Abstract
A review of the research on infant vagal tone suggests that vagal activity is associated with both infant growth and infant socioemotional development. Vagal activity has been noted to increase following the stimulation of pressure receptors as in massage therapy. Vagal activity, in turn, stimulates gastric motility which mediates weight gain in infants. Vagal activity has also been notably elevated during synchronous mother-infant interactions and positive affect, providing confirmatory data for the Porges “Social Engagement System” model. In contrast, low vagal activity has been noted in prenatally depressed mothers (and prenatally angry and anxious mothers) and their infants, as well as in children with autism. These studies highlight the relations between vagal activity and the social behaviors of attentiveness, facial expressions and vocalizations.
The vagus nerve is a key component in the regulation of the autonomic nervous system and socioemotional function. It is composed of afferent (sensory) and efferent (motor) fibers that innervate most organs in the body including the gastrointestinal and cardiovascular systems as well as the ears, mouth and voice (Chang, Mashimo & Goyal, 2003; Kandel, Schwartz & Jessel, 2000). Heart rate variability has been used to estimate vagal activity and serves as a non-invasive measure of autonomic nervous system function and maturation that has been shown to reflect vagal regulation of the heart and the gastro-intestinal system (Fox & Porges, 1985; Katoh, Nomura, Iga, Hiasa, Uehara, Harada et al., 2003; Task Electrophysiology, 1996). Several time and frequency domain methods have been used to estimate vagal activity from heart rate variability, yielding comparable results and include the Bohrer and Porges (1982) algorithm to estimate vagal tone, Lorenz plots on interbeat intervals to estimate the Toichi Cardiac Vagal Index (CVI), and spectral analyses to estimate the high frequency component of heart rate variability (HF, RSA) (Allen Chambers, Towers, 2006; Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology, 1996).
Estimates of vagal activity from heart rate variability have been widely used in studies assessing infant development, affect and social interactions. In these studies, vagal tone, specifically baseline vagal tone has been variously treated as a correlate, a predictor, a mediator, a marker variable and even a causal variable of normal and abnormal behaviors and conditions. The primary theme of the review paper is that baseline vagal activity may be considered a stable physiological state just as expressivity has been considered a primary behavior component of individual infant temperament. Secondly, low baseline vagal activity appears to be a marker of infant risk conditions such as prematurity and depression. Third, vagal stimulation in the form of therapies like massage may increase vagal activity, which then may partially mediate increased expressivity, growth and development.
Infant Growth and Development
Several studies have documented a relationship between vagal activity and infant development. Baseline vagal activity in preterm and full term infants is positively correlated with age, paralleling the normal maturation of the autonomic nervous system (Longin, Gerstner, Schaible, Lenz & Konig, 2006; Sahni, Schulze, Kashyap, Ohira-Kist, Fifer, Myers, 2000). Similarly, infant vagal activity is associated with the degree of maturation and integrity of the autonomic nervous system. For example preterm infants exhibit lower baseline vagal activity than full term infants, and infants who exhibit lower levels of baseline vagal activity are more likely to also exhibit less optimal neurodevelopmental outcomes (DiPietro, Caughy, Cusson & Fox, 1994; Doussard-Roosevelt, Porges, Scalon, Alemi & Scanlon, 1997; Fox & Porges, 1985; Porges,1995).
Low Vagal Activity in Preterm Infants
Vagal regulation of heart rate has been used as a predictor of outcomes for risk infants including very low birthweight preterm infants. Vagal activity and maturational shifts in vagal activity between 33 and 35 weeks gestational age were recorded in a study on very low birthweight infants (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997). These vagal activity measures predicted three year outcomes beyond the effects of birthweight, medical risks and socioeconomic status. Higher vagal activity was associated with better social skills, whereas greater vagal activity maturation was associated with better mental processing and gross-motor skills. When the sample was divided into those with birthweights of less than one-thousand grams versus those with birthweights greater than one-thousand grams, vagal activity maturation emerged as a strong predictor of mental processing, knowledge base and gross-motor skills in the less than one-thousand gram group. In a six-to-nine-year follow-up of a sub-sample of this very low birthweight infant group, neonatal risk measures were not related to school-age outcome measures, although vagal maturation was correlated with social competence, as measured by the Child Behavior Checklist (Doussard-Roosevelt, McClenny & Porges, 2001)
A similar assessment of very early vagal activity has also been conducted with low-risk fetuses between 36 and 40 weeks gestation (Groome, Loizou, Holland, Smith & Hoff, 1999). The authors used respiratory sinus arrhythmia to measure vagal activity, and the efficiency of homeostatic control was quantified for each infant by the slope (SRSA) and correlation coefficient (RRSA) of the regression line relating fluctuations in heart period and fluctuations in RSA. To test their hypothesis, they examined the relationship between RSA and both SRSA and RRSA in low-risk fetuses. They found that the fetuses who were parasympathetic-dominated had larger SRSA and RRSA values and were more efficient regulators of homeostasis than those who were sympathetic-dominated.
Vagal Stimulation for Preterm Infants
Vagal stimulation may promote growth and development in infants, and stimulation like kangaroo care and massage therapy may be non-invasive methods for increasing baseline vagal activity in infants. For example, a recent study on kangaroo care accelerated the maturation of vagal activity (Feldman & Eidelman, 2003). In this study, mother-infant skin-to-skin (kangaroo care) effects on autonomic functioning, state regulation and neurobehavior status were examined in preterm infants who received kangaroo care over a period of 24 days. Baseline vagal activity was calculated from ten minutes of heart rate before the kangaroo care started and again at 37 weeks gestational age. Infants receiving the kangaroo care showed more rapid maturation of vagal activity between 32 and 37 weeks gestational age, as well as more rapid state organization including longer periods of quiet sleep and alert wakefulness and shorter periods of active sleep. Performance on the habituation and orientation items of the Brazelton Neonatal Behavior Assessment Scale also suggested a more mature neurodevelomental profile.
Using a different form of skin contact, namely infant massage (tactile and kinesthetic stimulation), a group in Korea measured responses to the stimulation including baseline vagal activity, heart rate, and oxygen saturation (Lee, 2005). Preterm infants were randomly assigned to receive massage twice daily for ten days or to a standard treatment control group. Vagal activity was significantly higher after massage than before massage in the experimental group, while no change occurred in the control group. The treatment group also spent significantly more time being awake and active. Thus, baseline vagal activity was associated with greater attentiveness and more organized behavior.
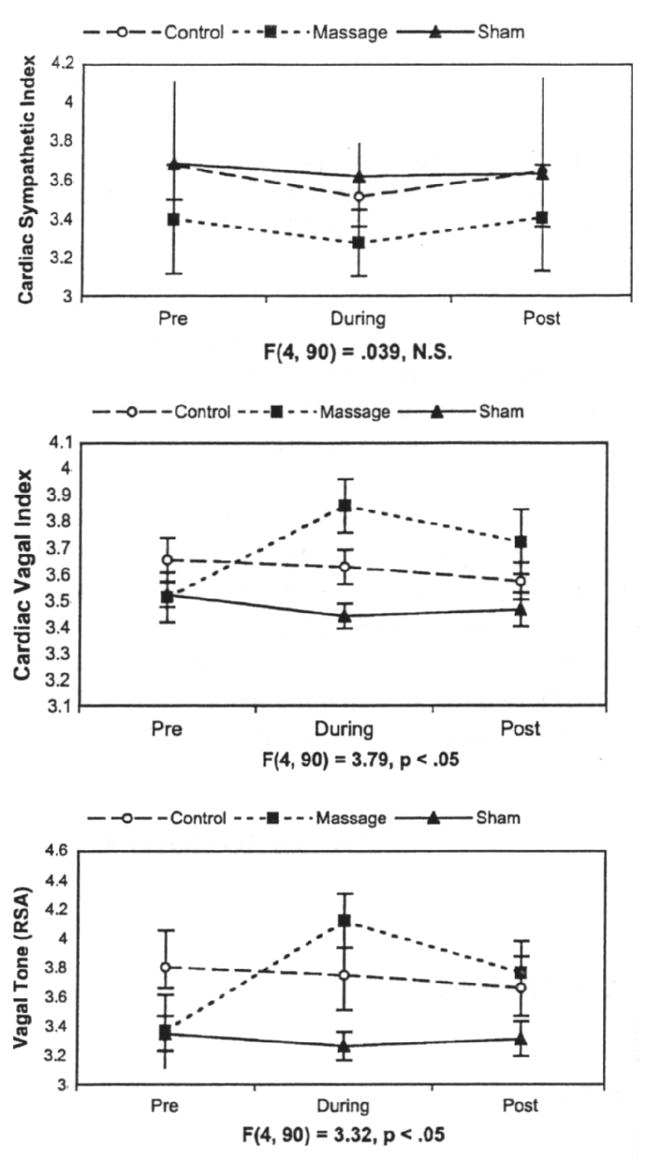
In many studies on massage therapy with preterm infants, significant weight gain has been reported (See Field, Diego & Hernandez-Reif, 2007 for a review). In these studies, the massaged preterm neonates did not consume more calories than the control neonates, nor did they conserve more calories by being more restful. After ruling out these potential underlying mechanisms, we explored the possibility that increased vagal activity following massage therapy would lead to increased gastric motility and, thereby, contribute to weight gain (Diego, Field & Hernandez-Reif, 2005). In this study, vagal activity was assessed along with gastric motility in response to massage therapy (a moderate pressure massage group) versus sham massage (a light pressure massage group) and a control group across a five-day period. Preterm neonates receiving the moderate pressure massage therapy exhibited greater weight gain (see table 1). Increased vagal tone and increased gastric motility were consistently noted for those infants during and immediately after the treatment sessions (see figures 1 and 2). Vagal tone and gastric motility during massage therapy were significantly related to weight gain.
Table 1.
Means for clinical outcomes (Diego et al., 2005).
| Control | Massage | Sham | F | p | |
|---|---|---|---|---|---|
| Days to discharge | 25.5 | 20.1 | 24.4 | 0.93 | NS |
| Weight gain (kg/day)* | 15.5 | 19.6 | 16.2 | 5.13 | <.01 |
| Caloric intake (kg/day) | 111.0 | 111.0 | 12.0¹ | 0.24 | NS |
Infants in the massage group gained significantly more weight than infants in the control or sham massage groups.
Figure 1.
Mean sympathetic and parasympathetic activity 15 minutes before, during, and after treatment (error bars denote ± 2 standard errors). Group-by-time interaction statistics for each measure are presented under each line plot (Diego et al., 2005).
Figure 2.
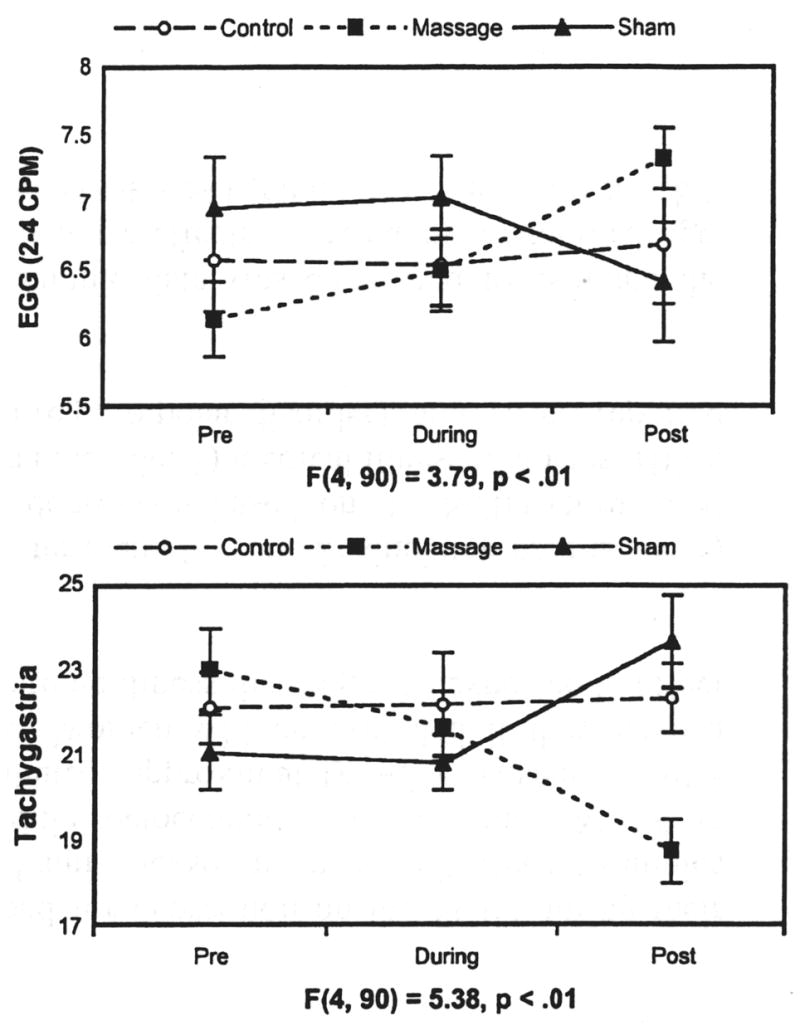
Mean gastric activity 15 minutes before, during, and after treatment (error bars denote ± 2 standard errors). Group-by-time interaction statistics for each measure are presented under each line plot (Diego et al., 2005).
Thus, the weight gain experienced by preterm neonates in the many previous studies reviewed by Field et al (2006) may have been mediated by increased vagal activity and, in turn, gastric motility. Along with enhanced gastric motility, one might expect that increased vagal activity would also lead to increased release of food absorption hormones, which would also contribute to weight gain. Vagal stimulation has been shown to release food absorption hormones (i.e. insulin) and digestive hormones (i.e. gastrin) (Chang et al., 2003’ Rozman, Bunc & Zorko, 2004). Further, tactile stimulation promotes the release of both food absorption (insulin) and digestive hormones (Marchini, Lagercrantz, Feuerberg, Winberg & Uvnas-Moberg, 1987; Uvnas-Moberg, 2004).
Infant Affect and Early Interactions
Although emotional expressions have been linked to autonomic activity in the theories of many renown scientists dating back to Charles Darwin (1872), Williams James (1884), and Walter Cannon (1927), autonomic activity has only been measured alongside of facial expressions in the last few decades (Ekman et al., 1983; Fox, 1989; Porges, 1991). In studies in the 80’s, for example, newborns who were more expressive were also noted to have greater heart rate variability (Field, 1982), and three-month-old infants with greater heart rate variability showed more interest expressions (Fox & Gelles, 1984). Older 5-month-old infants with greater vagal activity showed more interest and joy expressions, while infants with lower vagal tone showed more looking-away behavior (Stifter, Fox & Porges., 1989).
Vagal activity has also been measured in the context of mother-infant face-to-face interactions. In a study we conducted, infant facial expressions were coded using the AFFEX facial expression coding system, and the infants’ EKG was recorded during their interactions with their mothers at three-months of age (Pickens & Field, 1995). Vagal activity was correlated with infants’ joy and interest expressions and with self-comfort behaviors (see table 2). The low vagal activity in infants of depressed mothers, in contrast, was associated with sad and angry facial expressions. In a similar study by our group, higher vagal activity was associated with more vocalizing (positive vocalizations such as cooing and babbling) (Field, Pickens, Fox, Nawrocki & Gonzalez, 1995).
Table 2.
Correlations between vagal tone of infants of depressed and non-depressed mothers and the proportion of interaction time that facial expressions occurred (interobserver reliability coefficients in parentheses) (Pickens & Field 1995)
| Infant Vagal Tone | ||
|---|---|---|
| Depressed | Non-Depressed | |
| Expression | ||
| Interest (.77) | .00 | .441 |
| Joy (.80) | .22 | .651 |
| Sad (.84) | −.391 | −.16 |
| Anger (.79) | −.421 | −.18 |
p<.05
In another study, positive relations were noted between infants’ baseline vagal activity and symmetrical features of interactions in mother-infant dyads (Porter, 2003). In the Porter study, EKG was collected from the infants followed by a 15-minute mother-infant face-to-face interaction. The interactions were videotaped and then coded for symmetrical patterns and disruptive patterns of coregulation. In another study, newborn vagal activity was predictive of 3-month-old mother-infant interaction synchrony (Feldman, 2006). In this study, newborns (both preterm and full-term newborns) were tested on infant orientation using the Brazelton Neonatal Behavior Assessment Scale and for sleep-wake cyclicity and vagal activity. At three-months, mother-infant synchrony was assessed from microanalyses of the videotapes and time-series analyses of the face-to-face interaction behaviors. Results suggested that sleep-wake cyclicity, vagal activity, orientation and arousal modulation at the newborn period were each uniquely predictive of mother-infant synchrony. In this sense vagal activity was one of many predictor variables.
Perturbations of mother-infant interactions such as the mother posing a still-face have been noted to affect vagal activity. In a study on still-face interactions between three-month-old infants and their mothers, the infants’ affective states and vagal activity were recorded (Moore & Calkins, 2004). The authors assessed the synchrony and the matching of mothers’ and infants’ affective states and monitored infant heart rate and vagal activity. The infants showed increased negative affect during the still-face which was accompanied by decreased vagal activity.
Infant Temperament
Vagal activity has also been related to infant temperament. Temperament may be thought of as the stable affective state of an infant. In one of our early studies, we examined newborns’ threshold to tactile stimulation (pinprick), their responses to modeled facial expressions (happy, sad and surprised) and how accurate the models’ expressions could be guessed based on their “imitative expressions” (experimenter standing behind model and looking at infant’s face) (Field, 1982). In addition, we measured habituation to the modeled faces and vagal activity. We noted that infants who we called externalizers (infants who had extremely expressive facial expressions) had greater heart rate variability as early as the newborn period In contrast, those infants who we called internalizers (infants who showed flat affect) had low heart rate variability (see table 3). In this sense, vagal activity might be considered a stable physiological state just as expressivity is considered a stable affective state.
Table 3.
Means for high- and low-expressivity newborns a (Field, 1982).
| Expressivity of Infant | ||
|---|---|---|
| Measures * | Low | High |
| Threshold | ||
| Pinpricks to response (#) | 1.2 | 1.9 |
| Expressivity | ||
| Accuracy of observers' guesses (%) | ||
| Happy (mouth widensed) | 5.0 | 14.0 |
| Sad (lip protrusion) | 8.0 | 22.0 |
| Surprise (mouth open) | 20.0 | 44.0 |
| Physiological responsivity | ||
| Heart rate variability (beats per minute) | 23.0 | 32.0 |
| Habituation | ||
| Trials to criterion | 7.0 | 11.0 |
| Looking time (seconds) | 10.0 | 16.0 |
All means significantly different at p< .05
The relations that have been noted between vagal activity and early infant temperament, are perhaps not surprising inasmuch as continuity and stability have been noted for both temperament and vagal activity across time. In a prospective longitudinal study, vagal activity was measured at 2-months and at 5-years in children and their mothers (Bornstein & Suess, 2000b). The authors reported that the baseline-to-task change in vagal activity was continuous in both the children and mothers. The children were also noted to reach adult levels of vagal activity by 5 years and did not differ from their mothers in baseline-to-task change in vagal activity. Baseline vagal activity tended to be stable, but baseline to-task-change in vagal activity was unstable in the children, while both were stable in the mothers. In a similar longitudinal study, but on high risk infants (low birthweight), vagal activity maturation was correlated with social competence as measured by the Child Behavior Checklist (Doussard-Roosevelt, McClenny & Porges, 2001). In contrast, neonatal risk measures (low birthweight, low socioeconomic status and high medical risk) were not correlated with any of the school-age measures.
Low Baseline Vagal Tone in Infants of Prenatally Depressed, Anxious and Angry Mothers
Low vagal activity has been reported for depressed mothers and their infants as early as the neonatal stage (Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn, Yando & Bendell, 2004; Jones, Field, Fox, Lundy & Hart, 1998). In the Jones et al. (1986) study, the newborns of depressed mothers had both low vagal activity and greater relative right frontal EEG activation (a marker for negative affect and consistently noted in depressed adults). In the Field et al. (2004) study, the depressed mothers had lower vagal tone than non-depressed mothers, and greater relative right frontal EEG activation (see table 4). Their newborns similarly had lower vagal tone as well as greater relative right frontal EEG activation. The mimicry of the mothers’ vagal tone and EEG by these newborns of depressed mothers may have related to their similar biochemical profiles. The mothers’ prenatal cortisol levels were elevated, and their dopamine and serotonin levels were lower than those of non-depressed mothers. Similarly, the newborns’ cortisol levels were elevated, and their dopamine and serotonin levels were lower than those of the newborns of non-depressed mothers. In the same studies, lower vagal activity was significantly correlated with elevated cortisol and lower levels of serotonin and dopamine. In this case, infant vagal activity was a marker variable for the risk conditions of prenatal depression, an outcome variable predicted by maternal vagal activity and a correlate of the biochemical variables including cortisol, serotonin and dopamine.
Table 4.
Means for neonatal sleep activity and mothers’ and newborns’ EEG and vagal tone (Field et al., 2004).
| Group | F | p | ||
|---|---|---|---|---|
| Depressed | Non-depressed | |||
| Indeterminate sleep (% time) | 54.0 | 35.5 | 9.50 | .005 |
| Movement (% time) | 71.0 | 62.0 | 5.80 | .05 |
| EEG in mothers | −0.015 | 0.01 | 14.60 | .001 |
| EEG in infants | −0.06 | 0.03 | 11.30 | .001 |
| Vagal tone in mothers | 3.7 | 4.1 | 7.90 | .05 |
| Vagal tone in newborns | 4.2 | 4.7 | 3.20 | .05 |
The lower vagal activity of these newborns may also explain their lesser expressivity, inasmuch as the vagus nerve innervates the facial nerve, which then innervates the face (Porges, 2001). In this case, vagal activity might be seen as causal or an underlying mechanism for expressivity. In a study on facial expressions of newborns of depressed mothers, we recorded their faces during the Brazelton Neonatal Behavior Assessment Scale and during the modeling of happy, sad, and surprised faces (Lundy, Field & Pickens, 1996) (see figure 3). The newborns of depressed mothers demonstrated inferior performance on the orientation cluster of the Brazelton scale (less attentiveness) and showed fewer interest and more pre-cry expressions during the Brazelton. During the facial expression modeling, they showed less orientation and fewer facial expressions in response to the modeled happy and surprised facial expressions (see table 5).
Figure 3.
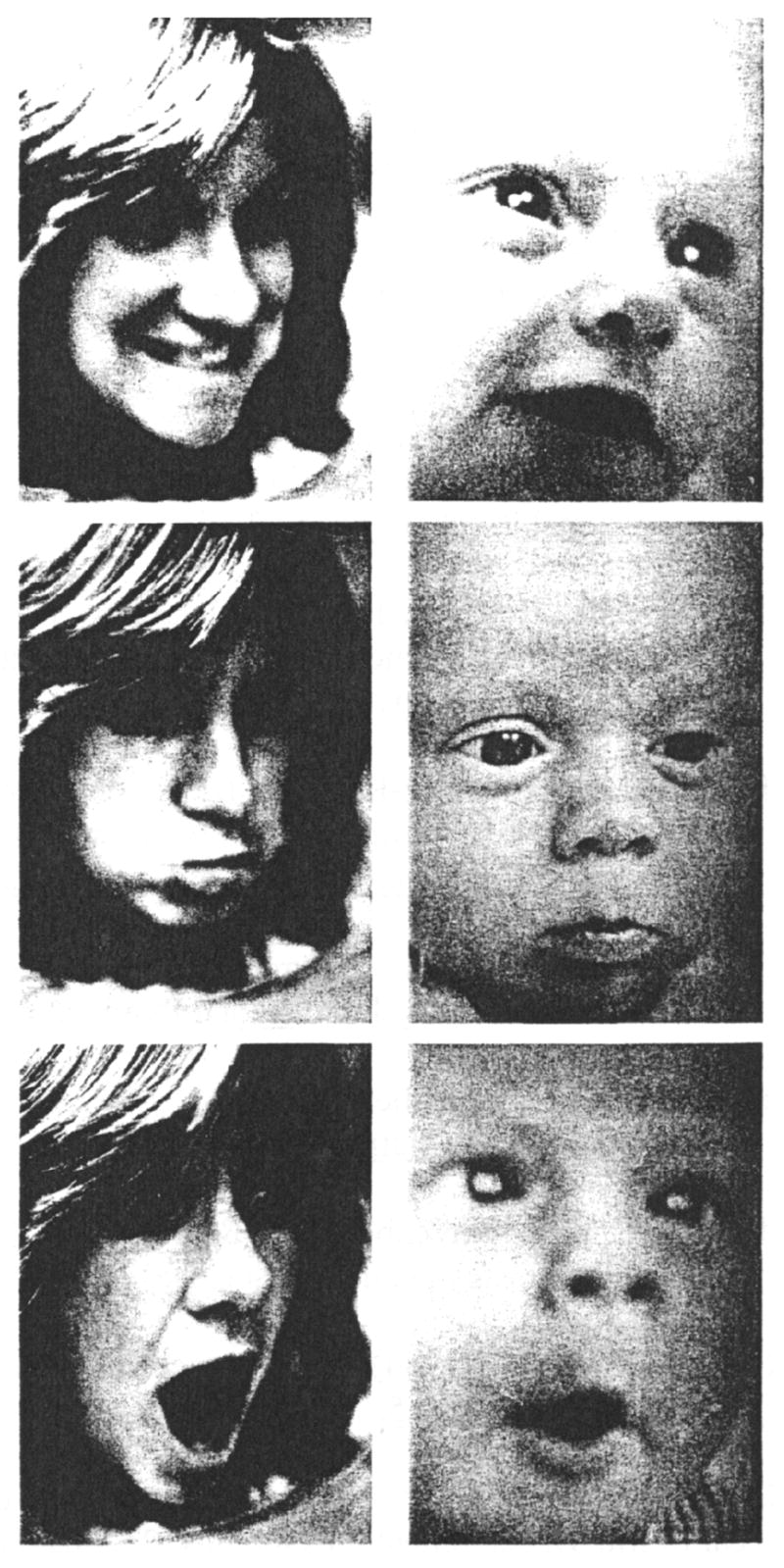
Sample photographs of model’s happy, sad, and surprised expressions and infant’s corresponding expressions (Field, 1982).
Table 5.
Means for orientation and responses to modeled facial expressions by newborns of depressed and non-depressed mothers (Lundy et al., 1996).
| Depressed | Non-Depressed | |||
|---|---|---|---|---|
| Expression | t | p | ||
| Happy | ||||
| Orientation | 3.6 | 4.6 | −2.21 | 0.05 |
| Responses to modeling | 2.4 | 3.8 | −2.50 | 0.01 |
| Sad | ||||
| Orientation | 3.2 | 4.0 | −1.31 | 0.10 |
| Responses to modeling | 1.9 | 2.2 | −0.60 | 0.28 |
| Surprise | ||||
| Orientation | 3.6 | 4.8 | −2.44 | 0.01 |
| Responses to modeling | 2.0 | 3.5 | −2.34 | 0.01 |
Porges (2001) has described activity of the “smart vagus” as a social engagement system that controls looking (somatomotor components of the ventral vagal complex, in this case cranial nerve XI), listening (cranial nerve VII), vocalizing (cranial nerves IX, X), and facial expressions (cranial nerve VII). Low vagal activity, then, may be accompanied by flat facial affect and vocalizations lacking intonation contour, as in depressed behavior.
The neonatal predisposition to lower baseline vagal activity may continue, inasmuch as we have noted that vagal activity was lower in both three and six-month old infants of depressed mothers, and, although a significant increase in baseline vagal activity occurred from three to six months for the infants of non-depressed mothers, that increase did not occur for infants of depressed mothers (Field, Pickens, Fox, Nawrocki & Gonzalez, 1995) (see table 6). In this study, higher vagal activity was associated with more vocalizing (positive vocalizations such as cooing and babbling). These data plus the data described earlier showing that 3-month-old infants of depressed mothers had significantly more sad and angry expressions and fewer interest expressions than infants of non-depressed mothers (Pickens & Field, 1995), suggesting that maternal depression affects infants’ emotional expressions as well as their vagal activity.
Table 6.
Means for vagal tone across early infancy in infants of depressed and non-depressed mothers (and mothers in parentheses).
| Infants | ||
|---|---|---|
| Depressed | Non-Depressed | |
| Neonatal Stage | 4.20 | 4.73¹ |
| (Field et al., 2004) | (3.70) | (4.12)² |
| 3 Months | 2.90 | 3.43² |
| (Field et al., 1995) | ||
| 6 Months | 2.90 | 3.71³ |
| (Field et al., 1995) | ||
p<.05,
p<.01,
p<.005
Lower vagal activity has also been noted for mothers who have high prenatal anxiety levels and those who are experiencing prenatal anger. In a study on prenatal anxiety effects, women were classified as experiencing high or low anxiety during the second trimester of pregnancy (Field, Diego, Hernandez-Reif, Schanberg, Kuhn, Yando & Bendell., 2003). The high anxiety women (based on a median split) also had high scores on depression and anger scales and lower postnatal vagal activity as well as greater relative right frontal EEG activity and elevated prenatal norepinephrine and low dopamine levels during the prenatal period. Subsequently, their newborns had lower vagal activity as well as lower dopamine and serotonin levels, and greater relative right frontal EEG activation, thus mimicking their mothers’ biochemical and physiological profiles (see table 7). Similarly, in a median split of the sample by high and low anger, the high anger mothers had low vagal tone and high prenatal cortisol and epinephrine as well as low dopamine and serotonin levels, which were, in turn, mimicked by their neonates (Field, Diego, Hernandez-Reif., Salman., Schanberg., Kuhn, Yando & Bendell, 2002) (see table 8).
Table 7.
Means for physiological variables in low and high anxiety groups (Field et al., 2003).
| Group | ||||
|---|---|---|---|---|
| Variables | Low Anxiety | High Anxiety | F | p |
| Frontal EEG mother | −0.02 | −0.09 | 1.15 | NS |
| Frontal EEG newborn | 0.03 | −0.04 | 5.60 | 0.05 |
| Vagal tone mother | 4.05 | 3.91 | 1.71 | NS |
| Vagal tone newborn | 4.72 | 4.25 | 3.08 | 0.05 |
Table 8.
Means for physiological variables in low and high anger groups (Field et al., 2002).
| Group | ||||
|---|---|---|---|---|
| Variables | Low Anger | High Anger | F | p |
| Frontal asymmetry-mother | 0.01 | −0.13 | 7.6 | 0.01 |
| Frontal asymmetry-infant | 0.03 | −0.05 | 4.93 | 0.05 |
| Vagal tone-mother | 4.11 | 3.84 | 6.66 | 0.01 |
| Vagal tone-infant | 4.70 | 4.22 | 2.82 | 0.05 |
Physiological and Biochemical Reactivity
As Porges (2001) has noted, vagal activity interacts with stress reactivity systems including the hypothalamic-pituitary-adrenal axis and the immune system. These interacting systems may underly those chemical, electrophysiological, expressivity and interaction differences noted in, for example, the depressed mothers and their infants. Physiological/biochemical reactivity, as in the greater relative right frontal EEG patterns and the high norepinephrine and cortisol levels noted in the depressed mothers and infants, would be exemplary of these interacting systems. Others have noted correlations between increased cortisol and decreased vagal activity (Cacioppo, Malaarkey, Kiecolt-Glaser, Uchino, Sgoutas-Emch, Sheridan, Berntson & Glaser, 1995; Gunnar, Porter, Wolf, Rigatuso & Larson, 1995).
The “Vagal Brake” and Individual Differences
The concept of the “vagal brake” (i.e. decreasing vagal activity) has also been introduced by Porges as a potential mechanism underlying the individual differences in vagal reactivity and in response to social/attention tasks (Porges, Doussard-Roosevelt, Portales & Greenspan, 1996). In this model, which at first glance would appear to be inconsistent with the baseline vagal activity model, infants who have difficulty regulating the vagal brake or decreasing cardiac vagal tone during social/attention tasks are thought to have difficulty developing strategies for reciprocally engaging and disengaging during social interactions. These investigators have, in fact, documented individual differences in vagal reactivity. For example, in the Porges et al, (1996) study, infants who had difficulties decreasing vagal tone during a social attention task at 9-months of age had significantly more behavior problems at 3 years of age (Porges et al., 1996). Baseline vagal tone at nine months was also related to less difficult three-year behavior (Porges, Doussard-Roosevelt, Portales & Suess 1994). This phenomenon has been reported even in younger 3-month-old infants (Huffman, Bryan, del Carmen, Pedersen, Doussard-Roosevelt & Porges, 1998). In the Huffman et al. study, vagal tone was measured during a resting baseline period and during a laboratory assessment of temperament. Infants with higher baseline vagal tone were rated as showing less negative behavior in the laboratory and were less disrupted by the temperament procedure. Infants who had decreased vagal tone during the temperament assessment were rated on maternal report temperament scales as having longer attention spans and being more easily soothed. Thus, higher baseline vagal tone and lower reactivity vagal tone appear to predict optimal development.
In another study, the still-face paradigm was used with five-month-old infants who were assessed during the “interaction challenge” (Bazhenova, Plonskala & Porges, 2001). When the infants were divided into two groups based on their vagal activity response to the still-face, those infants who showed a decrease in vagal activity to the still-face followed by an increase vagal activity during the subsequent normal interaction showed organized behavior. The authors suggested that their findings provided additional support for the relationship between vagal activity and affective behavior. High vagal tone would appear to be optimal in calm interactions and low vagal tone in stressful interactions that elicit vigilant behavior.
The “vagal brake” has been referred to as suppression of respiratory sinus arrhythmia (RSA) by others (Calkins & Keane, 2004) In their study, two-year-old children were assessed for the effects of emotional and behavioral challenges on their cardiac activity, and then a number of these children were seen again at 4.5 years of age. The ability to decrease vagal activity (suppress RSA), across the challenging tasks showed cross-age stability, although the magnitude of vagal suppression significantly decreased across age (Calkins & Keane, 2004). The children who displayed a pattern of stable and high suppression across the preschool period appeared to be less emotionally negative and had fewer behavior problems and better social skills than the other children, as measured by parent-report. This stability appears to start at an early age, as was noted in another study from another lab (Stifter & Jain, 1996). In their study, although no stability was found from 5 to 10 months, some stability of behavior and autonomic patterning was identified from 10 to 18 months. Infants who had higher vagal activity responded negatively to frustration but also displayed more regulatory behavior.
Decreased vagal activity also appears to occur during information processing or habituation tasks. In a study on habituation, vagal activity suppression was measured at baseline and during an habituation task (Bornstein & Suess, 2000a). Those who habituated had a greater decrease in vagal activity suppression during habituation. A regression on vagal activity predicted total looking time. Thus, the authors concluded that the self-regulation provided by the vagal system appeared to play a role in information processing as well as in habituation. In another paper, these authors reported that the baseline to habituation task change in vagal activity showed stability and consistent child-mother concordance across the first five years of development. (Bornstein & Suess, 2000b).
Therapies for Increasing Baseline Vagal Activity
The low vagal tone we have consistently noted in depressed individuals (infants, adolescents and mothers) may be effectively treated by therapies that enhance vagal activity such as massage therapy (see Field, Diego, Hernandez-Reif, 2006 for a review). AS noted earlier, increased vagal activity has occurred following massage therapy (Diego et al., 2005; Lee, 2005). Vagal stimulation has been similarly used with depressed adults (George, Sackeim, Rush, Marangell, Nahas, Husain, Lisanby, Burt, Goldman & Ballenger, 2000).
Children with autism also benefit from massage therapy (Escalona, Field, Singer-Strunck, Cullen & Hartshorn, 2001; Field, Lasko, Mundy& Henteleff, Kabot et al., 1997). Autism is another condition marked by low vagal activity and associated gaze aversion and flat facial expressions. In these ways, infants of depressed mothers may be similar to children with autism. They may also share difficulties with speech perception. Infants of depressed mothers show less recognition of their mothers’ voices (Hernandez-Reif, Field, Diego & Largie, 2002). Porges (2001) has referred to children with autism being unable to extract the high frequency sounds of the human voice from the background low frequency sounds. The similarly low vagal tone and expressivity in these two groups of children may be similarly treated by therapies that increase their expressivity such as imitation therapy (Field, Field, Sanders & Nadel, 2001) and increase their vagal activity such as massage therapy (Escalona et al., 2001; Field et al., 1997).
Summary and Suggestions for Future Research
This body of research, then, suggests the importance of vagal activity for both infant growth and infant social and emotional development. Vagal activity has been shown to increase following stimulation of pressure receptors and, in turn, to stimulate gastric activity and to mediate growth (see Field, et al 2007 for a review). Vagal activity has also been elevated in synchronous mother-infant interactions and has accompanied more positive affect, providing confirmatory data for the Porges (2001) social engagement system model. Data on prenatally depressed mothers (and prenatally angry and anxious mothers) and their infants (Field et al., 2003) as well as data on children with autism(Porges, 2001) are examples of low expressive vagal tone. The “vagal brake” or decreased vagal activity in response to social or cognitive tasks (Porges et al., 1996) has also been empirically supported. These studies highlight the relations between baseline vagal activity and social behaviors of attentiveness, facial expressions and vocalizations and, in general, the social engagement system that has been described by Porges (2001).
Some shortcomings of the literature on infant vagal activity are that: 1) vagal activity is often used as a “pet variable” or a primary mediating or outcome variable. But in studies using multiple variables, it is found to be, instead, one of many mediating variables; 2) a related issue is that vagal activity is often implied to be a causal variable, e.g. low vagal tone explaining low expressivity in infants of depressed mothers and children with autism, even though the data are based on correlations. And, again, another variable, for example, depleted serotonin may be a critical mediating variable; 3) vagal brake theory suggesting that low vagal activity is optimal during attention tasks seems inconsistent with high baseline vagal activity being optimal for cognitive development. Some inconsistencies in the data across infant labs may relate to that incongruity; and 4) like any other phenomenon, and as in an inverted U function, a moderate amount of vagal activity may be, in fact, the optimal level, and high as well as low amounts non-optimal levels.
Many of the individual differences in the literature have been partially attributed to baseline vagal activity and/or vagal brake (decreasing vagal activity) differences. However, much of the variance has been left unexplained. In a review of this literature, Beauchaine (2001) has argued that the Porges polyvagal model could be combined with motivational theory, which contrasts activation with inhibition to explain the wide individual differences and the differences across psychological disorders. Gray’s model has been empirically tested not only by behavior but also by EEG patterns, and a large literature has evolved on greater relative right frontal versus greater relative left frontal EEG activation (Fox, 1984; Davidson, 1992) including our work on depressed mothers and infants who are noted to have greater relative right frontal EEG activation (Field et al. 2003). We have argued elsewhere for a profile model that combines these approach/withdrawal behaviors, right/left frontal EEG activation, vagal tone and biochemistry (cortisol, catecholamines and serotonin), (Field et al 2005).
As was noted in our multivariable study on newborns of depressed mothers (Field et al., 2004), the neonates not only had lower vagal activity but also greater relative right frontal EEG activation, higher cortisol and lower dopamine and serotonin levels. Multivariable-profile analysis studies might more accurately classify individuals and their developmental courses than one variable alone such as vagal activity. Combining these measures in studies on infants as well as studies on disorders, such as depression and autism, would not only help us further understand infant development and individual disorders, but it might also help elucidate the interactions of these complex systems, as in a polysystems theory.
Acknowledgments
We would like to thank the mothers and infants who participated in these studies. This research was supported by a Merit Award (MH # 46586), and Senior Research Scientist Awards (MH# 00331 and AT# 001585) an NIH grant (#AT00370), a March of Dimes Grant (# 12-FYO3-48) and funding from Johnson & Johnson Pediatric Institute. Correspondence and requests for reprints should be sent to Tiffany Field, Ph.D., Touch Research Institutes, University of Miami School of Medicine, PO Box 016820, Miami, Florida, 33101. Business phone number (305) 243-6781. E-mail tfield@med.miami.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazhenova O, Plonskaia O, Porges S. Vagal reactivity and affective adjustment in infant during interaction challenges. Child Development. 2001;72:1314–26. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Developmental Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. a) Physiological self-regulation and information processing in infancy: cardiac vagal tone and habituation. Developmental Psychology. 2000;71:273–87. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. b) Child and mother cardiac vagal tone: continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36:54–65. [PubMed] [Google Scholar]
- Cacioppo J, Malaarkey W, Kiecolt-Glaser J, Uchino B, Sgoutas-Emch S, Sheridan J, Berntson G, Glaser R. Heterogeneity in neuroendocrine and immune reponses to brief psychological stressors as a function of autonomic carciac activation. Psychosomatic Medicine. 1995;57:154–164. doi: 10.1097/00006842-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Calkins S, Keane S. Cardiac vagal regulation across the preschool period: stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–12. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cannon W. The wisdom of the body. London: Kegan Paul; 1932. [Google Scholar]
- Chang H, Mashimo H, Goyal R. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. American Journal Physiology and Gastrointestinal Liver Physiology. 2003;284:357–66. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Expression of Emotions in Man and Animals. New York: D. Appleton; 1872. [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:121–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Diego M, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain massaged preterm neonates. Journal of Pediatrics. 2005;147:50–5. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Joe C, Bazhenova O, Porges S. Mother-child interaction in autistic and nonautistic children: characteristics of maternal approach behaviors and child social responses. Developmental Psychopathology. 2003;15:277–95. doi: 10.1017/s0954579403000154. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38:56–66. [PubMed] [Google Scholar]
- Doussard-Roosevelt J, Porges S, Scanlon J, Alemi B, Scanlon K. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68:173–86. [PubMed] [Google Scholar]
- Escalona A, Field T, Singer-Strunck R, Cullen C, Hartshorn K. Journal of autism and developmental disorders. 2001;31:513–516. doi: 10.1023/a:1012273110194. [DOI] [PubMed] [Google Scholar]
- Ekman F, Levenson R, Friesen W. Emotions differ in autonomic nervous system activity. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Feldman R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Developmental Psychology. 2006;42:175–88. doi: 10.1037/0012-1649.42.1.175. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Developmental Medicine and Child Neurology. 2003;45:274–81. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- Field T. Individual differences in the expressivity of neonates and young infants. In: Feldman R, editor. Development of nonverbal behavior in Children. New York: Springer-Verlay; 1982. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Massage Therapy Research. Developmental Review. 2007;27:75–89. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Deeds O, Figueiredo B, Ascencio A. Moderate Versus Light Pressure Massage Therapy Leads to Greater Weight Gain in Preterm Infants. Infant Behavior and Development. 2006;29:574–578. doi: 10.1016/j.infbeh.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Salman F, Schanberg S, Kuhn C, Yando R, Bendell D. Prenatal anger effects on the fetus and neonate. Journal of Obstetrics and Gynaecology. 2002;22:260–6. doi: 10.1080/01443610220130526. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27:216–229. [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depression and Anxiety. 2003;17:140–51. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- Field T, Field T, Sanders C, Nadel J. Children with autism display more social behaviors after repeated imitation sessions. Autism: The International Journal of Research and Practice. 2001;3:317–23. doi: 10.1177/1362361301005003008. [DOI] [PubMed] [Google Scholar]
- Field T, Fox NA, Pickens J, Nawrocki T. Relative right frontal EEG activation in 3–6 month-old infants of depressed mothers. Developmental Psychology. 1995;31:358–363. [Google Scholar]
- Field T, Hernandez-Reif M, Vera Y, Gil K, Diego M, Bendell D, Yando R. Anxiety and anger effects on depressed mother-infant spontaneous and imitative interactions. Infant Behavior and Development. 2005;28:1–9. [Google Scholar]
- Field T, Lasko D, Mundy P, Henteleff T, Kabot S, Talpins S, Dowling M. Brief report: Autistic children's attentiveness and responsivity improved after touch therapy. Journal of Autism & Developmental Disorders. 1997;27(3):333–338. doi: 10.1023/a:1025858600220. [DOI] [PubMed] [Google Scholar]
- Field T, Pickens J, Fox N, Nawrocki T, Gonzalez J. Vagal tone in infants of depressed mothers. Development and Psychopathology. 1995;7:227–231. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- Fox NA. The psychophysiological correlates of emotional reactivity during the first year of life. Developmental Pyshology. 1989;25:364–372. [Google Scholar]
- Fox NA, Gelles M. Face-to-face interaction in term and preterm infants. Infant Mental Health Journal. 1984;5:192–205. [Google Scholar]
- George M, Sackeim H, Rush A, Marangell L, Nahas Z, Husain M, Lisanby S, Burt T, Goldman J, Ballenger J. Vagus nerve stimulation: A new tool for brain research therapy. Biological Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Loizou P, Holland S, Smith L, Hoff C. High vagal tone is associated with more efficient regulation of homoeostasis in low-risk human fetuses. Developmental Psychobiology. 1999;35:25–34. doi: 10.1002/(sici)1098-2302(199907)35:1<25::aid-dev4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Porter F, Wolf C, Rigatuso J, Larson M. Neonatal stress reactivity: predictions to later emotional temperament. Child Development. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Largie S. Depressed mothers’ newborns show inferior face discrimination. Infant Behavior & Development. 2002;23:643–653. doi: 10.1016/j.infbeh.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Development. 1998;69:624–35. [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, Hart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior and Development. 1998;53:687–692. [Google Scholar]
- Lee HK. The effect of infant massage on weight gain, physiological and behavioral responses in premature infants. Taehan Kanho Hakhoe Chi. 2005;35:1452–60. doi: 10.4040/jkan.2005.35.8.1451. [DOI] [PubMed] [Google Scholar]
- Lundy B, Field T, Pickens J. Newborns of mothers with depressive symptoms are less expressive. Infant Behavior and Development. 1996;19:419–424. [Google Scholar]
- Marchini G, Lagercrantz H, Feuerberg Y, Winberg J, Uvnas-Moberg K. The effect of non-nutritive sucking on plasma insulin, gastrin, and somatostatin levels in infants. Acta Paediatrica Scandinavica. 1987;76:573–578. doi: 10.1111/j.1651-2227.1987.tb10523.x. [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40:1068–80. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Pickens J, Field T. Facial expressions and vagal tone of infants of depressed and non-depressed mothers. Early Development and Parenting. 1995;4:83–89. [Google Scholar]
- Porges SW. Heart rate patterns in neonates: A potential diagnostic window to the brain. In: Field T, Sostek AM, editors. Infants Born at Risk: Physiological and Perceptual Responses. New York: Grune and Stratton; 1983. pp. 3–22. [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in a periodic physiological response systems. United States Patent Nuber: 4,510,944. 1985 April 16, 1985
- Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Development. 1998;69:624–35. [PubMed] [Google Scholar]
- Porges SW. Vagal tone: A mediator of affect. In: Garber JA, Dodge KA, editors. Development of Affect Regulation and Dysregulation. New York: Cambridge University Press; 1991. pp. 111–128. [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic substrates of a social nervous system. Psychoneuroendocrinology. 2001;23:837–861. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales A, Greenspan SI. Infant regulation of vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales A, Suess P. Cardiac vagal tone: stability and relation to difficultness in infant and 3-years-olds. Developmental Psychobiology. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Porges SW, McCabe P, Yongue B. Respiratory-heart rate interactions: Psychophysiological implications for pathophysiology and behavior. In: Cacioppo J, Patty R, editors. Perspectives in Cardiovascular Psychophysiology. New York: Guilford Press; –1982.pp. 223–264. [Google Scholar]
- Porter CL. Coregulation in mother-infant dyads: links to infants’ cardiac vagal tone. Psychological Reports. 2003;92:307–19. doi: 10.2466/pr0.2003.92.1.307. [DOI] [PubMed] [Google Scholar]
- Rozman J, Bunc M, Zorko B. Modulation of hormone secretion by functional electrical stimulation of the intact and incompletely dysfunctional dog pancreas. Brazilian Journal Medicine and Biology Research. 2004;37:363–70. doi: 10.1590/s0100-879x2004000300012. [DOI] [PubMed] [Google Scholar]
- Stifter C, Fox NA, Porges S. Facial expressivity and vagal tine in 5-and 10-month-old infants. Infant Behavior and Development. 1989;12:127–137. [Google Scholar]
- Stifter C, Jain A. Psychophysiological correlates of infant temperament: stability of behavior and autonomic pattering from 5 to 18. Developmental Psychobiology. 1996;29:379–91. doi: 10.1002/(SICI)1098-2302(199605)29:4<379::AID-DEV5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg MD. Massage Relaxation and well-being: a possible role for oxytocin as an integrative principle? In: Field T, editor. Touch and massage in early child development. Johnson & Johnson Pediatric Institute; 2004. [Google Scholar]