Summary
We studied major histocompatibility complex (MHC) class I expression in 12 tumor cell culture lines established from patients with metastatic renal cell carcinoma (RCC). In one of these cell culture lines, UOK 123, we found no surface expression of β2-microglobulin (β2m) and MHC class I by flow cytometry. Immunofluorescence staining using three different monoclonal antibodies to β2m revealed no detectable β2m in the endoplasmic reticulum (ER), Golgi apparatus, cytoplasm, or on the cell surface. There was no evidence of folded class I molecules inside or on the surface of the cells; however, the ER stained intensively for unfolded class I molecules. Transient expression of β2m by UOK 123 after infection with a recombinant vaccinia virus containing the gene for β2m resulted in normal expression of both β2m and class I (HLA-A, B, C) determinants assessed by flow cytometry analysis. No expression of class I or β2m was seen with the recombinant vaccinia vector carrying a control gene. The inability of class I molecules to reach the cell surface is due to the requirement of β2m for proper folding and presentation of the class I MHC complex. The failure to assemble and express MHC class I complex on the cell surface renders these cells incapable of antigen presentation to cyto-toxic T cells and provides a mechanism for escape from immune recognition by the tumor.
Keywords: MHC class I, Renal cell carcinoma, β2-microglobulin
The development of malignant tumors may not depend only on neoplastic transformation but also on the failure of host resistance to eliminate aberrant cells. Because neoplastic cells may express abnormal surface antigens, they may be recognized as foreign and eliminated by immunosurveillance by the host (1). Effective immunosurveillance requires not only an immunocompetent host but also expression of major histocompatibility (MHC) class I antigens in association with the tumor antigen (2). Properties that allow a tumor to evade recognition by the host’s immune system may ultimately be responsible for their tumorigenicity (1).
The recognition of non-self or altered-self molecules is in part a function of T cells. T cells encounter antigen not as whole foreign molecules but as short peptide fragments presented in the context of MHC glycoproteins. MHC class I molecules are heterodimers that present peptide fragments to CD8+ T cells. They are composed of an integral membrane glycoprotein, termed the α-chain, that is noncovalently associated with β2-microglobulin (β2m). The α-chain, β2m, and peptide are assembled in the endoplasmic reticulum (ER) and transported to the cell surface as a complex. Without β2m the α-chain and peptide are not assembled and not transported to the cell surface and may be rapidly degraded in the ER β–5).
MHC class I presents endogenous antigens to CD8+ T cells. These endogenous antigens are derived from proteins manufactured by the cells. In contrast, CD4+ T cells recognize exogenous antigen, taken up from outside the cell by antigen-presenting cells and presented as part of the MHC class II complex. If intact tumor cells are to be directly recognized by T cells, they must express class I or class II MHC and be able to process and present antigen to the T-cell receptor. Human T-cell recognition of tumors in an antigen-specific MHC-restricted fashion has been described in patients with melanoma (6,7). Moreover, tumor-infiltrating lymphocytes (TIL) can be obtained from tumor digests cultured in interleukin-2 (8,9) and such TIL from melanoma tumors have been shown to specifically recognize self tumor in a MHC class I restricted fashion (6,7). This in vitro recognition has proven to be a useful predictor of subsequent clinical response to adoptive immunotherapy (10).
MHC restricted, antigen-specific recognition of renal cell carcinoma (RCC) by TIL from patients with RCC has been described (11,12) but is observed at a much lower frequency than in melanoma. To determine if MHC expression in RCC cell lines may contribute to this distinction we studied MHC expression in a series of RCC tumor cell lines. In the course of these studies, we identified one cell line lacking MHC class I on its surface. We found the mechanism for the failure of class I expression to be a lack of β2m expression. This defect may explain how some RCC tumor cells evade immune recognition.
MATERIALS AND METHODS
Cell Culture
Complete medium (CM) consisted of RPMI 1640 (Biofluids, Rockville, MD, U.S.A.) with 10% heat-inactivated fetal bovine serum (FBS, Biofluids) supplemented with 2 mM L-glutamine (NIH Media Unit, Bethesda, MD, U.S.A.) and 0.5 μg/ml amphotericin B (Biofluids), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were passaged in CM and harvested by trypsinization. Cell lines were established, characterized, and shown to be tumorigenic in nude mice (13).
For MHC class II induction we used interferon-γ (Genentech, South San Francisco, CA, U.S.A.) at a final concentration of 1,000 U/ml for 72 h before flow cytometric analysis.
Flow Cytometry
Confluent layers of cell lines were trypsinized, single cell suspensions were washed in flow cytometry buffer (FB) consisting of phosphate-buffered saline (PBS), pH 7.4 with 10% FCS and 0.1% sodium azide, and 106 cells were stained for 30 min at 4°C with 20 μl of labeled monoclonal antibodies. Antibodies used were W6/32 (Sera Lab, Sussex, England), anti-β2m (ICN Biomedicals, Costa Mesa, CA, U.S.A.), anti-HLA-DR, anti-HLA-DQ, and anti-HLA-DP (all from Becton-Dickinson, Mountain View, CA, U.S.A.). Control murine IgG1, and IgG2a fluorescein isothiocyanate were obtained from Becton-Dickinson. Cells were washed once in FB and 10,000 events were analyzed on a FACScan (Becton Dickinson) after nonviable cells were gated out with propidium iodide.
Tissue Sections
Formalin-fixed, paraffin-embedded tissue sections were obtained from the Department of Pathology, National Cancer Institute, from patients who had undergone nephrectomy for renal cell carcinoma. Histologic examination was performed on hematoxylin and eosin (H&E)-stained tissue sections and morphologically categorized as clear cell, granular cell, or sarcomatoid cell type.
Immunoperoxidase Staining
Monoclonal antibodies used were as follows: W6/32 (IgG2a) binds determinants on the heavy chain of MHC Class I antigens and was obtained from Sera-Lab (Westbury, NY, U.S.A.). NAMB-1 (IgG1) binds to β2m, and was obtained from Boehringer Mannheim (Indianapolis, IN, U.S.A.). Rabbit anti-β2m (Ig fraction) was obtained from either Dako (Carpintera, CA, U.S.A.) or Accurate (Westbury, NY, U.S.A.). The optimal dilution of each antibody was determined by staining sections of normal kidney. Negative controls were stained with equivalent dilutions of affinity-purified monoclonal antibody IA14, a mouse IgG2a that binds to the murine cell surface marker Thy 1.1.
Immunoperoxidase staining was performed using the Vectastain avidin-biotin complex immunoperoxidase technique (Vector Laboratories, Burlingame, CA, U.S.A.) or the Ventana 320 automated slide stainer (Ventana Medical Systems, Tucson, AZ, U.S.A.). Manual immunoperoxidase staining was performed using the manufacturer’s directions (Vectastain elite ABC kit). Tris, 0.05 M, pH 7.2, containing 0.05 mg/ml diaminobenzidine (Sigma) and 0.01% hydrogen peroxide (DABS) was used as substrate. Briefly, serial sections from formalin-fixed, paraffin-embedded tissue were deparaffinized with xylene and graded alcohols. After rehydration in distilled water, the sections were incubated in methanol containing 0.3% hydrogen peroxide for 30 min to quench any endogenous peroxidase activity. The sections were then washed in 0.05 M Tris buffered saline, pH 7.5 (TBS) and endogenous biotin was blocked with 1% horse serum supplied in the Vectastain kit. The sections were then incubated 30 min at 25°C with antibodies to β2m or MHC class I antigens and appropriate controls. After three 5-min washes in TBS, the sections were incubated 30 min at 25°C with either anti-mouse Ig or anti-rabbit biotinylated secondary antibody, then washed three times as previously described and incubated 30 min at 25°C in DABS. The sections were then counterstained with hematoxylin and coverslips were applied. Automated slide staining using the Ventana 320 automated slide stainer was accomplished using the manufacturer’s directions after deparaffinization and rehydration.
Cytofluoroscopy
Monoclonal antibody W6/32 (ATCC HB95) is a murine IgG2a that binds conserved determinants of the heavy chain of HLA-A, B, C. Three antibodies to human β2m were used as follows: L268 (IgG1, ATTC H149), BBM.1 (ATTC HB28), and BB7.7 (ATTC HB94). The monoclonal antibody HC-10 binds to unfolded MHC class I and was obtained from Hidde Ploegh (Massachusetts Institute of Technology, Cambridge, MA, U.S.A.). This antibody binds to the free heavy chains of most HLA-B loci, many A loci, and some C loci but not with β2m or heavy chains associated with β2m (14).
For cytofluoroscopy staining, 5 × 104 cells were transferred to round coverslips in 24-well plates (Costar, Cambridge, MA, U.S.A.) in CM and allowed to adhere overnight. Coverslips were washed in PBS, and fixed in 1% formaldehyde for 20 min at room temperature. Cells were washed three times on coverslips in PBS containing 0.5% saponin (Sigma). Antibodies were added at a final concentration of 25 μg/ml. Cells were stained with monoclonal antibodies for 45 min at 4°C in media containing 0.5% saponin. Cells were washed twice with PBS/0.5% saponin. Cells were counterstained with labeled goat anti-mouse Ig antibody for 45 min. Images were recorded using confocal microscopy (15).
Vaccinia Transfection
Vaccinia virus constructs containing the human β2m gene or the murine H-2Kd gene (control vaccinia) were kindly provided by Jon Yewdell and Jack Bennink, National Institute of Allergy and Infectious Diseases. The production and use of these virus constructs have been described (16). Confluent monolayers of UOK 123 cells were trypsinized, washed, and suspended at 1 × 106 cells/ml. The cells were infected with vaccinia virus at a multiple of infection of 10:1 for 60 min in a total volume of 15 ml of CM. Cells were then diluted to 5 × 105/ml and incubated for an additional 8 h. Cells were removed to 4°C and analyzed by flow cytometry.
RESULTS
Cell Surface Expression of β2m, MHC Class I, and MHC Class II by Flow Cytometry
We examined by flow cytometry the expression of β2m, MHC class I, and MHC class II in 12 cell lines established from patients with metastatic renal cell carcinoma. Cell lines were established from primary tumors of varying histologic subtypes including clear cell, granular cell, sarcomatoid, and mixed. All but one of the lines expressed HLA class I (W6/32) and β2m (Table 1). Some expressed low amounts of MHC class II (HLA-DR), and none expressed HLA-DP and HLA-DQ (data not shown). The one cell line not expressing class I or β2m and designated UOK 123 was subjected to further study.
TABLE 1.
Surface expression of MHC class I, class II, and β2m by renal cell carcinoma (RCC) cell lines
| % Events expressing:
|
||||
|---|---|---|---|---|
| Patient | Histology | W6/32 | HLA-DR | β2m |
| RC-1 | Clear | 96.0 | 0.28 | 99.9 |
| RC-2 | Clear | 42.6 | 23.3 | 66.7 |
| RC-3 | Clear | 93.0 | 0.00 | 92.4 |
| RC-4 | Clear | 67.8 | 1.20 | 67.8 |
| RC-5 | Clear | 26.1 | 0.33 | 42.6 |
| RC-6 | Clear, granular | 70.3 | 4.9 | 69.2 |
| RC-7 | Clear, granular | 42.6 | 23.3 | 66.7 |
| RC-8 | Clear | 43.8 | 0.68 | 47.1 |
| RC-9 | Clear, sarcomatoid | 70.4 | 3.18 | 59.5 |
| RC-10 | Clear, granular, and sarcomatoid | 32.6 | 1.20 | 56.8 |
| RC-11 | Clear | 92.6 | 9.63 | 92.2 |
| UOK 123 | Sarcomatoid, clear | 0.3 | 0.01 | 2.8 |
Cultured RCC cell lines derived from primary tumors of patients were analyzed by flow cytometry for class I expression (W6/32), class II expression (HLA-DR), and surface expression of β2m. Data are the percentage of acquired events expressing the marker compared with subtype-matched control antibodies.
To determine whether class I or II MHC markers could be induced on UOK 123 by cytokines, monolayers of cells were incubated with 1,000 U/ml interfere n-γ for 72 h before analysis. The cells were then trypsinized and studied by flow cytometry. The results are shown in Fig. 1. UOK 123 did not express HLA-A, B, C, HLA-DR, or β2m by flow cytometry. The lack of MHC class I and β2m expression could not be overcome by interferon-γ treatment. HLA-DR expression, however, did increase with the addition of interferon-γ to the media. Thus, the cells are responsive to interferon-γ as they upregulated HLA-DR but cannot upregulate expression of HLA-A, B, C.
FIG. 1.
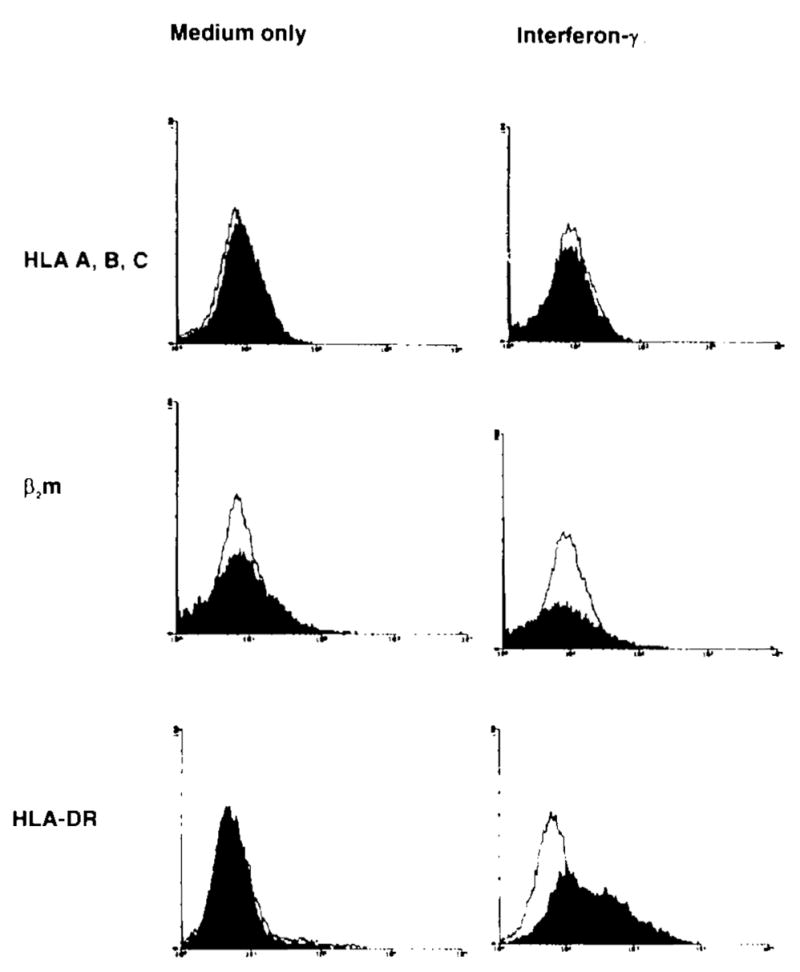
Flow cytometric analysis of UOK 123. Confluent layers of UOK 123 were trypsinized, washed, and stained with the antibodies shown (filled histograms) or subtype-matched control antibodies (unfilled histograms). Some monolayers were treated with interferon-γ for 72 h before analysis. UOK 123 failed to express HLA-A, B, C, β2-microglobulin (β2m), or HLA DR. Treatment with interferon-γ resulted in moderate expression of HLA-DR but no other changes.
Confocal Cytofluoroscopy to Detect Intracellular β2m and Class I Heavy Chains
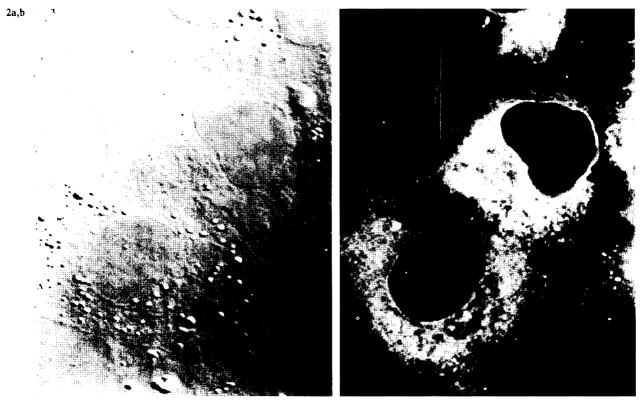
Because UOK 123 failed to express properly assembled MHC class I on the cell surface, we wished to determine whether the components of the class I complex could be detected inside the cell. To do this we used the method of Cox et al. (15). Cytospin preparations of UOK 123 were fixed, permeabilized, and stained for intracellular β2m, unfolded HLA class I α-chain, and assembled class I complexes. Staining with three different antibodies to β2m revealed no detectable β2m in the ER, Golgi apparatus, cytoplasm, or on the cell surface. In addition, there was no evidence of folded class I inside or on the surface of the cells. However, the ER stained intensively for unfolded class I, as shown in Fig. 2. This demonstrates that the cells make large amounts of class I α heavy chain, but are unable to assemble and transport mature class I molecules to the cell surface.
FIG. 2.
Confocal microscopy of permeabilized UOK-123 cells. Cytospin preparations of UOK 123 cells were permeabilized and stained with monoclonal antibody HC 10, which binds to unfolded class I heavy chains. Immunofluorescence images (right panel) demonstrated a reticular staining pattern in the cytoplasm consistent with reactivity in the endoplasmic reticulum. No surface staining was observed. A phase-contrast image of the same field is shown in the left panel.
Infection with Recombinant Vaccinia Virus
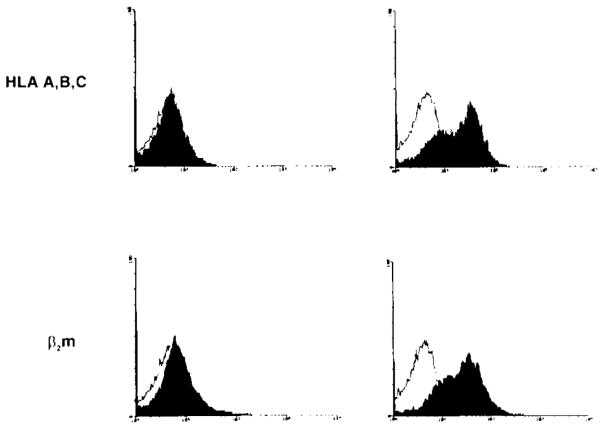
We next asked whether restoration of β2m to UOK 123 cells could overcome the apparent defect in assembly and surface expression of class I. We therefore transfected the cell line with a vaccinia virus construct containing the intact gene for β2m. This resulted in normal expression of both β2m and MHC class I (HLA-A, B, C) as assessed by flow cytometry analysis; no expression of class I or β2m was seen with the control vaccinia vector containing a nonfunctional gene (Fig. 3). This demonstrates that the lack of expression of MHC class I in UOK 123 was due to low or defective expression of the β2m gene product.
FIG. 3.
Flow cytometric analysis of UOK 123 infected with vaccinia virus. UOK 123 cells were infected with a recombinant vaccinia virus construct containing either the β2-microglobulin (β2m) gene or a control gene. The cells were then stained with antibodies to the surface markers shown (filled histograms) or subtype-matched control antibodies (unfilled histograms). Infection with a vaccinia virus containing the gene for β2m resulted in the surface expression of β2m and class I (HLA-A, B, C) but no expression of HLA-DR.
Immunoperoxidase Staining of the Tumor
The original paraffin blocks from patient UOK 123’s tumor were stained for MHC class I and β2m using immunohistochemistry. H&E staining revealed both clear cell and sarcomatoid foci within the primary tumor. The clear cell regions of the tumor demonstrated staining for class I and β2m. The sarcomatoid regions of the tumor, however, lacked staining for β2m but had focal weak expression of MHC class I. Within each section, endothelial cells as well as lymphocytes all stained positive for β2m and MHC class I serving as an internal positive control. Thus, the lack of expression of β2m was not an artifact of cell culture, but a defect of cells found in the tumor from which the cell line UOK 123 was derived.
DISCUSSION
We describe a cell line, UOK 123, derived from the primary tumor of a patient with metastatic renal cell carcinoma of primarily sarcomatoid histology. The cells were unusual compared with other RCC cell lines we examined because they failed to express assembled class I complexes on their surface and also did not express β2m inside or on the surface of the cells. Treatment with interferon-γ did not result in expression of class I by the cells, although the cells were responsive to interferon-γ because they upregulated class II expression after exposure to the cytokine in vitro. Large amounts of unfolded class I heavy chains were found in the endoplasmic reticulum of these cells. The defect in expression of class I was due to a defect in the natural expression of β2m because transient expression of β2m in the cells by infection with a recombinant vaccinia virus containing an intact gene for β2m resulted in the surface expression of class I as well as β2m.
A central question of tumor immunology is whether human tumors contain antigens that can be recognized by the immune system. Antigens recognized by T lymphocytes are short peptide fragments derived from proteins expressed by the cell and presented in the context of autologous MHC molecules on the surface of the cell. If tumor cells are to present antigens to T lymphocytes, then tumor cells must have an intact mechanism for expressing and assembling peptide-MHC complexes, a process termed antigen processing (17). In human melanoma it is clear that tumor antigens are present and can be expressed by the tumor cell for recognition by T lymphocytes (18–22). Interestingly, the antigens in melanomas that have been characterized to date are all derived from the products of normal genes, and some of these proteins are also expressed in mature normal cells of the tumor-bearing individual.
The expression of tumor antigens in RCC is much less clear. Specific, MHC-restricted recognition of RCC tumor lines by TIL cultured in interleukin-2 has been described (11,12). However, the nature of the antigen in RCC tumor cells recognized by such T cells remains unknown. TIL from RCC typically contain heterogeneous populations of CD4+ and CD8+ T cells, and cells derived from the NK lineage (23). The reason for the distinction between melanoma TIL and RCC TIL is unknown.
Immunohistochemical staining of the primary tumor from which UOK 123 was derived was consistent with the findings in vitro in that the sarcomatoid areas of the tumor did not express β2m and had only weak and focal expression of class I. The clear cell regions of the tumor expressed β2m and class I. This heterogeneity is typical of RCC, which can contain mixtures of histologic pattern, areas of necrosis, and inflammatory infiltrate. The cell line that grows from the tumor in vitro may or may not be representative of the population of tumor cells in vivo. The clones of cells that are capable of proliferating in vitro probably represent a minority of the total population of tumor cells. For example, in patient RC-9 the sarcomatoid area of the tumor failed to express β2m or class I by immunohistochemistry as did UOK 123, but the cell line derived from RC-9 expressed both. In patient RC-10 the sarcomatoid region of the tumor was positive for class I and weakly positive for β2m and the cell line expressed both. Although the immunohistochemical data are not conclusive, they are consistent with the in vitro data and the distinction between cell line UOK 123 and the 11 other cell lines was striking and consistent.
Interferon-γ has previously been shown to increase surface expression of a variety of markers (24). In the cell lines we studied we found all but a few to increase expression of MHC class I, β2m, and HLA-DR when cultured in the presence of interferon-γ for 72 h before analysis. However, interferon-γ failed to increase expression of β2m or MHC class I in UOK 123. Treatment with interferon-γ did, however, increase expression of HLA-DR glycoproteins on the cell surface. The cells thus are responsive to interferon-γ but did not upregulate class I because of a lack of β2m.
Defective HLA class I and II expression has been described in colon cancers (25) and melanoma (26). Reduction or loss of HLA class I expression has been found to occur in ~30% of surgically removed melanoma lesions (26). Cell lines established from advanced lesions more frequently show defective antigen processing compared with those established from early lesions (27). Some defects in antigen processing have been attributed to defects in β2m expression. Specific deletions and mutations have been described in melanoma cell lines. The FO-1 line described by D’Urso et al. has a deletion of the 5′ with a loss of a portion of the coding sequence for the β2m gene (28). More recently, this same group reported another melanoma cell line deficient in β2m caused by a reading frameshift in β2m messenger RNA (29).
Few studies have examined antigenic expression of HLA markers for RCC. In one series looking at primary and metastatic sites in 30 patients with various stages of RCC, 5 patients were found to have diminished expression of HLA class I by immunohistochemical staining (30). Three of the five patients were diagnosed as having granular cell histology (30). Granular cell histology is thought to be more aggressive than clear cell and perhaps less aggressive than the sarcomatoid histology. Sarcomatoid histology is relatively uncommon, but in the three patients we examined with mixed histology including sarcomatoid regions, differences in expression of class I and β2m were observed. These qualitative observations of differential expression within tumors of mixed histologies may suggest that defects in the assembly and transport of MHC class I may play a role in the clinical behavior of the tumor and may explain the aggressive clinical course observed in the patient from which UOK-123 was established.
Downregulation of MHC class I surface expression may represent one mechanism through which tumor cells evade immune recognition. UOK 123 fails to express intact class I because of a lack of β2m. The exact defect in β2m expression in these cells remains to be determined.
References
- 1.Tanaka K, Isselbacher KJ, Khoury G, Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985;228:26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- 2.Hammerling GJ, Klar D, Pulm W, Momburg F, Modenhauer G. The influence of major histocompatibility complex class I antigens on tumor growth and metastasis. Biochim Biophys Acta. 1987;907:245–59. doi: 10.1016/0304-419x(87)90008-4. [DOI] [PubMed] [Google Scholar]
- 3.Elliott T, Cerundolo V, Elvin J, Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991;351:402–6. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- 4.Townsend A, Ohlen C, Bastin J, Ljunggren HG, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443–8. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 5.Townsend A, Elliott T, Cerundolo V, Foster L, Barber B, Tse A. Assembly of MHC class I molecules anaylzed in vitro. Cell. 1990;62:285–95. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–25. [PubMed] [Google Scholar]
- 7.Hom SS, Schwartzentruber DJ, Rosenberg SA, Topalian SL. Specific release of cytokines by lymphocytes infiltrating human melanomas in response to shared melanoma antigens. J Immunother. 1993;13:18–30. doi: 10.1097/00002371-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Spiess P, LaFreniere R. A new approach to adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;223:1318–21. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzentruber DJ, Hom SS, Dadmarz R, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;12:1475–83. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 11.Finke JH, Rayman P, Edinger M, et al. Characterization of a human renal cell carcinoma specific cytotoxic CD8+ T cell line. J Immunother. 1992;11:1–11. doi: 10.1097/00002371-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Schendel DJ, Gansbacher B, Oberneder R, et al. Tumor-specific lysis of human renal cell carcinomas by tumor-infiltrating lymphocytes: HLA-A2-restricted recognition of autologous and allogeneic tumor lines. J Immunol. 1993;151:4209–20. [PubMed] [Google Scholar]
- 13.Anglard P, Trahan E, Liu S, et al. Molecular and cellular characterization of human renal cell carcinoma cell lines. Cancer Res. 1992;52:348–56. [PubMed] [Google Scholar]
- 14.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–306. [PubMed] [Google Scholar]
- 15.Cox JH, Bennick JR, Yewdell JW. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991;174:1629–37. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neil BH, Kawakami Y, Restifo NP, Bennick JR, Yewdell JW, Rosenberg SA. Detection of shared MHC-restricted human melanoma antigens after vaccinia virus-mediated transduction of genes coding for HLA. J Immunol. 1993;151:1410–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Restifo NP. Antigen processing and presentation: an update. In: DeVita VT, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer updates. Philadelphia: Lippincott; 1992. pp. 1–10. [Google Scholar]
- 18.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 19.Brichard V, Van Pel A, Wolfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–95. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaugler B, Van den Eynde B, van der Bruggen P, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–30. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:717–9. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–9. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belldegrun A, Muul LM, Rosenberg SA. Interleukin-2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988;48:306–14. [PubMed] [Google Scholar]
- 24.Nouri AME, Hussain RF, Dos Santos AVL, Gillott DJ, Oliver RTD. Induction of MHC antigen by tumor cell lines in response to interferon. Eur J Cancer. 1992;28:1110–5. doi: 10.1016/0959-8049(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 25.McDougall CJ, Ngoi SS, Goldman IS, et al. Reduced expression of HLA class I and II antigens in colon cancer. Cancer Res. 1990;50:8023–7. [PubMed] [Google Scholar]
- 26.Ruiter DJ, Mattijssen V, Broecker EB, Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991;2:35–45. [PubMed] [Google Scholar]
- 27.Alexander MA, Bennicelli J, Guerry D. Defective antigen presentation by human melanoma cell lines cultured from advanced, but not biologically early, disease. J Immunol. 1989;142:4070–8. [PubMed] [Google Scholar]
- 28.D’Urso CM, Wang Y, Cao Y, et al. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in β2m gene expression. J Clin Invest. 1991;87:284–92. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Cao Y, Albino AP, et al. Lack of HLA class I antigen expression by melanoma cells SK-MEL-33 caused by a reading frameshift in β2-microglobulin messenger RNA. J Clin Invest. 1993;91:684–92. doi: 10.1172/JCI116249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomita Y, Nishiyama T, Fujiwara M, Sato S. Immunohistochemical detection of major histocompatibility complex antigens and quantitative analysis of tumour-infiltrating mononuclear cells in renal cell cancer. Br J Cancer. 1990;62:354–9. doi: 10.1038/bjc.1990.296. [DOI] [PMC free article] [PubMed] [Google Scholar]