Abstract
Lens regeneration in newts is a remarkable process, whereby a lost tissue is replaced by transdifferentiation of adult tissues that only a few organisms possess. In this review, we will touch upon the approaches being used to study this phenomenon, recent advances in the field of lens regeneration, similarities and differences between development and regeneration, as well as the potential role stem cells may play in understanding this process.
Keywords: eye, lens, regeneration, transdifferentiation, development
Introduction
The ability of some organisms to regenerate body parts is one that has both fascinated and plagued scientists for hundreds of years. All animals exhibit some level of regenerative ability with some able to regenerate entire body parts and organs. Regeneration of body parts in invertebrates and amphibia has been documented for over two hundred years. In 1712 Reaumur, perhaps the father of regeneration, noted the talents of crayfish in their ability to regenerate a new limb upon amputation (Dinsmore, 1991). Another major contributor was Trembley and his elegant studies on Hydra in the 1740s (Dinsmore, 1991). By far the most impressive organisms in regenerative abilities are the amphibians. Many amphibians are capable of regenerating limbs, tails, nervous tissue, muscle, and in certain species lens and retina. Colucci (1891) and Wolff (1895) first observed, independently, lens regeneration in the adult newt which eventually resulted in the term Wolffian regeneration. This review will focus specifically on newt lens regeneration; why it is advantageous to use as a model for examining regeneration, how it occurs, what approaches have been taken in studying it, and what progress has been made in inducing lens regeneration. We will also touch upon stem cells and what help they might possibly provide in solving the regeneration mystery.
Why Study the lens?
As discussed above the study of regeneration has been studied for years using different approaches to the problem. During some types of regeneration, terminally differentiated cells lose their characteristics or dedifferentiate and finally differentiate into another cell type. The term that was adopted to denote this change in cell differentiation, that is a differentiated cell type giving rise to a totally different cell type, is transdifferentiation (Selman and Kafatos, 1974). Depending on the type of regeneration being studied the number of cell types that needs to be replaced can vary from many cell types to just one. What is even more fascinating is that in some cases of complex regeneration, as in the limb and lens, an exact replica is formed via the mechanism of pattern formation. For instance in the process of limb regeneration, all of the tissues of the stump (bone, muscle, cartilage) undergo dedifferentiation. This is followed by the formation of the blastema, which is a region of dedifferentiated mesenchymal cells, and subsequently proliferation and redifferentiation of the lost limb (Tsonis, 1996; Tsonis,2000). In contrast to limb regeneration, retina regeneration via transdifferentiation, as seen in chicks and amphibia, occurs through the transdifferentiation of a single cell type. That is the retinal pigment epithelial cells undergo dedifferentiation and redifferentiation to all of the neural retina cell types. In chicks, some neural retina must be left behind or the eye must be treated with fibroblast growth factor (FGF) (Park and Hollenberg, 1989). So far we have briefly examined two cases of regeneration in which one or more cell types transdifferentiate to form multiple cell types. In order to delineate the mechanism of transdifferentiation, we and others examine the process of lens regeneration in the newt. There are several advantages for using the lens as a model system for studying transdifferentiation. First, the process of lens regeneration occurs via the transdifferentiation of one cell type (iris pigmented epithelial cells) to another cell type (lens cells). Secondly, as will be discussed in more detail below, lens regeneration occurs from only the pigmented epithelial cells (PECs) of the dorsal iris and not the ventral. Therefore understanding this restriction could help determine why lens regeneration is not possible in other animals. Transdifferentiation of PECs has been studied both in vivo and in vitro (Eguchi et al, 1974; Mizuno et al, 1999; Tsonis and Del Rio-Tsonis, 2004). While knowledge has been gained through these studies the exact mechanism of transdifferentiation still eludes scientists today. Today the number of cell types being reported as being capable of transdifferentiation is ever increasing making it ever more important to delineate the mechanism of transdifferentiation. To help in understanding the mechanism of the transdifferentiation/regeneration process the rest of this review will focus on the lens.
Process of Lens Regeneration
Histological, cellular, and molecular events all take place during the complex process of regeneration. Soon after removal of the lens, the PECs of the dorsal iris dedifferentiate, that is they lose cellular characteristics such as pigmentation that define their cell type. Macrophages recruited to the area help mediate this process. Dedifferentiation also marks the initiation of cell cycle re-entry, which is paramount to proliferation. The first peak of proliferation is observed at about four days post lentectomy. At approximately 10 days post-lentectomy, the depigmented cells are visible as a vesicle, containing both an inner and outer layer, at the tip of the dorsal iris (Eguchi, 1963; Tsonis, 2000; Tsonis and Del Rio-Tsonis, 2004) (Figure 1A). The inner layer of the vesicle begins to thicken at 12-16 days post lentectomy as the cells elongate and differentiate into primary lens fiber cells (Figure 1B). It is also at this time point that the second peak of proliferation is observed and the synthesis of crystallins begins. As regeneration ensues primary lens fibers continue to form in the inner layer, while non-dividing secondary fibers start to form in the external layer of the lens vesicle (day 15-19). At 18-20 days post lentectomy crystallins are continually synthesized (Figure 1C). A complete lens is formed with a layer of lens epithelial cells on the anterior surface and lens fibers cells on the interior of the lens 25 days after lens removal (Eguchi 1964, Yamada 1977).
Figure 1.
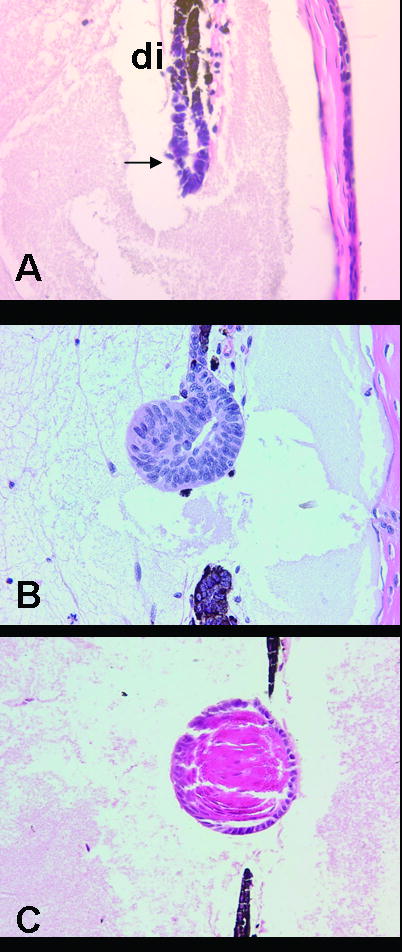
Lens regeneration in the newt stemming from the PECs of the dorsal iris (di). A. 10 days post-lentectomy. Note the formation of a lens vesicle (arrow). B. 15 days post-lentectomy. Cells are elongating into lens fibers. C. 20 days post-lentectomy. The lens is well differentiated with lens fibers.
Classical Approaches
The observations of Colucci and Wolff led to a new era of studies on lens regeneration. These classical studies, a series of experimental approaches including transplantations attempted in order to understand exactly which cells were involved in lens regeneration, are some of the most fascinating in regenerative science. Many questions were answered by these studies, however, many more remained. The most basic experiment showed that when grafts of dorsal iris were placed into lentectomized eyes they gave rise to a lens (Wachs, 1914; Sato, 1930, 1935; Mikami, 1941). When dorsal irises were placed into lentectomized eyes from non-regenerating salamanders they were also able to form a lens (Ikeda, 1934; Amano and Sato, 1940; Reyer, 1956). Conversely, regeneration competent dorsal irises that were implanted into the body cavity or subcutaneously in both lens regenerating and non lens regenerating animals did not form a lens (Ikeda, 1935, 1936; Stone, 1958a; Reyer, 1953, 1954). This suggested that perhaps something else was necessary for lens regeneration to take place. Studies went on to show that the neural retina was sufficient to rescue regeneration of the lens in the previous attempts (Stone, 1958a). It was further shown that by separating the neural retina from the iris, lens regeneration could be prevented (Stone, 1958b). Obviously, one might conclude that there is a certain factor that is provided by the retina that plays a role in lens regeneration. Speculation on the identity of this factor includes FGFs and FGFRs because of their known roles in lens development and polarity (see below). Several classical experiments have shown that the lens itself might provide a factor that is involved. If the lens is removed from lens regeneration competent animals and then replaced near the dorsal iris no regeneration occurs or it is minimal depending on the distance from the dorsal iris (Eguchi, 1961). The same lack of regeneration is seen from a dorsal iris explant that is placed in the anterior part of the eye and the lens is either left in or removed and then replaced (Reyer, 1961). These classical approaches led to a greater understanding of the parameters of lens regeneration and helped pave the way to the molecular era with many questions looming, mainly that of the actual molecular mechanism.
Modern Approach
In order to understand the molecular aspects of lens regeneration, many scientists begin their quest by examining molecules which are known to play a role in vertebrate lens development. Do the same factors that play a role in developing body parts also play a role in regeneration? In other words, does regeneration recapitulate development? It is in this mode of thinking that some of the strongest data has been generated. To fully understand this modern approach, we will first briefly explain the process of lens development.
Vertebrate lens development is brought about by a series of inductive interactions in the embryo which leads to the initiation of differentiation of the head ectoderm (Coulombre and Coulombre 1963). Three primordial tissues (ectoderm from the neural tube, surface ectoderm, and mesoderm) are required for formation of the vertebrate eye. The lens as well as the corneal epithelium is derived from the surface ectoderm. In most vertebrates, lens development is initiated by proliferation of the ectodermal cells overlying the optic vesicle, which forms the lens placode. The placode then invaginates along with the optic vesicle to form the lens pit and optic cup, respectively (Figure 2A). The lens pit deepens and is eventually closed off to form a lens vesicle. The lens vesicle soon separates from the overlying ectoderm forming the lens.
Figure 2.
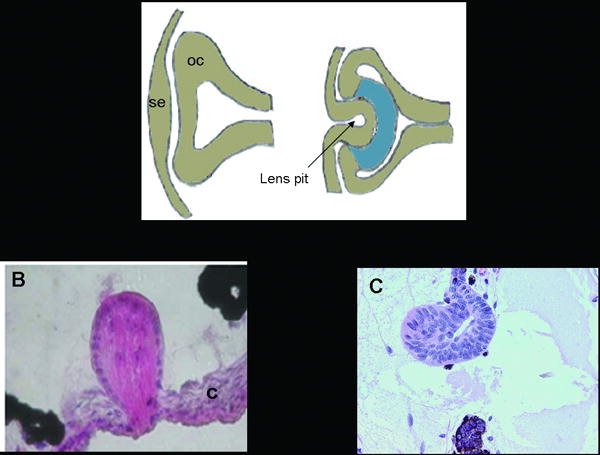
Comparison of lens development with two methods of lens regeneration. A. Schematic showing lens development, which involves a series of inductive interactions between the surface ectoderm and the optic cup. The lens pit eventually gives rise to the lens. se: surface ectoderm, oc: optic cup. B. Lens regeneration in Xenopus laevis. The regenerated lens comes from transdifferentiation of cells in the outer cornea (c). C. Lens regeneration in the newt. The regenerated lens comes from transdifferentiation of cells in the dorsal iris.
During lens regeneration events of lens differentiation are initiated after the formation of the lens vesicle. At this point, the process of lens regeneration and development in the newt are very similar in terms of differentiation and even that of crystallin synthesis. β-crystallin and γ-crystallin proteins are the first to be detected (McDevitt and Brahma, 1982) in the lens vesicle followed by α-crystallin (Takata et al, 1964). It is not until later stages that crystallins are detected in the lens epithelial cells (Takata et al, 1966). The similarities in crystallin gene expression between regeneration and development have been shown via in situ hybridization as well (Mizuno et al., 2002). Another similarity between lens regeneration and lens development is seen in the expression pattern of two important eye genes, Pax-6 and Prox 1 (Mizuno et al., 1999). Pax-6 was expressed soon after lentectomy in a broad region that includes both the dorsal and ventral iris. As regeneration continues, Pax-6 expression becomes restricted to the dorsal iris. Eventually Prox 1 becomes expressed within the Pax-6 expressing tissue. This sequential expression of Pax-6 and Prox 1 is also seen in the lens placode during lens development indicating that there may be a common genetic program to both development and regeneration (Mizuno et al, 1999). Due to the apparent similarities between lens development and regeneration it is quite clear to see why scientists have engaged in the modern approach to this problem.
Retinoids
Retinoids and their receptors play major roles in both lens development and regeneration. Retinoic acid is thought to play a role in inducing the inductive signal during lens development. Retinoic acid deficiency in mouse embryos leads to a failure to form the lens placode and optic vesicle invagination, which leads to a small eye phenotype (Bavik et al, 1996; Grindley et al, 1995). Retinoic acid receptors play a role in transcriptional control of αB and E crystallins (Gopal-Srivastava et al, 1998) during development. It also appears that retinoic acid and its other analogs regulate gene expression in lens cells and plays an important role in maintaining the epithelial layer (Lovicu and Robinson, 2004). Retinoic acid has also been shown to control the fate of neural retinal cells during development (McCaffrey et al, 1993; Wagner et al, 2000). In addition to these roles in development, exogenous retinoic has led to the formation of ectopic lens differentiation (Manns and Fritzsch, 1991).
Retinoic acid and retinoic acid receptors not only play a role in development but they also play a crucial role in newt lens regeneration. Treatment of newts, following removal of the lens, with an antagonist to the retinoic acid receptors or with disulfiram (a chemical which inhibits the synthesis of retinoic acid) severely retards the regenerative capability of the dorsal iris. While inhibition was the most prevalent outcome there were also a few cases of ectopic lenses being formed (Tsonis et al, 2000; Tsonis et al, 2002).
Fibroblast Growth Factors
Fibroblast growth factors (FGFs) and their receptors are critical for lens development. As was the case for retinoic acid, FGFs play dominant roles in controlling crystallin gene expression and regulating the spatial and temporal pattern of expression (deIongh et al., 1997; Lang, 1999). In addition, they also play a role in lens fiber differentiation and maintenance. In chicks, FGF-8 expression in the distal optic vesicle leads to the expansion of the lens field (Vogel-Höpker et al., 2000). Targeted overexpression of FGFs in transgenic mice leads to inappropriate differentiation of the lens epithelium (Robinson et al., 1995, 1998; Lovicu and Overbeek, 1998).
In regeneration, several FGFs and their receptors are expressed but only FGFR-1 was present in the dorsal iris during dedifferentiation (Del Rio-Tsonis et al., 1997; Del Rio-Tsonis et al., 1998; McDevitt et al., 1997). Further examination showed that FGFR-1 plays a role in regulating lens differentiation. This was shown by inhibiting the function of FGFR-1, which in turn led to inhibition of lens regeneration and lens fiber differentiation (Del Rio-Tsonis et al., 1998).
Homeobox-Containing Genes
Pax6 has long been known to be one of the most important determinants for eye formation. Mutations in Pax-6 cause aniridia in humans and the “small eye” phenotype in mice and rats. In mice it is expressed in the head ectoderm early in development and at later stages (E10.0) is expressed in the lens vesicle and optic cup. At E13.5 Pax-6 is expressed in the proliferating anterior epithelial cells, but is not detected in the lens fiber cells beyond this stage (Koroma et al., 1997). Another homoedomain protein important in lens development is Prox-1. Prox-1 is first detected at E9.5 in the lens placode with subsequent expression in the lens vesicle, anterior epithelium and lens fibers (Oliver et al., 1993; Tomarev et al, 1996; Glasgow and Tomarev, 1998). A Prox-1 mutation in mice leads to death due to lymphatic vessel development. These mutant mice also show a defect in lens differentiation due to a defect in fiber cell elongation, which is in turn due to the absence of cyrstallin (Wigle et al., 1999). In fact both Pax-6 and Prox-1, regulate crystallin expression (Cvekl and Piatigorsky, 1996; Tomarev et al., 1996).
During newt lens regeneration Pax-6 and Prox-1 are expressed in a pattern which is very similar to that seen in development. Following lentectomy, Pax-6 is expressed in both the dorsal and ventral PECs. However, once dedifferentiation is apparent the expression of Pax-6 is localized to the dorsal iris PECs and subsequently becomes restricted to the lens epithelium of the regenerating lens (Del Rio-Tsonis et al., 1995; Mizuno et al., 1999). Prox-1 is expressed specifically in the dorsal iris during regeneration and not in the regeneration incompetent ventral iris (Del Rio-Tsonis et al., 1999).
Stem Cells
The most recent approach to regeneration is seen in stem cell research. In the past few years studies have shown that stem cells, those reserved and used for repair, may play more of a role in regeneration than originally thought. The ability to take stem cells and coax them into differentiating into the tissue of choice is very alluring to those in the regenerative medicine field. In this case the stem cells involved are local (i.e, located in the brain and involved in nervous tissue repair) or non-local (i.e., hematopoietic and involved in repair of several tissues such as liver, nervous, or cardiac) multipotent cells which differ from the urodele repair strategy in that they do not undergo dedifferentiation. It has been hypothesized that there may also be similarities between stem cells and transdifferentiating cells (Tsonis, 2000; Tsonis and Del Rio-Tsonis, 2004). For instance, mesenchymal stem cells located in the bone marrow can differentiate to any number of cells (chondrocytes, myocytes, osteoblasts, or adipocytes) much like that of the cells of the blastema. It has been shown that mammalian myotubes can transdifferentiate through the generation of progenitor cells (Chen et al, 2004). In doing this study they screened over 50,000 discrete small molecules and found a compound that reversed a terminally differentiated cell into progenitor cells, which were then able to differentiate to osteocytes or adipocytes (Chen et al, 2004; Tsonis, 2004). These findings are of great importance in the regeneration field. If these compounds can induce dedifferentiation in multiple cell types then it begs the question of there being a common signal for dedifferentiation. One of the more popular questions these days in the regeneration field asks if cells capable of regeneration such as those in the limb that form the blastema actually revert back to a “younger” state that resembles developing cells or if these cells have a unique quality in which they are multipotent but do not resemble their “younger” selves. Sustar and Schubiger tackled this question using Drosophila imaginal disc cells and found that in fact, the cells in the imaginal discs that are capable of transdetermination do not revert back to their “younger” selves, but instead convert into a unique cell type (Sustar and Schubinger, 2005). These findings will have a large impact on the mechanisms of the two strategies of regeneration, that of the urodeles and transdifferentiation and that of recruitment of stem cells.
Lens Regeneration in Other Vertebrates
Lens regeneration is seen predominantly in amphibians, but most spectacularly in some species of urodeles as this feat can occur in an adult organism (as discussed with the newt). The newt is not, however, the only organism that can regenerate its lens. Anurans such as Xenopus laevis can also regenerate a lens through the process of transdifferentiation. Unlike the newt, which regenerates the lens through transdifferentiation of the dorsal iris pigmented epithelial cells, Xenopus regenerates a lens from the inner layer of the outer cornea (Freeman, 1963; Filoni et al., 1997) (Figure 2B and C). This process appears to be facilitated by a factor secreted from the retina. Following removal of the lens, this factor is no longer hindered from making contact with the outer cornea (Filoni el al., 1982). Besides the type of cell that undergoes transdifferentiation, another difference exists between the newt and Xenopus and that is the stage at which regeneration is possible. As was mentioned earlier, the newt can regenerate a lens throughout its lifespan, which is one of the reasons that scientists use this animal as a model system for studying regeneration. Xenopus can only regenerate during early stages of life. The capacity for regeneration is lost after metamorphosis (Freeman, 1963).
Lens regeneration is not restricted to only amphibians but has been shown to take place in other vertebrates as well. Lens regeneration has been reported to occur from a layer of cells found at the border of the iris near the choroid in the chick embryo (Deth, 1940). These findings, however, are very controversial. The controversy lies in the fact that scientists are not sure if the newly formed lens is a result of transdifferentiation of the cells near the iris or if it is merely an inductive response due to competent ectoderm being left behind after surgery (McKeehan, 1961). The lack of markers to follow the process was a major problem with these studies. Similar to salamanders, some species of adult fish can regenerate a lens through transdifferentiation of cells of the dorsal iris (Sato, 1961; Mitashov, 1966). Other vertebrates, such as mice, rabbits, and cats can also undergo regeneration. Unlike the other organisms examined, these mammals do not regenerate a lens through the process of transdifferentiation. Instead regeneration occurs from lens epithelial cells remaining on the capsular bag following removal of the lens. Without the capsular bag, regeneration in these organisms will not take place (Gwon et al., 1989, 1990; Call et al., 2004). As was illustrated above, regeneration occurs via many different mechanisms and even within transdifferentiation different tissues are utilized (cornea in the Xenopus and dorsal iris in the newt). With this in mind, is there a common strategy used in regeneration or does each organism employ a unique strategy to regeneration? This question is one that stumps researchers and, of course, the hope would be for a more common mechanism shared amongst organisms. There is some evidence (discussed below) that organisms that do not normally regenerate still maintain the ability to do so if the right buttons are pushed. The key to answering this must lie in elucidating the mechanism in regeneration competent animals and then applying those findings to non-competent ones.
Through the use of in vitro cell culture systems, it has been demonstrated that PECs from many organisms can undergo the transdifferentiation process to form lentoids, lens-like structures. Tissues that were once thought to lack regenerative capabilities have been shown to have the potential for regeneration through these culturing systems. Eguchi showed that PECs from the ventral iris of the newt could undergo transdifferentiation to form a lentoid (Eguchi et al, 1974). This potential for transdifferentiation is also seen with retinal PECs of chick embryos (Eguchi and Okada, 1973) as well as in human iris and retinal PECs from adult and fetal eyes (Eguchi, 1988; Tsonis et al., 2001). Knowing that this potential is present in a wide variety of species raises some very interesting questions. Why does transdifferentiation occur in vitro but not in vivo in non-regenerating species? How can we unlock this potential in these species? Perhaps the answers to these questions lie in the cells' extracellular matrix (ECM). The presence of collagen has been shown to inhibit dedifferentiation of PECs (Eguchi, 1979; Yasuda, 1979). In addition, localization of β1 integrin at the focal contact sites in PECs is lost during the process of dedifferentiation and is thought to be the result of phosphorylation. Cultured PECs treated with an antibody against β1 integrin results in morphological changes as well as changes in the pattern of gene expression. These changes are remarkably similar to the changes observed during dedifferentiation of PECs (Mazaki et al., 1996). Integrins, in addition to other ECM molecules, may be necessary for maintaining the differentiated state of PECs which may be inhibited or blocked in vivo. A molecule known as 2NI-36 may be responsible for stabilization of the extracellular matrix (Eguchi, 1988). This glycoprotein has been shown to disappear from the dorsal iris of the newt upon regeneration (Eguchi, 1988; Imokawa and Eguchi, 1992; Imokawa et al., 1992). Understanding the role the ECM plays during lens regeneration may provide the key to unlocking the mechanism of lens regeneration. With this key induction in higher organisms may be one step closer.
Induction of Lens Regeneration
It has been clearly established that in vivo lens regeneration in urodele amphibians comes from the dorsal iris only. One of the most exciting prospects in studying lens regeneration is inducing it from the incompetent ventral iris. Treatment with the potent carcinogen MNNG (Methyl-Nitro-Nitrosoguanidine) has shown that such induction is possible. MNNG was added to regenerating eyes and the result was ectopic lenses from the ventral iris (Eguchi and Watanabe, 1973). This was also done on cultured ventral irises in vitro that were treated with the carcinogen and implanted back into a lentectomized eye. Some of the implants produced a lens in this study as well (Eguchi and Watanabe, 1973). While the mechanism is still unknown, it was suggested in that study that MNNG altered the cell surface properties of the ventral PECs. However, this has not been documented. Nevertheless, these experiments clearly show that induction is possible and remains one of the greatest challenges in the field.
Acknowledgments
This work was supported by NEI grant EY10540 to PAT
Bio Sketches
Dr. Tsonis is a Professor of Molecular Biology and the Leonard A. Mann Chair in the sciences at the University of Dayton, Ohio. He is very interested in many issues of tissue regeneration, the mechanisms of transdifferentiation, and induction of regeneration in mammals. Ms. Call and Mr. Grogg candidates in the laboratory of Dr. Tsonis at the University of Dayton.
References
- Amano U, Sato J. Uber die xenoplastische implantation der larvalen des Triturus pyrrhogaster in das entlinste Auge der Larven des Hynobius nebulosus. Jpn J Med Sci I Anat. 1940;8:75–81. [Google Scholar]
- Bavik C, Ward S, Chambon P. Developmental abnormalities in cultured mouse embryos deprived of retinoic acid by inhibition of yolk-sac retinol binding protein synthesis. Proc Natl Acad Sci USA. 1996;93:3110–3114. doi: 10.1073/pnas.93.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA. Lens regeneration in mice: implications in cataracts. Exp Eye Res. 2004;78:297–299. doi: 10.1016/j.exer.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang Q, Wu X, Schultz PG, Ding S. Dedifferentiation of lineage-committed cells by a small molecule. J Am Chem Soc. 2004;126:410–411. doi: 10.1021/ja037390k. [DOI] [PubMed] [Google Scholar]
- Colucci VL. Sulla rigenereazione parziale dell'occhio nei Tritoni-Istogenesi e sviluppo: Studio sperimentale. Mem R Acad Sci Ist Bologna. 1891;Ser 51:593–629. [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1490. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Piatigorsky J. Lens development and crystalline gene expression: many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- deIongh RU, Lovicu FJ, Chamberlin CG, McAvoy JW. Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest Ophthalmol Vis Sci. 1997;38:1688–1699. [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Washabaugh CH, Tsonis PA. Expression of Pax-6 during urodele eye development and lens regeneration. Proc Natl Acad Sci USA. 1995;92:5092–5096. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Jung JC, Chiu IM, Tsonis PA. Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci USA. 1997;92:5092–5096. doi: 10.1073/pnas.94.25.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Trombley MT, McMahon G, Tsonis PA. Regulation of lens regeneration by fibroblast growth factor receptor 1. Dev Dyn. 1998;213:140–146. doi: 10.1002/(SICI)1097-0177(199809)213:1<140::AID-AJA14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tomarev SI, Tsonis PA. Regulation of Prox-1 during lens regeneration. Invest Ophthalmol Vis Sci. 1999;40:2039–2045. [PubMed] [Google Scholar]
- Van Deth JHMG. Induction et regeneration du cristallin chez l'embryon de la poule. Acta Neerland Morph. 1940;3:151–169. [Google Scholar]
- Dinsmore CH, editor. A history of regeneration research. Cambridge University Press; New York: 1991. [Google Scholar]
- Eguchi G. The inhibitory effect of the injured and displaced lens on the lens-formation in Triturus larvae. Embryologia. 1961;6:13–35. [Google Scholar]
- Eguchi G. Electron microscopic studies on lens regeneration I: Mechanism of depigmentation of the iris. Embryologia. 1963;8:45–62. [Google Scholar]
- Eguchi G. Electron microscopic studies on lens regeneration II: Formation and growth of lens vesicle and differentiation of lens fibers. Embryologia. 1964;8:247–287. [Google Scholar]
- Eguchi G. “Transdifferentiation” in pigmented epithelial cells of the vertebrate eye in vitro. In: Ebert JD, Okada TS, editors. Mechanisms of Cell Change. New York: Wiley; 1979. pp. 273–291. [Google Scholar]
- Eguchi G. Cellular and molecular background of Wolffian lens regeneration. In: Eguchi G, Okada TS, Saxen L, editors. Regulatory Mechanisms in Developmental Process. Amsterdam: Elsevier; 1988. pp. 147–158. [DOI] [PubMed] [Google Scholar]
- Eguchi G. Transdifferentiation as the basis of eye lens regeneration. In: Ferreti P, Geraudie J, editors. Cellular and Molecular Basis of Regeneration: From Invertebrates to Humans. Chichester, England: Wiley; 1998. pp. 207–228. [Google Scholar]
- Eguchi G, Okada TS. Differentiation of lens tissue from the progeny of chick retinal pigment cell cultured in vitro: A demonstration of a switch of cell types in clonal cell culture. Proc Natl Acad Sci USA. 1973;70:1495–1499. doi: 10.1073/pnas.70.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi G, Watanabe K. Elicitation of lens formation from the ventral iris epithelium of the newt by a carcinogen, MNNG. J Embryol Exp Morphol. 1973;30:63–71. [PubMed] [Google Scholar]
- Eguchi G, Abe SI, Watanabe K. Differentiation of lens-like structures from the newt iris epithelial cells in vitro. Proc Natl Acad Sci USA. 1974;71:5052–5056. doi: 10.1073/pnas.71.12.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C. The role of neural retina in lens regeneration from cornea in larval Xenopus laevis. Acta Embryol Morphol Exp. 1982;3:15–28. [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, D'Alessio A. Lens regeneration in larval Xenopus laevis: experimental analysis of the decline in the regenerative capacity during development. Dev Biol. 1997;187:13–24. doi: 10.1006/dbio.1997.8598. [DOI] [PubMed] [Google Scholar]
- Freeman G. Lens regeneration from the cornea in Xenopus laevis. J Exp Zool. 1963;154:39–66. doi: 10.1002/jez.1401540105. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Tomarev SI. Restricted expression of the homeobox gene prox1 in developing zebrafish. Mech Dev. 1998;76:175–178. doi: 10.1016/s0925-4773(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorksy J. Involvement of retinoic acid/retinoid receptors in the regulation of murine αB-crystallin/small heat shock protein gene expression in the lens. J Biol Chem. 1998;273:17954–61. doi: 10.1074/jbc.273.28.17954. [DOI] [PubMed] [Google Scholar]
- Gwon A, Enomoto H, Horowitz J, Garner MH. Induction of de novo synthesis of crystalline lenses in aphakic rabbits. Exp Eye Res. 1989;49:913–926. doi: 10.1016/s0014-4835(89)80016-8. [DOI] [PubMed] [Google Scholar]
- Gwon AE, Gruber LJ, Mundwiler KE. A histologic study of lens regeneration in aphakic rabbits. Invest Ophthalmol Vis Sci. 1990;31:540–547. [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Ikeda Y. Beitrage zur Analyse der Wolffschen Linsenregerneration durch xenoplastische implantation der iris is das entlinste auge bei Triton und Hynobius. Arb Anat Inst Sendai. 1934;16:69–82. [Google Scholar]
- Ikeda Y. Uber die regeneration von Augenbechern an verschiedenen Korperstellen durch isolierte Irisstucke. Arb Anat Inst Sendai. 1935;17:11–54. [Google Scholar]
- Ikeda Y. Neue versuche zur analyse deer Wolffschen Linsenregeneration. Arb Anat Inst Sendai. 1936;18:1–16. [Google Scholar]
- Imokawa Y, Eguchi G. Expression and distribution of regeneration responsive molecule during normal development of the newt, Cynops pyrrhogaster. Int J Dev Biol. 1992;36:407–412. [PubMed] [Google Scholar]
- Imokawa Y, Ono S, Takeuchi T, Eguchi G. Analysis of a unique molecule responsible for regeneration and stabilization of differentiated state of tissue cells. Int J Dev Biol. 1992;36:399–405. [PubMed] [Google Scholar]
- Koroma BM, Yang JM, Sundin OH. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- Lang RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest Ophthalmol Vis Sci. 1999;40:3075–3078. [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiationin transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Robinson ML. Development of the ocular lens. Cambridge University Press; 2004. [Google Scholar]
- Manns M, Fritzsch B. The eye in the brain: retinoic acid effects morphogenesis of the eye and pathway selection of axons but not differentiation of the retina in Xenopus laevis. Neurosci Lett. 1991;127:150–154. doi: 10.1016/0304-3940(91)90782-o. [DOI] [PubMed] [Google Scholar]
- Mazaki Y, Mochii M, Kodama R, Eguchi G. Role of integrins in differentiation of chick retinal pigmented epithelial cells in vitro. Dev Growth Differ. 1996;38:429–437. doi: 10.1046/j.1440-169X.1996.t01-3-00011.x. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Posch KC, Napoli JL, Gudas L, Drager UC. Changing patterns of the retinoic acid system in the developing retina. Dev Biol. 1993;158:390–399. doi: 10.1006/dbio.1993.1197. [DOI] [PubMed] [Google Scholar]
- McDevitt DS, Brahma SK. Alpha-, beta-, and gamma-crystallins in the regenerating lens of Notophthalmus viridescens. Exp Eye Res. 1982;34:587–594. doi: 10.1016/0014-4835(82)90032-x. [DOI] [PubMed] [Google Scholar]
- McDevitt DS, Brahma SK, Courtois Y, Jeanny JC. Fibroblast growth factor receptors and regeneration of the eye lens. Dev Dyn. 1997;208:220–226. doi: 10.1002/(SICI)1097-0177(199702)208:2<220::AID-AJA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McKeehan MS. The capacity for lens regeneration in the chick embryo. Anat Rec. 1961:227–230. [Google Scholar]
- Mikami Y. Experimental analysis of the Wolffian lens-regeneration in the adult newt, Triturus pyrrhogaster. Jpn J Zool. 1941;9:269–302. [Google Scholar]
- Mitahov VI. Comparative study of lens regeneration in Cobitid fishes [in Russian] Dokl Akad Nauk SSSR. 1966;170:1439–1442. [PubMed] [Google Scholar]
- Mizuno N, Mochii M, Yamamoto TS, Takahashi TC, Eguchi G, Okada TS. Pax-6 and Prox 1 expression during lens regeneration from Cynops iris and Xenopus cornea: evidence for a genetic program common to embryonic lens development. Differentiation. 1999;65:141–149. doi: 10.1046/j.1432-0436.1999.6530141.x. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Agata K, Sawada K, Mochii M, Eguchi G. Expression of crystalline genes in embryonic and regenerating newt lenses. Dev Growth Diff. 2002;44:251–256. doi: 10.1046/j.1440-169x.2002.00639.x. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenber MJ. Basic fibroblast growth factor induces retina regeneration in vivo. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Reyer RW. Lens regeneration from heterolastic iris grafts between Triturus viridescens and Amblystoma punctatum. Anat Rec. 1953;115:362–363. [Google Scholar]
- Reyer RW. Regeneration of the lens in the amphibian eye. Q Rev Biol. 1954;29:1–46. doi: 10.1086/399936. [DOI] [PubMed] [Google Scholar]
- Reyer RW. Lens regeneration from homoplastic and heteroplastic implants of dorsal iris into the eye chamber of Triturus viridescens and Amblystoma punctatum. J Exp Zool. 1956;133:145–190. [Google Scholar]
- Reyer RW. Lens regeneration from intra-ocular, iris implants in the presence of the host lens. Anat Rec. 1961;139:267. [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/Int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- Sato T. Beitrage zur Analyse der Wolffschen Linssenregeneration. Pt. I. Wilhem Roux Arch Entwickl-Mech Org. 1930;122:451–493. doi: 10.1007/BF00573589. [DOI] [PubMed] [Google Scholar]
- Sato T. Beitrage zur Analyse der Wolffschen Linssenregeneration. Pt. 3. Wilhem Roux Arch Entwickl-Mech Org. 1935;133:323–348. doi: 10.1007/BF00596524. [DOI] [PubMed] [Google Scholar]
- Sato T. Uber die linsen-regeneration bei den Cobitiden Fischen Misgurnus Anguillicaudatus. Embryologia. 1961;6:251–291. [Google Scholar]
- Selman K, Kafatos FC. Transdifferentiation in the labial gland of silk moth: Is DNA synthesis required for cellular metamorphosis? Cell Differ. 1974;3:81–94. doi: 10.1016/0045-6039(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Stone LS. Lens regeneration in adult newt eyes related to retina pigment cells and the neural retina factor. J Exp Zool. 1958a;139:69–84. doi: 10.1002/jez.1401390106. [DOI] [PubMed] [Google Scholar]
- Stone LS. Inhibition of lens regeneration in newt eyes by isolating the dorsal iris from the neural retina. Anat Rec. 1958b;131:151–172. doi: 10.1002/ar.1091310203. [DOI] [PubMed] [Google Scholar]
- Sustar A, Schubiger G. A transient cell cycle shift in Drosophila imaginal disc cells precedes multipotency. Cell. 2005;120:383–393. doi: 10.1016/j.cell.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Takata C, Albright JF, Yamada T. Lens antigens in the lens-regenerating system studied by immunofluorescent technique. Dev Biol. 1964;9:385–397. doi: 10.1016/0012-1606(64)90032-6. [DOI] [PubMed] [Google Scholar]
- Takata C, Albright JF, Yamada T. Gamma-crystallins in Wolffian lens regeneration demonstrated by immunofluorescence. Dev Biol. 1966;14:382–400. [Google Scholar]
- Tomarev SI, Sundin O, Banerjeebasu S, Duncan MK, Yang JM, Piatigorsky J. Chicken homeobox gene Prox1 related to Drosophila prospero is expressed in the developing lens and retina. Dev Dyn. 1996;206:354–367. doi: 10.1002/(SICI)1097-0177(199608)206:4<354::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Limb Regeneration. Cambridge: Cambridge Univeristy Press; 1996. [Google Scholar]
- Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Stem cells from differentiated cells. Mol Interv. 2004;4:81–83. doi: 10.1124/mi.4.2.4. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Jang W, Del Rio-Tsonis K, Eguchi G. A human pigment epithelium cell line as a model for lens transdifferentiation. Int J Dev Biol. 2001;45:753–758. [PubMed] [Google Scholar]
- Tsonis PA, Tsavaris M, Call MK, Chandraratna RAS, Del Rio-Tsonis K. Expression and role of retinoic acid receptor alpha in lens regeneration. Dev Growth Diff. 2002;44:391–394. doi: 10.1046/j.1440-169x.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: transdifferentiation, stem cells and clinical applications. Exp Eye Res. 2004;78:161–172. doi: 10.1016/j.exer.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Vogel-Höpker A, Momose T, Rohrer H, Yasuda K, Ishihara L, Rapaport DH. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mech Dev. 2000;94:25–36. doi: 10.1016/s0925-4773(00)00320-8. [DOI] [PubMed] [Google Scholar]
- Wachs H. Neue versuche sur Wolffschen linsenregeneration. Wilhelm Roux Arch Entwickl-Mech Org. 1914;39:384–451. [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wolff G. Entwicklungsphysiologische Studien. I. die Regeneration der Urodelenlinse. Wilhelm Roux Arch Entwickl-Mech Org. 1895;1:380–390. [Google Scholar]
- Yamada T. Monographs in Dev Biol. Vol. 13. Krager; Basel: 1977. Control mechanisms in cell-type conversion in newt lens regeneration. [PubMed] [Google Scholar]
- Yasuda K. Transdifferentiation of “lentoid” structures in cultures derived from pigmented epithelium was inhibited by collagen. Dev Biol. 1979;68:618–623. doi: 10.1016/0012-1606(79)90231-8. [DOI] [PubMed] [Google Scholar]


