Reactive oxygen species (ROS) alter nuclear histone acetylation and deacetylation (chromatin remodeling) leading to increased NF-κB-dependent gene expression of proinflammatory mediators. Naturally occurring dietary polyphenols such as curcumin (diferuloylmethane, an active component of spice turmeric) and resveratrol (phytoalexin, a flavanoid found in red wine) can directly scavenge ROS and modulate signaling pathways mediated via NF-κB and MAP kinase pathways, and upregulate glutathione biosynthesis gene via Nrf2 activation. They also downregulate expression of proinflammatory mediators, matrix metalloproteinases, adhesion molecules, and growth factor receptor genes by inhibiting histone acetyltransferase activity and activating histone deacetylase/sirtuins. Thus, these polyphenolic compounds have therapeutic value as antioxidant and anti-inflammatory therapy against chronic inflammatory epigenetically regulated diseases.
Reactive oxygen species play a key role in enhancing the inflammation through sustained activation and phosphorylation of MAP kinases and redox-sensitive transcription factors, such as NF-κB and AP-1, in various inflammatory diseases.1 Oxidative stress also alters nuclear histone acetylation and deacetylation (chromatin remodeling) leading to increased gene expression of proinflammatory mediators.2,3 Recent studies from our laboratory show that oxidative stress enhances lung inflammation via expression of proinflammatory mediators through the activation of intrinsic histone acetyltransferase (HAT) activity of coactivator molecules.4–7 Increased histone acetylation was associated with increased activation of IκB kinase-α and interaction of NF-κB with CBP, leading to increased acetylation of RelA/p65 subunit of NF-κB. Oxidative stress also inhibits the activity of histone deacetylase (HDACs) (decreased HDAC2 levels), activates cells for NF-κB transactivation, and enhances inflammatory gene expression, which leads to chronic inflammatory response both in monocytes and epithelial cells in vitro, and in lungs in vivo.5–7 Oxidative stress also plays a role in poor efficacy of corticosteroids in various chronic inflammatory diseases.2,8 Thus, oxidative stress-mediated aberrant chromatin remodeling or histone modifications can lead to heightened abnormal inflammatory response. These changes, despite being heritable and stably maintained, are also potentially reversible; therefore, there is scope for the development of ‘epigenetic therapies’ for such diseases. However, there is no specific therapeutic agent currently available to effectively inhibit both oxidative stress and inflammatory responses in a variety of inflammatory conditions/disorders.
Polyphenols in food plants are a versatile group of phytochemicals with many potentially beneficial activities in terms of disease prevention. Dietary polyphenols (bioflavanoids) have antioxidant and anti-inflammatory properties that might explain their beneficial effects. However, the actual therapeutic potential of these compounds remains to be translated for human use due to lack of knowledge of their complex mode of absorption, biotransformation, and bioavailability. Although several in vitro studies have yielded excellent results using polyphenols from plants, more detailed investigations are still required to extrapolate these results to in vivo conditions. In this minireview, antioxidant and anti-inflammatory properties of some of the dietary polyphenols are briefly described in the context of nuclear chromatin remodeling.
Curcumin
Curcumin (diferuloylmethane, a principal and active component of turmeric) is a yellow-colored polyphenolic pigment obtained from the rhizome of Curcuma longa Linn (Family-Zingiberaceae) and a member of the curcuminoid family of compounds. Several of its pharmacological properties and medicinal applications have been reported previously.9 The hydroxyl and methoxy groups of curcumin have been considered to render antioxidant and anticarcinogenic activities, respectively. About 40–85% of the total amount of curcumin ingested remains unaltered in the gastrointestinal tract, it is, however, metabolized in the intestinal mucosa and liver.10 Consumption of up to 10 g curcumin/day have been reported to be devoid of any direct toxicity in humans11 and its bioavailability has been found to be increased 20-fold when consumed along with piperin (an active ingredient of pepper).12 A recent surge in research on oxidative stress-related diseases and the possibility that antioxidants may help control such diseases (particularly in susceptible populations) have triggered a remarkable increment in scientific investigations and knowledge regarding the antioxidant and anti-inflammatory roles of dietary polyphenols.
Antioxidant properties of curcumin
Free radicals (ROS and reactive nitrogen species) such as superoxide anion (O2–), hydrogen peroxide (H2O2), and nitric oxide (NO) have now been reported to be scavenged by curcumin (in the micro to millimolar range) both in vitro and in vivo.9 Findings from our own laboratory indicate that curcumin (1–50 μM) could scavenge ROS in 1–4 hours, as determined by electron paramagnetic resonance spectroscopy.13 Curcumin was found to be much faster in terms of quenching ROS when compared to other polyphenols tested (resveratrol and quercetin). More recently, curcumin has been demonstrated to induce antioxidant defenses through increases in glutathione production via Nrf2 activation and induction of glutamate cysteine ligase transcription. Similarly, expression of phase II enzymes such as glutathione-S-transferase is also induced by curcumin. The antioxidant properties of curcumin are evident from its ability to lower lipid peroxidation and maintain the activity status of various antioxidant enzymes. Since ROS have been implicated in the pathogenesis of various chronic and inflammatory conditions, curcumin therefore has the potential to control these diseases through its potent antioxidant activity.
Anti-inflammatory property of curcumin: its role in chromatin remodeling
Curcumin has been reported to have both anti-cancer and anti-inflammatory properties and inhibit a wide range of inflammatory and signaling molecules.9 Recent studies have reported that curcumin inhibits NF-κB expression/activation, IL-8 release, and neutrophil recruitment in lung cells.13 Curcumin inhibits NF-κB transactivation by inhibiting the nuclear translocation of the p65 subunit of NF-κB, in association with the sequential suppression of IκB kinase phosphorylation, IκB-α degradation, p65 phosphorylation, and p65 acetylation9,13 (Figure 1). Since NF-κB regulates expression of a wide variety of genes that are intimately involved in the process of inflammation, inhibition of NF-κB by curcumin may be an interesting prospect for controlling chronic inflammatory diseases involving the NF-κB signaling pathway. In addition to the suppression of proinflammatory genes, curcumin also downregulates the expression of iNOS, MMP-9, TNF-α, chemokines, cell surface adhesion molecules, and growth factor receptors (such as epidermal growth factor receptor). In addition, curcumin modulates a number of key kinase signaling pathways such as mitogen-activated protein kinases (MAPK) and protein kinase C (PKC) in a wide variety of different cell types. The pleiotropic nature of curcumin in targeting so many cell signaling pathways complicates the process of identifying which pathway is essential for the anti-inflammatory effects. On the other hand, it may be that the ability to prevent cross talk between the myriad signaling pathways is a prerequisite for its anti-inflammatory properties.
Figure 1. Impact of oxidative stress and dietary polyphenols on the regulation of chromatin modifications and proinflammatory gene expression.
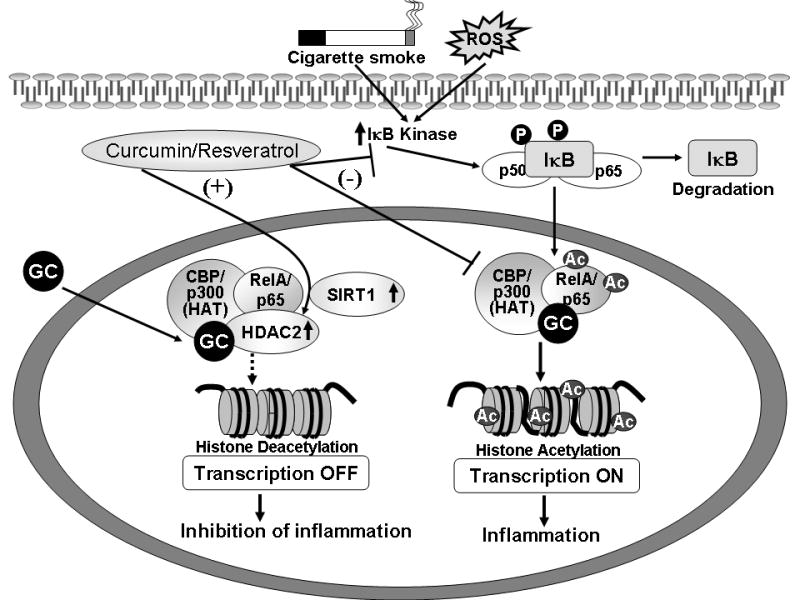
Oxidants and proinflammatory cytokines activate transcription factors, such as NF-κB, by recruitment of transcriptional coactivator molecules CBP/p300, which possess intrinsic histone acetylase (HAT) activity, resulting in histone acetylation and DNA unwinding, allowing DNA polymerases access to the DNA leading to proinflammatory gene expression. Direct interaction between coactivators (such as HAT), histone deacetylase, and the glucocorticoid receptor (GR) may result in repression of expression of proinflammatory genes. Histone deacetylase type II (HDAC2) forms a bridge with HAT and RelA/p65 to inhibit gene transcription. However, when the HDAC2 (by post-translational modification/disruption) is inhibited by cigarette smoke/oxidants and/or the NF-κB subunit RelA/p65 is acetylated, steroids may not be able to recruit HDAC2 into the transrepressor complex to inhibit proinflammatory gene expression. Dietary polyphenols, such as curcumin and resveratrol inhibit NF-κB activation, CBP-HAT activity, and restore glucocorticoid efficacy by upregulating HDAC2 and sirutin activity, culminating in inhibition of proinflammatory gene expression.
We recently observed that curcumin can inhibit inflammation and restore glucocorticoid efficacy (which is lost under oxidative stress) through upregulation/restoration of HDAC2 activity in monocytes/macrophages (U937 and MonoMac6 cells). Curcumin restored both HDAC2 activity and corticosteroid resistance in a concentration-dependent manner with an EC50 of 15 nM and 200–300 nM, respectively, in the monocytes and macrophages. This restoration facilitated steroid-mediated HDAC2 recruitment in the co-repressor complex attenuating NF-κB-mediated chromatin acetylation and subsequent proinflammatory gene expression. Interestingly, it has recently been suggested that the anti-inflammatory actions of curcumin at 50 μM are propagated through inhibition of HAT activity, preventing NF-κB-mediated chromatin acetylation.14 Hence, it might be reasonable to propose that in addition to its role as an antioxidant/anti-inflammatory agent, curcumin may also assist in increasing the efficacy of steroids via modulation of HDAC and HAT activity. Clearly, clinical trials using a combination approach of a steroid with curcumin are warranted.
Resveratrol
Resveratrol (3, 4′-5- trihydroxystilbene), which is a phytoalexin found in the skin and seeds of grapes and produced by some spermatophytes, such as grapevines, in response to injury or fungal attack, has been reported to possess antioxidant, anti-inflammatory, and anticarcinogenic properties.15 Studies have shown that resveratrol is more effective in inhibiting the oxidative damage than the conventional antioxidants and has also been shown to scavenge free radicals such as lipid hydroperoxyl, hydroxyl, and superoxide anion radicals.16 Our data show that resveratrol is a potent ROS scavenger and exerts antioxidant properties through modulation of glutathione biosynthesis via Nrf2 antioxidant response element signaling.
Anti-inflammatory property of resveratrol
Resveratrol is an effective inhibitor of inflammatory cytokine release from macrophages in patients with chronic obstructive pulmonary disease.17 This anti-inflammatory property of resveratrol may be due to its ability to induce sirtuins and HDAC activity. A recent in vivo study has shown that resveratrol inhibits inflammatory cytokine expression in response to lipopolysaccharide in rat lungs.18 Furthermore, in both monocytic U937 cells and alveolar epithelial A549 cells, resveratrol inhibited NF-κB and AP-1 activation.19 The mechanism through which this occurs is still unclear. It is possible that resveratrol could induce histone deacetylase activity via SIRT1 (sirtuin1) and inhibit proinflammatory genes.20
Conclusions
Dietary polyphenols such as curcumin and resveratrol have been shown to act both as an antioxidant as well as an anti-inflammatory agent. These polyphenols inhibit oxidant-induced NF-κB activation, histone acetylation, and proinflammatory cytokine release, and they restore glucocorticoid functions via a mechanism involving upregulation of HDAC2/sirtuin activity. Thus, regulation of inflammatory response by dietary polyphenols and restoration of glucocorticoid efficacy at the molecular level are possible ways forward to design therapeutic strategies for the treatment of chronic inflammatory diseases, particularly in susceptible populations. However, future studies on the bioavailability, absorption, tissue distribution, and understanding of the in vivo molecular effects of curcumin and resveratrol are needed in order to consider these dietary polyphenols as natural therapy ‘nutraceuticals’ for chronic inflammatory disorders.
Acknowledgments
Funding. NIH R01-HL085613, NIEHS Grant ES-01247, and NIEHS Toxicology Training grant # ES07026.
Abbreviations
- GC
glucocorticoid
- Ac
acetylation
- P
phosphate
- CBP
CREB (cAMP response element binding protein)-binding protein
- RelA/p65
NF-κB subunit
- SIRT1
Sirtuin1
- ROS
reactive oxygen species
- +
inducting effects of polyphenols
- –
inhibitory effects of polyphenols
References
- 1.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung disease. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 2.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 3.Rahman I, Marwick JA, Kirkham PA. Redox modulation of histone acetylation and deacetylation in vitro and in vivo: modulation of NF-κB and pro-inflammatory genes. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Rahman I, Gilmour P, Jimenez LA, Mac Nee W. Oxidative stress and TNF-α induce histone acetylation and AP-1/NF-κB in alveolar epithelial cells: potential mechanism in inflammatory gene transcription. Mol Cell Biochem. 2002;234/235:239–248. [PubMed] [Google Scholar]
- 5.Marwick JA, Giddings J, Butler K, et al. Cigarette smoke induces inflammatory response and alters chromatin remodeling in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 6.Moodie F, Marwick JA, Anderson C, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and pro-inflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1909. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 7.Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Lim G, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15:1110–1112. [PubMed] [Google Scholar]
- 9.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 10.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 12.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 13.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits oxidant- and TNF-α-mediated NF-κB activation and chromatin remodeling in alveolar epithelial cells. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem Pharmacol. 2005;69:1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 16.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;14:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 17.Culpitt SV, Rogers DF, Fenwick PS, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-KappaB-independent mechanism. FASEB J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 20.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]


