Abstract
T cell factor (TCF) family of transcription factors and β-catenin critically regulate T cell development as demonstrated by the deletion of the tcf gene, which results in a block early in development that becomes complete in mice bearing tcf/lef double deletion. However, the role of β-catenin, a major TCF cofactor, remains controversial. To directly address this, we have generated transgenic mice expressing Inhibitor of β-catenin and TCF (ICAT), a naturally occurring inhibitor that specifically disrupts TCF and β-catenin interactions. In this report, we demonstrate that disrupting the interaction of β-catenin with TCF renders adult thymocytes and activated T cells highly susceptible to apoptosis. In contrast to previously reported observations during fetal thymocyte development, these data show that in adult mice, survival and not differentiation of thymocytes, depends on transcription by TCF and β-catenin. Indeed, we demonstrate that expression of ICAT impedes thymocyte survival by reducing the expression of BclxL in thymocytes below a critical threshold. Survival of activated mature T cells was also impaired due to diminished expression of activation-induced BclxL. Accordingly, expression of transgenic Bcl-2 rescued activated ICAT-Tg CD4 T cells from apoptosis. Thus, disruption of TCF-β-catenin interactions specifically impairs the survival of thymocytes and activated T cells.
Keywords: apoptosis, β-catenin, TCF, ICAT, thymus
Introduction
Thymocytes develop from bone marrow-derived progenitor cells in an ordered manner characterized by the expression of specific surface markers CD4 and CD8. The most immature thymocytes do not express CD4 and CD8 and form the CD4−CD8− double negative (DN) sub-population. DN thymocytes progress to the CD4 and CD8 expressing CD4+CD8+ double-positive (DP) stage followed by the CD4 or CD8 expressing single positive (SP) mature stage. DN population of thymocytes can be further divided into four sub-populations, which bear a precursor–product relationship, based on cell surface expression of CD44 and/or CD25: DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+) and DN4 (CD44−CD25−). At the DN2-DN3 stage of development thymocytes rearrange and express TCRβ chain in conjunction with a non-rearranging pre-Tα chain to form the pre-TCR (1, 2). Pre-TCR regulates β-selection, which chain regulates β-selection, which ensures expression of a functional TCRβ chain, mediates cellular proliferation and transition to DP stage where TCRα chain is rearranged (3). αβTCR along with the CD4 and CD8 molecules interact with self-MHC complexes to eliminate self-reactive cells and generate MHC-restricted mature SP thymocytes (4, 5). Mature T cells migrate to colonize the peripheral immune system and become available for immune response.
In the thymus, thymic epithelial cells and developing thymocytes express many proteins that make up the Wnt–β-catenin–TCF pathway, implicating its participation in the inductive thymic microenvironment (6). Consistent with this, Wnt1 and Wnt4 double deletion results in significantly reduced thymic cellularity (7). The canonical Wnt-signaling pathway activates gene transcription by TCF and Lymphoid Enhancer Factor (LEF) family of transcription factors in conjunction with β-catenin. Loss of transcription factor TCF impairs proliferation, survival and differentiation of thymocytes (8) and a TCF and LEF double deletion completely blocks development to the DP stage (9). Over-expression of inhibitors of the Wnt-signaling pathway such as modified Frizzled, Dickkopf or ICAT blocks the development of T cell precursors in fetal thymic organ cultures (FTOCs) (10, 11). Accordingly, T cell specific deletion of β-catenin results in a block in development at the DN3–DN4 stage of development and reduced proliferation of DN4 thymocytes (12). Consistent with this, dramatically high-level over-expression of β-catenin in RAG2-deficient thymocytes permits maturation of DN thymocytes to the DP stage without expression of the TCRβ chain or proliferation perhaps in a manner similar to that observed after a sub-lethal irradiation in RAG-deficient mice (13–15). Alternatively, modest transgenic expression of β-catenin in developing thymocytes enhances the generation of CD8SP thymocytes and accelerates thymic involution (16, 17). TCF-deficient DP thymocytes are highly susceptible to apoptosis due to diminished expression of BclxL. TCF-dependent survival of DP thymocytes requires β-catenin function as the survival of TCF−/− DP thymocytes was rescued by transgenic expression of TCF containing an intact β-catenin-binding site but not a mutant TCF lacking this site (18). Notably, stabilization of endogenous β-catenin is regulated in a developmentally significant manner, suggesting that intrathymic signals stabilize β-catenin during normal development (19). Together, these data suggest that the Wnt–β-catenin–TCF pathway facilitates multiple events during thymocyte maturation and the level of β-catenin expression differentially affects the biology of the thymus.
The role of β-catenin in T cell development remains controversial, despite the observations noted above, mainly because deletion of β-catenin in the precursor hematopoietic stem cells (HSCs) or at the DN3 stage of thymocyte maturation showed discrepant results. Deletion of β-catenin in HSCs followed by bone marrow transplantation shows no defect in T cell development (20) while deletion at the DN3–DN4 stage of development results in a partial but significant block at the DN3–DN4 transition and decreased thymic cellularity due to decreased proliferation (12). One explanation for the discrepancy was believed to be redundancy with γ-catenin. However, this option was eliminated when double deletion of both genes failed to disrupt hematopoietic development (21, 22). Interestingly, Jeannet et al. (22) demonstrated that the regulation of the Wnt–β-catenin signaling pathway was more complex in hematopoietic cells as the pathway remained active even when both γ- and β-catenin are deleted. Therefore, at this time, more information is required to define the Wnt-β-catenin-TCF-dependent mechanisms that regulate hematopoiesis and lymphopoiesis.
ICAT is a naturally occurring small protein that inhibits β-catenin-TCF, but not γ-catenin-TCF or β-catenin–cadherin interactions (23). Binding of β-catenin to ICAT does not protect it from degradation (23). Therefore, over-expression of ICAT specifically disrupts β-catenin–TCF interactions and gene expression without affecting other aspects of TCF or β-catenin expression. This strategy was exploited by Jenkinson et al. (11) who demonstrated that expression of ICAT in thymocytes using retroviral constructs blocked T cell development at the DN to DP transition in FTOCs. FTOCs are excellent tools for analysis of early stages of thymocyte maturation but these studies did not address the role of ICAT at late stages of T cell development. In addition, the effect of ICAT expression on thymocyte survival could not be assessed as FTOCs naturally have high level of apoptosis. Finally, the role for ICAT in the development and function of mature T cells could not be assessed in FTOCs. To address these issues, we have generated transgenic mice (ICAT-Tg) that express ICAT in thymocytes and T cells.
In this report, we show that intrathymic signals regulate expression of endogenous ICAT in a developmentally significant manner, indicating a role for ICAT during normal thymocyte development. ICAT-Tg mice express ICAT in all thymocyte subsets and show enhanced apoptosis and reduced thymic cellularity. Mature T cells were also reduced in ICAT-Tg mice and the T cells that did develop were susceptible to apoptosis after activation. Apoptosis of ICAT-Tg T cells was due to reduced induction of BclxL after TCR signaling and was rescued by enforced expression of Bcl-2. Together, these data suggest that β-catenin–TCF interactions are primarily required for cell survival during T cell development and in antigen-activated mature T cells.
Methods
Mice
ICAT cDNA (24) was PCR amplified from thymocyte cDNA of a C57BL/6 mouse with primers designed to introduce XbaI at 5′ and BamHI at the 3′ end and cloned into pCGN to introduce the HA tag. Another BamHI site was introduced at the 5′ end and BamHI cut HA-ICAT fragment was cloned into the BamHI site in p1017 vector (25). HA-ICAT DNA sub-cloned in p1017 was sequenced to ensure the absence of mutations. NotI cut DNA fragment containing the proximal Lck promoter, the ICAT gene, and human growth hormone sequences was injected in C57BL/6-recipient mice (University of Connecticut Health Center Gene Targeting and Transgenic Facility). Transgenic mice were identified by PCR analysis of DNA extracted from tail cuts using standard protocols. Transgenic founders (ICAT-Tg 101, ICAT-Tg 102, ICAT-Tg 103) were maintained as heterozygous for the transgene. The mice were bred and maintained in animal facility at Gerontology Research Center according to the National Institutes of Health regulations.
Flow cytometry
Thymocytes, splenocytes or lymph node cells were harvested, stained and analyzed on a FACSCalibur (Becton Dickinson). Antibodies with the following specificities were used for staining: FITC-lineage cocktail (B220, Mac-1, Gr-1, NK1.1, CD4, CD8, TCRβ and TCRγδ), PE-CD44 (IM7), APC-CD4 (GK1.5), PerCP-Cy5.5-CD25 (PC61), c-kit-APC (2B8), PE-CD8α or PerCP-Cy5.5-CD8α (53–6.7), FITC-TCRβ (H57-597), PE-TCRγδ (GL3), FITC-CD122 (TM-β1), FITC-CD5 (53–7.3), FITC-CD62L (MEL-14), FITC-CD24 (M1/69), FITC-CD69 (H1.2F3) and PE-CD25 (PC61) (all from BD Pharmingen, San Diego, CA, USA). Where indicated, thymocytes were sorted into TCRβ−CD4−8− (DN), DP, CD4SP and TCRhiCD8SP sub-populations on a DakoCytomation Moflo (Fort Collins, CO, USA). For intracellular staining, freshly isolated thymocytes were fixed and permeabilized first with 4% paraformaldehyde and then with methanol/acetone mixture (1:1, v/v). Cells were then stained with anti-Bcl-xL antibody (Southern Biotech) or anti-Bcl-2 antibody (BD Pharmingen).
Annexin V staining
Annexin V staining of freshly isolated thymocytes, splenocytes or LN cells was performed with Annexin-V-FLUOS staining kit according to the manufacturer’s protocol (Roche) after cells had been stained for surface TCR®, CD4 and CD8 or Lin, CD25 and CD44.
In vivo 5-bromo-2-deoxyuridine labeling
To examine in vivo cell proliferation, mice were intra-peritoneally injected with 5-bromo-2-deoxyuridine (BrdU) and 2 or 6 h later, mice were sacrificed and thymocytes, splenocytes or LN cells were stained for BrdU incorporation after cells had been stained for surface TCRβ, CD4 and CD8 or Lin, CD25 and CD44. Intracellular staining for BrdU was performed with FITC-BrdU Flow Kit according to the manufacturer’s protocol (BD Pharmingen).
T cell activation, apoptosis and proliferation in in vitro cultures
To assess in vitro T cell expansion, splenocytes were stimulated with anti-CD3 antibody (1 μg ml−1). IL-2 was used at a final concentration of 10 ng ml−1 where indicated. At the appointed time, cells were counted and stained with antibodies to CD4, CD8, CD25 and CD69 and analyzed by flow cytometry. To assess apoptosis in in vitro cultures, thymocytes were plated in medium at 37°C in CO2 incubator and assayed at noted time points by trypan blue exclusion or Annexin V staining.
Biochemical assays
For quantitative (real-time) reverse transcription (RT)–PCR, total RNA from sorted thymocyte sub-populations was reverse transcribed using poly (dT) and Superscript III reverse transcriptase (Invitrogen). The cDNA was subjected to real-time RT–PCR amplification (Applied Biosystems) for 40 cycles with annealing and extension temperature at 60°C. For RT–PCR, total RNA from thymocytes or purified CD4 T cells was reverse transcribed as above and the cDNA was subjected to PCR amplification. Primer sequences used in RT–PCR reactions were: β-actin (5′): 5′-TGGATGACGA-TATCGCTGCG-3′, (3′): 5′-AGGGTCAGGATACCTCTCTT-3′, product size: 199 bp; ICAT-endogenous (5′): 5′-GAGTTGAG-CATCTGTTTGCCT-3′, (3′): 5′-CACGCGGACCTTCTGTTGAA-3′, product size: 132 bp; ICAT transgenic (5′): 5′-ACCTGGAT-CCACCATGGCTTCTAGCTATCCTTATGAC-3′, (3′): 5′-CACG-CGGACCTTCTGTTGAA-3′, product size: 141 bp. β-catenin (5′): 5′-GCCTGCAGAACTCCAGAAAG-3′, (3′): 5′-GTGG-CAAAAACATCAACGTG-3′, product size: 400 bp; TCF (5′):5′-CGCTGCATAACAAGCC-3′, (3′): 5′-CCAGCTCACAGTAT-GGG-3′, product size: 143 bp. Bax (5′): 5′-AATTGGAGATG-AACTGGACAGC-3′, (3′): 5′-CCAGTTGAAGTTGCCATCAGC-3′, product size: 125 bp. BclxL (5′): 5′-CAGCTAGAGCCTTGG-ATCCA-3′ (3′): 5′-TTGAAGCGCTCCTGGCCTTT-3′ product size: 114 bp. Western blot analysis was done with thymocytes, purified B cells or T cells lysed in SDS sample buffer. Cell lysates were resolved on 4–12% SDS–PAGE (Invitrogen) and then transferred to polyvinylidene fluoride membrane. Blots were incubated with anti-HA antibody (Sigma), anti-PKCμ antibody (Santa Cruz Biotechnology), anti-Bcl-xL antibody (Cell Signaling Technology) or anti-β-actin antibody (Sigma) followed by HRP-conjugated anti-mouse-IgG or anti-rabbit-IgG (Santa Cruz). Reactivity was revealed by enhanced chemiluminescence.
Retroviral transduction of purified CD4 T cells
Mouse CD4+ T cells were isolated with CD4+ T cell isolation kit (Miltenyi Biotec). CD4+ T cells were activated with plate-bound anti-CD3 (1 μg ml−1) and anti-CD28 (1 μg ml−1) antibodies. Cells were then infected with MSCV-based retrovirus co-expressing GFP and ICAT. At noted time points after infection, GFP+ cells were analyzed.
Results
ICAT expression is developmentally regulated in thymocytes
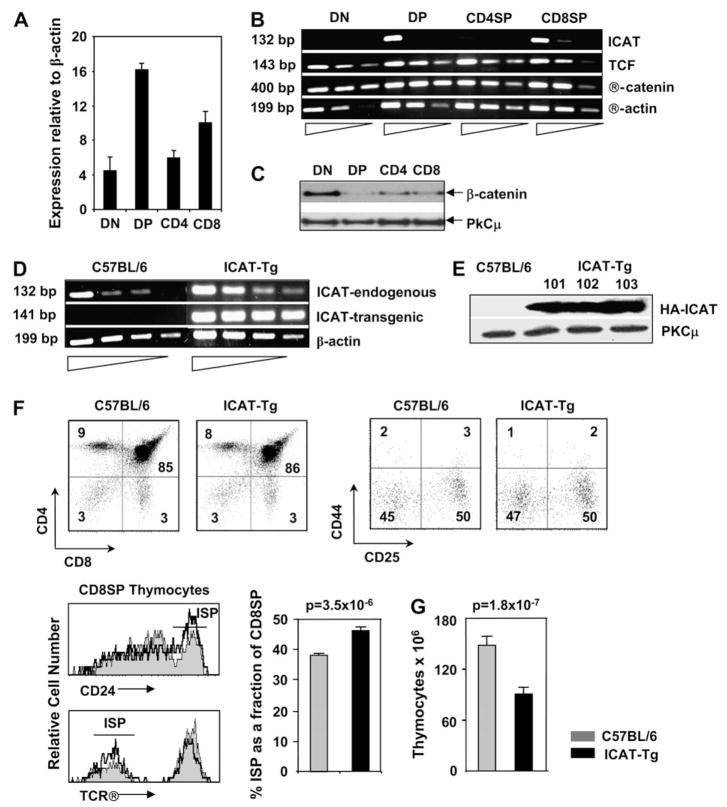
To study the function of ICAT, a naturally occurring inhibitor of β-catenin–TCF interactions, we determined the regulation of its expression during T cell development. Ex vivo DN thymocytes showed low levels of ICAT mRNA while DP thymocytes showed high levels of ICAT expression (Fig. 1A). These data reveal that ICAT expression is up-regulated by intrathymic signals that mediate DN to DP transition. At the DP to SP transition, ICAT expression is down-regulated with higher levels observed in CD8SP thymocytes and relatively lower levels in CD4SP thymocytes (Fig. 1A). Next, we compared the abundance of ICAT mRNA with β-catenin and TCF mRNAs in thymocyte subsets (Fig. 1B). While ICAT mRNA was developmentally regulated, mRNA corresponding to TCF and β-catenin were expressed in all thymocyte subsets (Fig. 1B). Regulation of ICAT expression is not well studied but it is believed to be regulated at the transcriptional level (26). We have previously demonstrated that β-catenin protein levels, but not mRNA levels, vary dramatically in thymocyte subsets [Fig. 1B and C, and (19)]. This is because β-catenin expression is largely regulated at the post-translational level by ubiquitin-mediated degradation. Thus, TCF and ICAT are primarily regulated at the transcription level while β-catenin is regulated by post-transcriptional mechanisms. Here, we note that when TCF and ICAT are both highly expressed in DP thymocytes, β-catenin is down-regulated. Similar inverse regulations of ICAT and β-catenin have been noted in skin development (26). We conclude that the inverse regulation of ICAT mRNA expression and β-catenin stabilization by intrathymic signals may be significant for thymocyte development.
Fig. 1.
Intrathymic signals regulate endogenous ICAT expression in the thymus. (A) Intrathymic signals regulate ICAT expression in thymocyte subsets. Real-time RT–PCR analysis of endogenous ICAT mRNA expression in sorted thymocyte subsets (two mRNA sets from independently sorted cell populations were analyzed). (B) Intrathymic signals regulate ICAT mRNA, but not TCF or β-catenin mRNAs, in thymocyte subsets. Serial dilution (5-fold) RT–PCR analysis of abundance of endogenous ICAT, TCF and β-catenin mRNA in thymocyte subsets (data are representative of two mRNA sets from independently sorted cell populations). (C) β-Catenin protein expression is regulated by intrathymic signals in thymocyte subsets. Total protein was extracted from sorted thymocytes and analyzed by western blot. (D) Transgenic ICAT is expressed in thymocytes. Serial dilution (3-fold) RT–PCR analysis of endogenous and transgenic ICAT mRNA expression in C57BL/6 and ICAT-Tg thymocytes (upper panels). (E) Thymocytes from three lines of ICAT-Tg mice express comparable levels of HA-ICAT protein. Western blot analysis of transgenic ICAT protein expression in thymocytes from three lines of ICAT-Tg mice. Data are representative of two independent experiments. (F) Transgenic ICAT expression does not impair T cell development. C57BL/6 and ICAT-Tg thymocytes were stained with antibodies to CD4 and CD8 (left panels, both n = 22) or with a lineage cocktail and CD25 and CD44 (right panels, both n = 13) and analyzed by flow cytometry. The numbers in quadrants show the % cells in that subset. Fraction of ISPs is modestly but significantly increased in ICAT-Tg mice (C57BL/6, n = 18 and ICAT-Tg, n = 20; P = 3.5 × 10−6). Overlay of TCRβ expression (lower panel; representative of n = 18 mice for both strains) and CD24 expression (upper panel; representative of n = 4 mice for both strains) on CD8SP thymocytes from control (shaded) and ICAT-Tg (line) mice. (G) Thymic cellularity is significantly decreased in ICAT-Tg mice. Total number of thymocytes in C57BL/6 and ICAT-Tg mice (C57BL/6, n = 25; ICAT-Tg, n = 26; P = 1.8 × 10−7)
Thymic cellularity is significantly diminished in ICAT-Tg mice
To study the role of β-catenin–TCF interactions in T cell development, we disrupted these interactions by over-expressing transgenic ICAT from the proximal Lck promoter. Three lines of transgenic mice (ICAT-Tg 101, 102 and 103) were analyzed and showed roughly comparable levels of transgenic HA-ICAT expression. mRNA for endogenous ICAT and for transgenic HA-ICAT from ICAT-Tg 103 line is shown (Fig. 1D). Because there is no antibody defined for ICAT protein, we used anti-HA antibody to demonstrate the expression of HA-tagged transgenic protein. HA-ICAT protein was expressed in thymocytes from three lines of ICAT-Tg mice but not in control thymocytes, and the level of expression was comparable in the three lines of transgenic mice analyzed (Fig. 1E). Thus, transgenic ICAT protein is expressed in ICAT-Tg thymocytes. Expression of BclxL has been shown to be strictly dependent on β-catenin–TCF interactions (18, 27). Therefore to determine whether the transgenic protein was functional, we assayed the expression of BclxL protein in ICAT-Tg DP thymocytes. The data demonstrated that BclxL expression was decreased in ICAT-Tg thymocytes compared with control thymocytes (see Fig. 3D). We conclude that the transgenic ICAT protein is expressed in thymocytes and is functional.
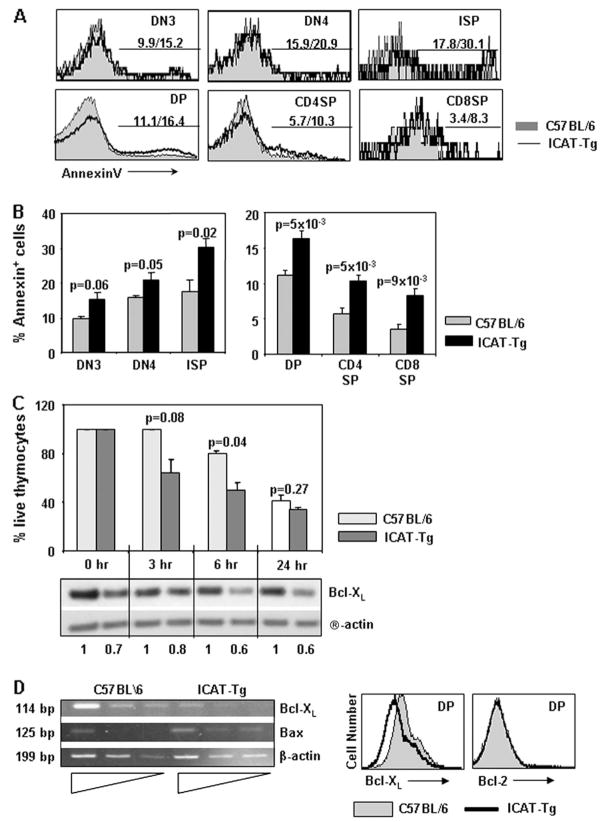
Fig. 3.
ICAT over-expression enhances thymocyte apoptosis. (A) Apoptosis is enhanced in all thymocyte subsets. Representative histogram overlays of Annexin V staining of electronically gated thymocyte subsets from C57BL/6 (shaded) and ICAT-Tg (line) mice. The numbers to the left represent percent Annexin V+ cells in C57BL/6 thymocytes and to the right Annexin V+ cells in ICAT-Tg thymocytes. (B) Increased apoptosis in ICAT-Tg thymocytes is statistically significant. Statistical analysis and graphical representation of Annexin V+ cells (C57BL/6 n = 4 and ICAT-Tg n = 4; DN3 P = 0.06; DN4 P = 0.05; ISP P = 0.02; DP P = 5 × 10−3; CD4SP P = 5 × 10−3; CD8SP P = 9 × 10−3). (C) ICAT expression enhances apoptosis of ICAT-Tg thymocytes in in vitro cultures due to decreased expression of BclxL. Thymocytes from C57BL/6 and ICAT-Tg mice were cultured in medium. Input thymocytes were assigned the value 100% and at each time point, apoptosis was assessed by trypan blue exclusion. The level of BclxL was determined by western blot analysis of protein extracts from cultured thymocytes (C57BL/6, n = 2 and ICAT-Tg, n = 2). (D) Ex vivo ICAT-Tg thymocytes express lower levels of BclxL. Abundance of mRNA from ex vivo thymocytes (left panels) and intracellular staining for BclxL protein (gated on DP thymocytes) (right panels) show lower level of BclxL expression in ICAT-Tg thymocytes. Data are representative of C57BL/6, n = 4 and ICAT-Tg, n = 4 mice.
Staining of ICAT-Tg thymocytes with antibodies to CD4 and CD8 showed that the distribution of thymocytes in the four subsets was similar to littermate control mice (Fig. 1F). DN1–4 subsets defined by CD44 and CD25 expressing lineage negative thymocytes were also comparable in control and ICAT-Tg mice (Fig. 1F). We conclude that expression of transgenic ICAT did not grossly disrupt thymocyte development. Immature Single Positive (ISP) thymocytes form an intermediate stage of development as cells transition from the DN to the DP stage. ISP cells are characterized by low levels of CD8 and TCRβ expression and high levels of CD24 expression (Fig. 1F). In ICAT-Tg mice, the proportion of ISP cells is modestly but significantly increased (Fig. 1F). The increase in the proportion of cells at the ISP stage probably results from a block in development of ISP cells to the DP stage as noted in TCF-KO mice (8) and in studies in which ICAT was expressed using retroviral vectors in FTOCs (11). Thus, expression of transgenic ICAT, which disrupts β-catenin–TCF interactions, phenocopy TCF-deficient mice (8). We suggest that β-catenin–TCF interactions are important for the maturation of ISP thymocytes to the DP stage.
Disruption of TCF and β-catenin interaction does not impair thymocyte proliferation in vivo
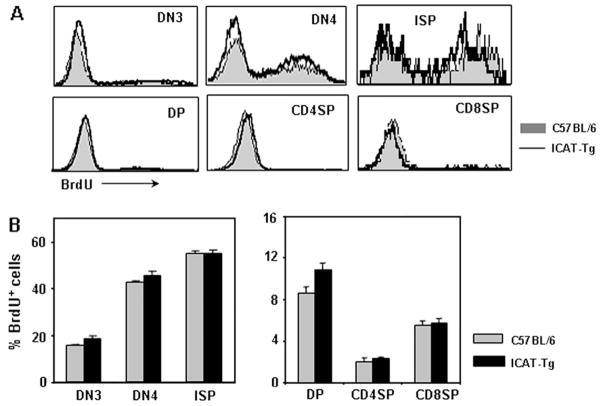
Thymic cellularity was significantly decreased in ICAT-Tg mice (Fig. 1G and average thymocyte number in ICAT-Tg 101 mice was 80.2 ± 5.2, n = 18 and in ICAT-Tg 103 mice was 90.6 ± 8.3, n = 8 compared with littermate controls 148.6 ± 9.96, n = 25). This could result from impaired proliferation of thymocytes. In the adult thymus, some cells in the DN compartment and ISP cells have been shown to proliferate and provide most of the cellularity (28). Despite higher proportion of ISP cells, ICAT-Tg mice showed hypocellular thymuses. To examine proliferation of ICAT-Tg thymocytes, we assessed BrdU incorporation in vivo. Mice were injected with BrdU and thymocytes were isolated after 2 h, stained with anti-BrdU antibody and analyzed. Representative histogram overlays (Fig. 2A) and statistical analysis of data from four independent experiments (Fig. 2B) show that proliferation of ICAT-Tg thymocytes was very comparable to control thymocytes. We conclude that β-catenin–TCF interactions are dispensable for proliferation of thymocytes.
Fig. 2.
Decreased thymic cellularity in ICAT-Tg mice is not due to impaired proliferation. (A) Representative histogram overlays of BrdU staining of electronically gated ex vivo thymocyte subsets from BrdU-injected C57BL/6 (shaded) and ICAT-Tg (line) mice. (B) Statistical analysis and graphical representation of BrdU+ cells (C57BL/6 n = 4 and ICAT-Tg n = 4; DN3 P = 0.13; DN4 P = 0.24; ISP P = 0.85; DP P = 0.06; CD4SP P = 0.51; CD8SP P = 0.83).
Disruption of TCF and β-catenin interactions results in apoptosis of thymocytes
Decreased thymic cellularity could also result from enhanced apoptosis of thymocytes. To dissect the role of ICAT in survival of thymocytes, we assessed apoptosis of thymocytes in all the subsets (Fig. 3). Ex vivo thymocytes were stained with antibodies to cell surface markers and Annexin V. Representative histogram overlays are shown and statistical analysis of data from several independent experiments is graphed (Fig. 3A and B). We observed statistically significant increase in apoptosis in all thymocyte subsets. We conclude that transcription regulated by the coordinated action of TCF and β-catenin plays an important role in maintaining the survival of thymocytes.
To address the molecular basis for apoptosis of thymocytes, we assayed for the expression of Bcl family proteins in control and ICAT-Tg thymocytes. Survival of thymocytes in different subsets is regulated by different members of the Bcl family proteins. ICAT-Tg thymocytes cultured in vitro showed enhanced apoptosis over a 6-h period. The extent of survival is correlated with the abundance of BclxL protein in the cells (Fig. 3C). At the 24-h time point, the difference in the proportion of apoptotic cells in control and ICAT-Tg thymocyte cultures decreased because control thymocyte cultures also began to accumulate apoptotic cells. Regardless, the amount of BclxL expression in the remaining ICAT-Tg thymocytes remained low compared with the remaining control thymocytes. We conclude that TCF and β-catenin are required to express BclxL in thymocytes. To determine if decreased BclxL might be the basis for increased Annexin V+ cells, we stained ex vivo ICAT-Tg thymocytes for expression of the protein (Fig. 3D). Ex vivo ICAT-Tg thymocytes showed demonstrably lower expression of BclxL mRNA (Fig. 3D, left panel). Intracellular staining experiments also showed lower level of BclxL protein in ex vivo DP thymocytes (Fig. 3D, right panel). Together, these data demonstrate that ICAT expression diminishes the expression of BclxL protein in thymocytes. We conclude that β-catenin–TCF interactions play a role in maintaining the expression of BclxL and survival of DP thymocytes.
ICAT-Tg mice have reduced peripheral CD4 and CD8 T cells
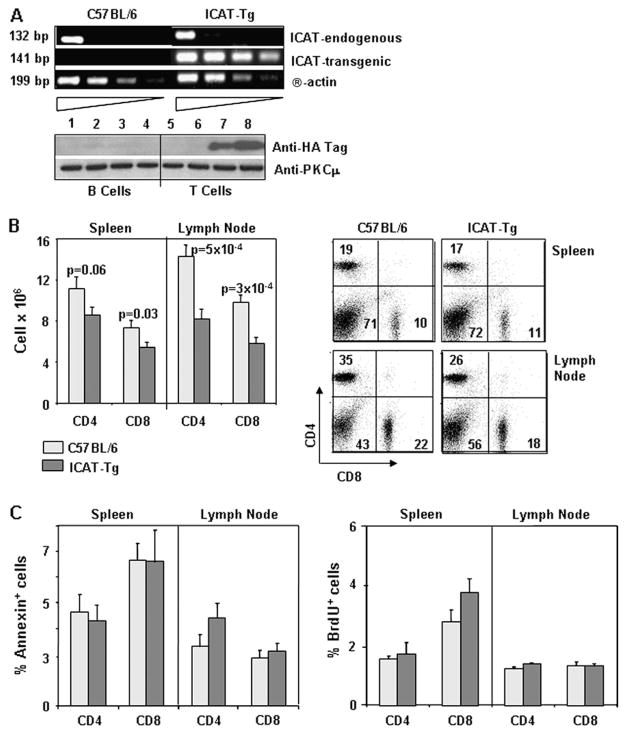
Next, we studied peripheral T cells in the spleen and the lymph nodes of ICAT-Tg mice. Analysis of endogenous and transgenic ICAT mRNA in CD4 T cells from ICAT-Tg mice shows that ICAT transgene is expressed in CD4 T cells (Fig. 4A, upper panels). As no antibody reagents are available for ICAT protein, we used anti-HA antibody to demonstrate the expression of HA-tagged transgenic protein in T but not B cells (Fig. 4A, lower panels). The proportion of CD4 and CD8 T cells in spleen and lymph nodes of ICAT-Tg mice was comparable to control mice (Fig. 4B, right panels). However, the absolute number of T cells was significantly decreased in ICAT-Tg mice compared with control mice (Fig. 4B, left panels). To determine the reason for decreased numbers of CD4 T and CD8 T cells, we assessed cell death by Annexin V staining of ex vivo cells and proliferation capacity by BrdU incorporation in vivo (Fig. 4C). Ex vivo Annexin V staining data and BrdU uptake data showed no consistent differences between control and ICAT-Tg T cells. Therefore, we suggest that the decreased number may result from reduced generation of thymocytes in ICAT-Tg mice.
Fig. 4.
ICAT expression in peripheral T cells and the characterization of apoptosis and proliferation of ICAT-Tg T cells. (A) Endogenous and transgenic ICAT is expressed in peripheral T cells. Serial dilution (3-fold) RT–PCR analysis shows endogenous and transgenic ICAT mRNA expression in control and ICAT-Tg CD4 T cells (upper panels). Western blot analysis of transgenic ICAT protein expression in B (lanes 1–4) and T (lanes 5–8) cells from C57BL/6 (lanes 1, 2, 5 and 6) and ICAT-Tg (lanes 3, 4, 7 and 8) mice (lower panels). Data are representative of two independent experiments. (B) The number of CD4 and CD8 T cells is reduced in spleen and lymph nodes. Splenic and lymph node cells were counted and stained with antibodies to CD4 and CD8 to determine the number of CD4 and CD8 T cells in ICAT-Tg (spleen, n = 18; LN, n = 12) and C57BL/6 (spleen, n = 15; LN, n = 12) mice. (C) ICAT-Tg T cells from the spleen or lymph nodes do not show enhanced apoptosis or proliferation in vivo. Percent Annexin V+ cells (left panels) and BrdU+ cells (right panels) was determined by assessing electronically gated ex vivo CD4 T and CD8 T cells from BrdU-injected C57BL/6 (light bars, n = 4) and ICAT-Tg (dark bars, n = 4) mice.
Enhanced apoptosis of activated ICAT-Tg T cells
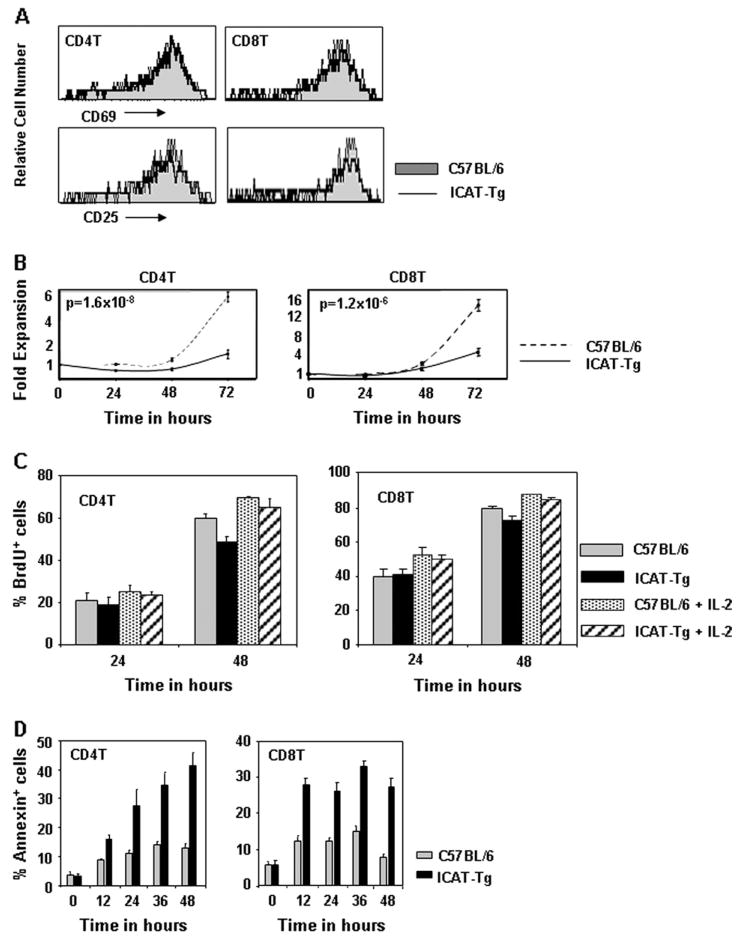
To examine the role of β-catenin–TCF interactions during T cell function, we assessed activation and proliferation of ICAT-Tg T cells in vitro. Because ICAT-mediated disruption of β-catenin–TCF interactions could affect cadherin-dependent cell surface events, we assayed T cell activation in whole spleen cell cultures (Fig. 5). For these assays, anti-CD3 antibody was added to cultures containing CD4 T, CD8 T and antigen-presenting cells from the spleen, as described in Methods. Early events in TCR-mediated activation of ICAT-Tg T cells and control T cells were similar, as shown by the up-regulation of cell surface markers of activation CD69 and CD25 (Fig. 5A). However, TCR-mediated expansion of CD4 and CD8 T cells was severely impaired (Fig. 5B). We conclude that T cells were activated normally but failed to expand when β-catenin–TCF interactions were disrupted by ICAT expression.
Fig. 5.
Enhanced apoptosis of ICAT-Tg T cells in activation cultures. (A) ICAT expression does not impair T cell activation. T cells in total spleen cultures were activated with anti-CD3 antibody, and after 24 h of culture, CD4 and CD8 T cells were analyzed for expression of early activation markers CD69 and CD25 by flow cytometry. (B) ICAT expression impairs expansion of activated T cells. The number of CD4 T and CD8 T cells was determined at 24-h intervals by counting the cells in the cultures and staining T cells with antibodies to CD4 and CD8. T cell expansion was evaluated by calculating fold expansion defined as the number of CD4 or CD8 T cells at a particular time point divided by the input number of CD4 or CD8 T cells (C57BL/6, n = 6 and ICAT-Tg, n = 6). (C) ICAT expression does not impair T cell proliferation in activation cultures. BrdU was added to activation cultures. CD4 and CD8 T cells that had incorporated BrdU were identified by staining with antibodies to CD4, CD8 and BrdU at indicated time points and analyzed by flow cytometry. (D) ICAT expression impairs the survival of activated T cells. Cells in culture were stained with Annexin V and anti-CD4 and CD8 at 12-h intervals to determine cell death in activation cultures.
To further dissect the basis for impaired expansion of T cells, we assessed proliferation and cell death in activation cultures. Briefly, BrdU was added to in vitro cultures to assess the extent of proliferation. The extent of BrdU incorporation by CD4 T and CD8 T cells was found to be comparable when ICAT-Tg and control cultures were compared (Fig. 5C). In addition, Annexin V-positive cells in the activation cultures were scored at regular intervals during the culture. The fraction of Annexin V+ cells were significantly increased in both ICAT-Tg CD4 T and CD8 T cells starting at 10–12 h after culture (Fig. 5D). Addition of exogenous IL-2 did not rescue cells from apoptosis in activation cultures (data not shown). Thus, activation and proliferation of mature ICAT-Tg T cells were comparable to control T cells. In contrast, survival in activation cultures was seriously impaired. Thus, disruption of β-catenin and TCF interaction results in apoptosis of TCR-activated mature T cells. We conclude that TCF and β-catenin cooperate to ensure survival of antigen-activated T cells.
Mechanism of apoptosis of ICAT-Tg T cells
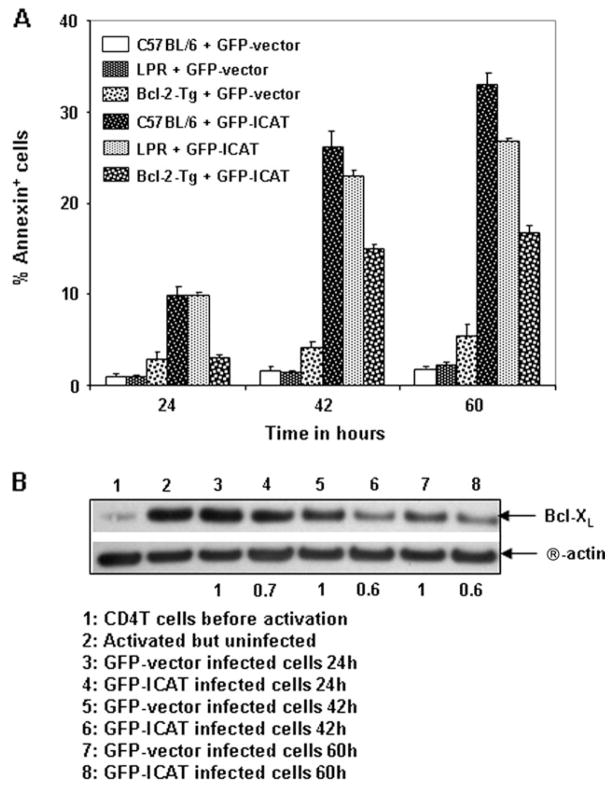
To determine the molecular basis for ICAT-dependent apoptosis of T cells in in vitro activation cultures, we utilized retroviral technology to express ICAT in CD4 T cells. If Fas/FasL played an essential role in ICAT-dependent apoptosis of activated T cells, expression of ICAT in LPR CD4 T cells would be expected to rescue the ICAT-expressing T cells from cell death. ICAT-dependent apoptosis was not rescued by the lpr mutation (Fig. 6A). BclxL is induced upon T cell activation and believed to participate in maintaining cell viability of activated T cells (29, 30). Interestingly, T cells expressing retrovirally transduced ICAT induced significantly lower levels of activation-induced BclxL protein upon TCR-driven activation (Fig. 6B). These data suggest that ICAT-dependent apoptosis involved Bcl family proteins. Accordingly, retroviral over-expression of ICAT in Bcl-2-Tg CD4 T cells resulted in a significant, though incomplete, rescue from apoptosis (Fig. 6A). Therefore, ICAT-induced apoptosis was partially rescued by transgenic expression of Bcl-2, indicating that other factors may also contribute to ICAT-mediated apoptosis of activated T cells. We conclude that β-catenin–TCF interactions regulate T cell survival during T cell activation in part by regulating the expression of Bcl family proteins.
Fig. 6.
ICAT-dependent apoptosis is due to diminished expression of activation-induced BclxL in ICAT-Tg T cells and is rescued by transgenic expression of Bcl-2. (A) Expression of ICAT in Bcl-2-Tg CD4 T cells, using retroviral technology, partially rescues ICAT-dependent apoptosis. Purified CD4 T cells from C57BL/6 and Bcl-2-Tg mice were activated and infected with GFP-ICAT or control GFP-vector. At indicated post-infection time points, cells were stained with Annexin V and anti-CD4 to determine cell death. Statistical analysis and graphical representation of Annexin V+ cells in electronically gated GFP+ CD4+ cell population are shown (C57BL/6, n = 4 and Bcl-2-Tg, n = 4; P = 1 × 10−3, 8 × 10−4 and 5 × 10−5 respectively at 24, 42 and 60 h time points). Expression of ICAT in LPR CD4 T cells, using retroviral technology, does not rescue T cells from ICAT-dependent apoptosis (C57BL/6, n = 4 and LPR, n = 4; P = 0.98, 0.13 and 3 × 10−3 respectively at 24, 42 and 60 h time points). (B) Expression of ICAT in CD4 T cells, using retroviral technology, results in enhanced apoptosis (A) that correlated with diminished expression of activation-induced BclxL. Purified CD4 T cells from C57BL/6 mice were activated and infected with GFP-ICAT or control GFP-vector and at indicated post-infection time points, cells were collected for protein analysis by western blots. Data are representative of two independent experiments.
Discussion
In this study, we investigated the consequences of interrupting β-catenin–TCF interactions by over-expressing a naturally occurring inhibitor of this interaction, ICAT, in thymocytes and T cells. We demonstrate that β-catenin–TCF interactions regulate thymocyte and T cell survival by regulating the expression of Bcl family proteins. We have previously demonstrated that TCR signals stabilize β-catenin and facilitate its localization to the nucleus (12). In this report, we propose that TCR-induced β-catenin works with TCF to provide survival signals to activated T cells.
ICAT was cloned as an inhibitor of β-catenin-TCF, but not γ-catenin-TCF or β-catenin-cadherin, interactions (23, 31, 32). Jenkinson laboratory showed that disruption of β-catenin and TCF interactions by ICAT leads to a block in the development of DN thymocytes to the DP stage in FTOCs (11). In contrast, in this study we demonstrate that during adult T cell development, the proportion of ISP thymocytes is slightly increased. However, DN to DP transition proceeds normally while survival of DP thymocytes is diminished. We propose that, at a mechanistic level, the block in development to the DP stage, observed in ICAT-expressing FTOCs (11), resulted from apoptosis of newly generated DP thymocytes and not due to a block in development. Furthermore, we show that transgenic over-expression of ICAT results in reduced thymic cellularity which was attributed to increased apoptosis of thymocytes in ICAT-Tg mice and not to diminished proliferation. These data complement previous experiments that showed that β-catenin-binding domain of TCF is essential for DP thymocyte survival. Expression of TCF, with β-catenin-binding site deleted, was inadequate to rescue TCF-deficient DP thymocytes from apoptosis (18). In contrast, over-expressing β-catenin specifically in DP thymocytes, using the CD4-promoter, enhanced survival of transgenic DP thymocytes (27). Together with our data, these observations propose that a finely balanced regulation of β-catenin and TCF interactions is important for the survival of short-lived DP thymocytes.
In this report, we also demonstrate that ICAT expression is regulated by intrathymic signals in different thymocyte subsets, suggesting a role for this protein during normal T cell development. ICAT is expressed at low levels in DN and SP thymocytes and highest level in DP thymocytes. Thus, ICAT is likely to have a major role in DP thymocytes. This pattern of regulation of endogenous ICATexpression is opposite of β-catenin expression, suggesting that β-catenin–TCF interactions are likely to be most critical at the DN and SP stages of development where both TCF and β-catenin are highly expressed and ICAT is expressed at low levels. Indeed, deletion of TCF (9, 33) and β-catenin (12) shows that DN compartment is most sensitive to the absence of these proteins. In contrast, at the DP stage, TCF (34) and ICAT are highly expressed (this study) while β-catenin expression is decreased (19) in response to intrathymic signals. We suggest that ICAT fine-tunes TCF and β-catenin signals that provide survival to short-lived DP thymocytes. This fine tuning seems essential for proper selection of thymocytes because over-expression of β-catenin from the CD4 promoter provided sustained survival to DP thymocytes but did not facilitate maturation to the SP stage (27). In contrast, expression of β-catenin from the Lck promoter, which allowed expression of transgenic β-catenin in a manner similar to endogenous β-catenin, facilitated generation of SP thymocytes (19, 35). We propose that TCF and β-catenin interactions must be fine-tuned to provide survival to short-lived DP thymocytes and must be appropriately regulated for thymocyte selection to proceed normally.
The role of gene expression mediated by β-catenin–TCF interactions in mature T cell function is poorly understood because TCF deletion blocks T cell development, resulting in few mature T cells. Cursory examination of TCF-deficient T cells suggested only a minor role for TCF-β-catenin-mediated gene expression (8, 33). T cell proliferation in TCF-deficient spleen cultures showed decreased thymidine uptake (8). The authors concluded that decreased numbers of T cells in the TCF-deficient spleen reflected the decreased thymidine uptake but did not study proliferation or apoptosis directly. In contrast, a role for TCF in the function of human CD8 T cells is proposed because resting human CD8 T cells express inhibitory isoforms of TCF and LEF and expression pattern shifts toward the stimulatory forms upon engagement of the TCR or IL-15 receptor (36). Examination of endogenous ICAT expression showed that the mRNA is up-regulated starting at ~22 h and maximum levels are reached at 42 h after TCR stimulation, suggesting that initial survival of antigen-activated T cells depends on TCF and β-catenin interaction. Analysis of ICAT-Tg T cells demonstrated that TCF and β-catenin signals specifically regulate survival of T cells after antigen-driven activation by regulating the expression of activation-induced BclxL. Accordingly, Bcl-2-Tg, but not Fas deficiency, rescued ICAT-dependent apoptosis in vitro. Therefore, β-catenin–TCF interactions regulate survival of activated T cells by regulating the expression of activation-induced BclxL.
In summary, we show that interruption of β-catenin and TCF interactions by over-expression of ICAT results in decreased survival of thymocytes due to diminished expression of BclxL in vivo. In activation cultures, mature T cell survival, but not proliferation, was significantly diminished due to impaired induction of BclxL in response to TCR stimulation. Together, these data demonstrate that gene expression mediated by β-catenin and TCF signaling regulates survival of thymocytes and T cells during immune response.
Acknowledgments
Funding
Intramural Research Program of the National Institute on Aging at the National Institutes of Health.
We thank Robert Wersto, Francis J. Chrest and Cuong Nguyen for expert cell sorting of thymocyte sub-populations; Donna Tignor, Dawn Phillips, Dawn Nines, Crystal Gifford, Anna Butler for maintaining animals; Shengyuan Luo and his group for genotyping animals.
Abbreviations
- BrdU
5-bromo-2-deoxyuridine
- DN
double negative
- DP
double positive
- FTOC
fetal thymic organ culture
- HSC
hematopoietic stem cell
- ICAT
inhibitor of β-catenin and TCF
- ISP
immature single positive
- LEF
lymphoid enhancer factor
- RAG
recombination activating gene
- RT
reverse transcription
- SP
single positive
- TCF
T cell factor
References
- 1.Hayday AC, Pennington DJ. Key factors in the organized chaos of early T cell development. Nat Immunol. 2007;8:137. doi: 10.1038/ni1436. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MK. At the crossroads: diverse roles of early thymocyte transcriptional regulators. Immunol Rev. 2006;209:191. doi: 10.1111/j.0105-2896.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 3.von Boehmer H, Aifantis I, Azogui O, et al. Crucial function of the pre-T-cell receptor (TCR) in TCR beta selection, TCR beta allelic exclusion and alpha beta versus gamma delta lineage commitment. Immunol Rev. 1998;165:111. doi: 10.1111/j.1600-065x.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 4.Laky K, Fleischacker C, Fowlkes BJ. TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol Rev. 2006;209:274. doi: 10.1111/j.0105-2896.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 5.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006;18:175. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Weerkamp F, Baert MR, Naber BA, et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA. 2006;103:3322. doi: 10.1073/pnas.0511299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984. [PubMed] [Google Scholar]
- 9.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 10.Staal FJ, Meeldijk J, Moerer P, et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur J Immunol. 2006;36:2376. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 13.Gounari F, Aifantis I, Khazaie K, et al. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat Immunol. 2001;2:863. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 14.Gounari F, Chang R, Cowan J, et al. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidos CJ, Williams CJ, Wu GE, Paige CJ, Danska JS. Development of CD4+CD8+ thymocytes in RAG-deficient mice through a T cell receptor beta chain-independent pathway. J Exp Med. 1995;181:1187. doi: 10.1084/jem.181.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulroy T, Xu Y, Sen JM. beta-Catenin expression enhances generation of mature thymocytes. Int Immunol. 2003;15:1485. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Sen J. Beta-catenin expression in thymocytes accelerates thymic involution. Eur J Immunol. 2003;33:12. doi: 10.1002/immu.200390002. [DOI] [PubMed] [Google Scholar]
- 18.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin–TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2:691. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 19.Yu Q, Sen JM. beta-Catenin regulates positive selection of thymocytes but not lineage commitment. J Immunol. 2007;178:5028. doi: 10.4049/jimmunol.178.8.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobas M, Wilson A, Ernst B, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of {beta}- and {gamma}-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2007;111:160. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 22.Jeannet G, Scheller M, Scarpellino L, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of {beta}-catenin and {gamma}-catenin. Blood. 2007;111:142. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 23.Gottardi CJ, Gumbiner BM. Role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions. Am J Physiol Cell Physiol. 2004;286:C747. doi: 10.1152/ajpcell.00433.2003. [DOI] [PubMed] [Google Scholar]
- 24.Tago K, Nakamura T, Nishita M, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14:1741. [PMC free article] [PubMed] [Google Scholar]
- 25.Garvin AM, Abraham KM, Forbush KA, Farr AG, Davison BL, Perlmutter RM. Disruption of thymocyte development and lymphomagenesis induced by SV40 T-antigen. Int Immunol. 1990;2:173. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Stow JL. ICAT is a multipotent inhibitor of beta-catenin. Focus on ‘‘role for ICAT in beta-catenin-dependent nuclear signaling and cadherin functions’’. Am J Physiol Cell Physiol. 2004;286:C745. doi: 10.1152/ajpcell.00563.2003. [DOI] [PubMed] [Google Scholar]
- 27.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 28.Paterson DJ, Williams AF. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. J Exp Med. 1987;166:1603. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636. [PubMed] [Google Scholar]
- 30.Boise LH, Minn AJ, Noel PJ, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 31.Graham TA, Clements WK, Kimelman D, Xu W. The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Mol Cell. 2002;10:563. doi: 10.1016/s1097-2765(02)00637-8. [DOI] [PubMed] [Google Scholar]
- 32.Daniels DL, Weis WI. ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell. 2002;10:573. doi: 10.1016/s1097-2765(02)00631-7. [DOI] [PubMed] [Google Scholar]
- 33.Verbeek S, Izon D, Hofhuis F, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 34.Yu Q, Erman B, Park JH, Feigenbaum L, Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORgammat: impact on thymocyte development. J Exp Med. 2004;200:797. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Q, Xu M, Sen JM. Beta-catenin expression enhances IL-7 receptor signaling in thymocytes during positive selection. J Immunol. 2007;179:126. doi: 10.4049/jimmunol.179.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]