1 μm oligo(dT) primer. PCR was performed using 2 μL of a 2-fold dilution of the cDNA, 10 pmol of each oligonucleotide primer, and 1 unit of Taq polymerase (Takara) in a 25-μL reaction volume.
Primers used were as follows: for CSDNAJ-1 (accession no. X67695), forward (5′-AGGGTAAGGGTTCTAAAT-3′) and reverse (5′-CGACGAGAGACAAGGTAT-3′), amplifying a 390-bp fragment; for CSCDPK1 (accession no. AJ312239), forward (5′-TGCACTGACAAACCAACT-3′) and reverse (5′-ACACTCACAATAACCCCT-3′), amplifying a 284-bp fragment; for CSCDPK5 (accession no. AY027885), forward (5′-CAACTTCCCACCTCCTCCG-3′) and reverse (5′-GCTTCCCCATCTTCTTTCA-3′), amplifying an 869-bp fragment; for 18s rRNA (accession no. AF206894), forward (5′-CCTGAGAAACGGCTACCACA-3′) and reverse (5′-GATCCCGAAGGCCAACAAAA-3′), amplifying a 456-bp fragment. To standardize the results, the relative abundance of 18s rRNA was determined and used as the internal standard.
The cycle numbers of the PCR were adjusted for each gene to obtain visible bands on agarose gels. Aliquots from the PCR were loaded on 1.2% agarose gels with the use of ethidium bromide. Specific amplification products of the expected size were observed, and their identities were confirmed by sequencing.
Data Analysis
Where indicated, results are expressed as mean values ± se of at least three independent experiments (n = 20). Statistical analysis was performed using SPSS 8.0 software. For statistical analysis, t test (P < 0.05 or P < 0.01) or Duncan's multiple test (P < 0.05) was chosen as appropriate.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers X67695, AJ312239, AY027885, and AF206894.
Acknowledgments
We thank Dr. Evan Evans from the University of Tasmania, Australia, for his kind help in writing the manuscript.
This work was supported by the Program for New Century Excellent Talents in University (grant no. NCET–07–0441 to W.-B.S.), the Natural Science Foundation of Jiangsu Province of China (grant no. BK2007157 to W.-B.S.), the 111 Project (grant no. B07030), the National Fund for Fostering Talents of Basic Science (grant no. J0730647 to J.-Y.Q.), and the Student Research Training Project of Nanjing Agricultural University, China (grant no. 0506B07 to W.X.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wen-Biao Shen (wbshenh@njau.edu.cn).
References
- Baudouin E, Frendo P, Le Gleuher M, Puppo A (2004) A Medicago sativa haem oxygenase gene is preferentially expressed in root nodules. J Exp Bot 55 43–47 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210 215–221 [DOI] [PubMed] [Google Scholar]
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4 57–62 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Cao ZY, Huang BK, Wang QY, Xuan W, Ling TF, Zhang B, Chen X, Nie L, Shen WB (2007. a) Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull 52 2365–2373 [Google Scholar]
- Cao ZY, Xuan W, Liu ZY, Li XN, Zhao N, Xu P, Wang Z, Guan RZ, Shen WB (2007. b) Carbon monoxide promotes lateral root formation in rapeseed. J Integr Plant Biol 49 1007–1016 [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coceani F (2000) Carbon monoxide in vasoregulation. Circ Res 86 1184–1186 [DOI] [PubMed] [Google Scholar]
- Davis SJ, Bhoo SH, Durski AM, Walker JM, Vierstra RD (2001) The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol 126 656–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Hargrove M (2002) Weedy adaptation in Setaria spp. V. Effects of gaseous environment on giant foxtail (Setaria faberii) (Poaceae) seed germination. Am J Bot 89 410–416 [DOI] [PubMed] [Google Scholar]
- Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8 390–396 [DOI] [PubMed] [Google Scholar]
- Doerner P (2000) Plant stem cells: the only constant thing is change. Curr Biol 10 201–203 [DOI] [PubMed] [Google Scholar]
- Dulak J, Józkowicz A (2003) Carbon monoxide: a “new” gaseous modulator of gene expression. Acta Biochim Pol 50 31–47 [PubMed] [Google Scholar]
- Ewing JF, Maines MD (1991) Rapid induction of heme oxygenase-1 mRNA and protein by hyperthermia in rat brain: heme oxygenase-2 is not a heat shock protein. Proc Natl Acad Sci USA 88 5364–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugis G, Mele G, Giannino D, Mariotti D (1999) MsJ1, an alfalfa DnaJ-like gene, is tissue-specific and transcriptionally regulated during cell cycle. Plant Mol Biol 40 397–408 [DOI] [PubMed] [Google Scholar]
- Gohya T, Zhang X, Yoshida T, Migita CT (2006) Spectroscopic characterization of a higher plant heme oxygenase isoform-1 from Glycine max (soybean): coordination structure of the heme complex and catabolism of heme. FEBS J 273 5384–5399 [DOI] [PubMed] [Google Scholar]
- Han Y, Xuan W, Yu T, Fang WB, Lou TL, Gao Y, Chen XY, Xiao X, Shen WB (2007) Exogenous hematin alleviates mercury-induced oxidative damage in the roots of Medicago sativa. J Integr Plant Biol 49 1703–1713 [Google Scholar]
- Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S, Ding X, She WB (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa L. New Phytol 177 155–166 [DOI] [PubMed] [Google Scholar]
- Hu XY, Neill SJ, Tang ZC, Cai WM (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer JK, Shi L, Shankar AH, Sullivan DJ Jr (2003) Zinc protoporphyrin IX binds heme crystals to inhibit the process of crystallization in Plasmodium falciparum. Mol Med 9 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Fukuda H (1994) Involvement of calmodulin and calmodulin-binding proteins in the differentiation of tracheary elements in Zinnia cells. Planta 194 388–394 [Google Scholar]
- Komatsu S, Masuda T, Abe K (1996) Phosphorylation of a protein (pp56) is related to the regeneration of rice cultured suspension cells. Plant Cell Physiol 37 748–753 [DOI] [PubMed] [Google Scholar]
- Kumar KGS, Ullanat R, Jayabaskaran C (2004) Molecular cloning, characterization, tissue-specific and phytohormone-induced expression of calcium-dependent protein kinase gene in cucumber (Cucumis sativus L.). J Plant Physiol 161 1061–1071 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Lamar CA, Mahesh VB, Brann DW (1996) Regulation of gonadotrophin-releasing hormone (GnRH) secretion by heme molecules: a regulatory role for carbon monoxide? Endocrinology 137 790–793 [DOI] [PubMed] [Google Scholar]
- Landaw SA, Callahan EW, Schmid R (1970) Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest 49 914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Reuter S, Buzescu T, August C, Heidenreich S (2005) Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol 17 155–165 [DOI] [PubMed] [Google Scholar]
- Lanteri ML, Laxalt AM, Lamattina L (2008) Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventitious root formation in cucumber. Plant Physiol 147 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57 1341–1351 [DOI] [PubMed] [Google Scholar]
- Liu K, Xu S, Xuan W, Ling T, Cao Z, Huang B, Sun Y, Fang L, Liu Z, Zhao N, et al (2007) Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci 172 544–555 [Google Scholar]
- Lüttge U, Fischer K (1980) Light-dependent net CO-evolution by C3 and C4 plants. Planta 149 59–63 [DOI] [PubMed] [Google Scholar]
- Morita T, Perrella MA, Lee ME, Kourembanas S (1995) Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 92 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T (2002) Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 130 1958–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström AC, Eliasson L (1991) Levels of endogenous indole-3-acetic acid and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings. Physiol Plant 82 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega GO, Balestrasse KB, Batlle A, Tomaro ML (2004) Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem Biophys Res Commun 323 1003–1008 [DOI] [PubMed] [Google Scholar]
- Noriega GO, Yannarelli GG, Balestrasse KB, Batlle A, Tomaro ML (2007) The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 226 1155–1163 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA (2002) Biological chemistry of carbon monoxide. Antioxid Redox Signal 4 259–270 [DOI] [PubMed] [Google Scholar]
- Preisig-Muller R, Kindl H (1993) Plant DnaJ homologue: molecular cloning, bacterial expression, and expression analysis in tissues of cucumber seedlings. Arch Biochem Biophys 305 30–37 [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AMK (2002) Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234/ 235 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade GW, Hofmann RM, Crutzen PJ (1999) CO emissions from degrading plant matter. I. Measurements. Tellus B 51 889–908 [Google Scholar]
- Siegel SM, Renwick G, Rosen LA (1962) Formation of carbon monoxide during seed germination and seedling growth. Science 137 683–684 [DOI] [PubMed] [Google Scholar]
- Song XG, She XP, Zhang B (2008) Carbon monoxide-induced stomatal closure in Vicia faba is dependent on nitric oxide synthesis. Physiol Plant 132 514–525 [DOI] [PubMed] [Google Scholar]
- Tarr MA, Miller WL, Zepp RG (1995) Direct carbon monoxide photoproduction from plant matter. J Geophys Res 100 11403–11413 [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH (1993) Carbon monoxide: a putative neural messenger. Science 259 381–384 [DOI] [PubMed] [Google Scholar]
- Watts RN, Ponka P, Richardson DR (2003) Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron. Biochem J 369 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks SS (1959) Carbon monoxide in green plants. Science 129 964–966 [DOI] [PubMed] [Google Scholar]
- Xie YJ, Ling TF, Han Y, Liu KL, Zheng QS, Huang LQ, Yuan XX, He Z, Hu B, Fang L, et al (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defense in wheat seedling roots. Plant Cell Environ (in press) [DOI] [PubMed]
- Xu J, Xuan W, Huang BK, Zhou YH, Ling TF, Xu S, Shen WB (2006) Carbon monoxide-induced adventitious rooting of hypocotyl cutting from mung bean seedling. Chin Sci Bull 51 668–674 [Google Scholar]
- Xuan W, Huang LQ, Li M, Huang BK, Xu S, Liu H, Gao Y, Shen WB (2007) Induction of growth elongation in wheat root segments by heme molecules: a regulatory role of carbon monoxide in plants? Plant Growth Regul 52 41–51 [Google Scholar]
- Xuan W, Xu S, Yuan XX, Shen WB (2008) Carbon monoxide: a novel and pivotal signal molecule in plants? Plant Signal Behav 3 381–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannarelli GG, Noriega GO, Batlle A, Tomaro ML (2006) Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 224 1154–1162 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Cho HS, Ha HJ, Liu JR, Lee HSP (1999) Characterization of NtCDPK1, a calcium-dependent protein kinase gene in Nicotiana tabacum, and the activity of its encoded protein. Plant Mol Biol 39 991–1001 [DOI] [PubMed] [Google Scholar]
- Zarzov P, Boucherie H, Mann C (1997) A yeast heat shock transcription factor (Hsf1) mutant is defective in both Hsc82/Hsp82 synthesis and spindle pole body duplication. J Cell Sci 110 1879–1891 [DOI] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD (1993) Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science 260 1946–1950 [DOI] [PubMed] [Google Scholar]
- Zimmerman PW, Crocker W, Hitchcock AE (1933) Initiation and stimulation of roots from exposure of plants to carbon monoxide gas. Contrib Boyce Thompson Inst 5 1–17 [Google Scholar]