Ghrelin is a 28 aminoacid peptide hormone implicated in the regulation of energy homeostasis[16, 17, 34]. Ghrelin is produced primarily in the stomach, and in neurons located in hypothalamic regions associated with the regulation of food intake and energy balance [9, 21]. Plasma ghrelin and ghrelin mRNA expression in the stomach and hypothalamus increase just prior to the onset of feeding and after fasting[11, 15, 30, 32], and both peripheral and central ghrelin administration increase adiposity and food intake in rodents[31], and effect independent from its ability to secrete growth hormone (GH)[31]. These data suggest that ghrelin, whether secreted from the stomach or within the brain, acts at central sites to regulate energy balance.
Lactation is the most energetically demanding period during the life of a female mammal. During this period, mothers increase their food intake and use their adipose reserves to obtain sufficient nourishment for themselves and their young [12, 39]. Lactating rats show a threefold increase in food intake and a dramatic loss of fat pad weight[13, 22]. These changes correlate with decreases in leptin levels, and with changes in the expression of hypothalamic peptides regulating food intake and energy balance[3, 5, 7, 19, 24, 27, 37]. Paradoxically, ghrelin levels diminish during the first week of lactation in rats, perhaps as a mechanism that enables the animal to divert energy for milk production[25]. Nevertheless, it remains possible that hypothalamic ghrelin levels are altered in lactation, and or that ghrelin sensitivity increases to produce the changes in food intake and energy expenditure seen during this period. To examine this hypothesis, we compared plasma ghrelin concentrations between lactating rats allowed to nurse their litters with those of lactating rats having their litters removed at different times of lactation. Given that changes in plasma ghrelin concentrations may also affect sensitivity to this hormone, we also evaluated the expression of the message for the active form of the ghrelin receptor, the growth hormone secretagogue receptor 1a (GHS-R 1a), in the brain, pituitary, stomach and mammary glands. Finally, because ghrelin is also synthesized in the hypothalamus[9, 15, 16], and its expression is modulated by changes in metabolic state[15], we analyzed differences in the expression of the ghrelin message in hypothalamic and stomach explants from the same experimental animals.
Timed-pregnant Sprague-Dawley rats purchased from Charles River Farms (Wilmington, MA) were housed individually in plastic cages under controlled conditions (12 : 12 h light/dark, lights on at 08.00 h; 24 26 °C room temperature; 70–80% humidity). All rats received water and rat chow ad libitum throughout the duration of the study. All procedures were performed under the guidelines prescribed by the Yale Animal Care Committee.
On day 1 postpartum (pp; day 0 pp= day of parturition) rats were assigned to one of three groups. The first group of dams had their litters removed on day 1 pp (NL), the second group had their litters removed on day 13 pp (D13), and the last group was allowed to remain with their litters until all animals were sacrificed (day 15 pp; D15). Animals in the latter two groups had their litters culled on day 1 pp so that they each nursed 12 pups. Animals whose litters were removed on day 1pp had shown a full estrous cycle by day 15pp. Only 3 dams from the D13 group showed a vaginal smear showing epithelial cell cornification prior to sacrifice. All Day 15 dams remained acyclic. On day 15 pp, dams were deeply anesthetized by isofluorane inhalation, and decapitated after a blood sample was collected from the atrium of the heart. Animals were killed between 10:00 AM and noon. The brain, pituitary, and samples of stomach and mammary gland were immediately removed, frozen in liquid nitrogen and stored at −80°C until RNA extraction.
Blood samples were collected in tubes containing 50µ 1 N HCl and addition of 10 μl of Phenylmethylsulfonyl fluoride (PMSF) per one ml of plasma to decrease sample degradation, and centrifuged. Plasma was collected, and stored at −20°C until assayed. Plasma ghrelin levels were measured using a commercially available RIA kit (linco) with antibodies specific for the 29-residue octanoylted active form of the ghrelin peptide. All plasma samples were assayed in duplicate yielding a within assay variability of 6%.
Detection of Ghrelin mRNA
Stomach and hypothalamic samples were homogenized for total RNA extraction by the guanidium thiocyanate-phenol-chloroform method using TRIzol reagent (Invitrogen) and one microgram of total RNA was reverse transcribed using first-strand cDNA synthesis kit (Pharmacia Biotech, Piscataway, NJ, USA). The total volume was adjusted to 15 μl with distilled H2O. The hypothalamic sample included both the arcuate nucleus (ARC) and the subparaventricular zone of the mediobasal hypothalamus, and was dissected so as to encompass about 1mm of tissue on each side of the third ventricle, and the rostrocaudal extent of the ARC. A 326 bp fragment of Ghrelin cDNA was amplified based on reverse transcription polymerase chain reaction (RT-PCR) using specific oligonucleotide primers derived from the coding region of the rat Ghrelin sequence (GenBank accession no. ABO29433). These primers had the following sequences: (sense 5’- TTGAGCCCAGAGCACCAGAAA -3’; and anstisense 5’ -AGTTGCAGAGGAGGCAGAAGCT -3’). The reaction was performed for 1 h at 37°C. Half of the RT reaction mixture was used directly for the PCR reaction in a total volume of 20 µl, containing, 0.25 U of Taq DNA polymerase, 0.5X PCR Buffer, 2.5 mM MgCl2 (Invitrogen), 2.0 mM dNTP (Roche) and 0.3 pmol of the relevant oligonucleotide primers. As an internal control, the same cDNAs were amplified using Cyclophilin oligonucleotide primers (sense 5’- AGCACTGGGGAGAAAGGATT-3’ and antisense 5’- CATGCCTTCTTTCACCTTCC-3’, GenBank accession no. M19533), generating a fragment of 252 bp. Parallel amplifications (20, 25, 30, and 35 cycles) of the same cDNA were used to determine the optimum number of cycles. After 35 cycles, a readily detectable signal within the linear range was observed. For the actual analysis, samples were heated for 5 min at 94°C, and then 35 cycles were performed, each consisting of 1 min at 94°C, 1 min at 60, and 1 min at 72. This was followed by a final 10- min extension at 72°C. PCR reaction products were separated on a 2% agarose gel containing ethidium bromide‥
Detection of Growth Hormone Secretagoge Receptor mRNA
A cDNA transcript containing a 312 bp fragment of cDNA of GHS-R 1a from hypothalamus, stomach pituitary and mammary gland were amplified using RT-PCR using specific oligonucleotide primers derived from the coding region of the rat GHS-R 1a sequence. The primers had the following sequences: (sense 5' -GAGATCGCTCAGATCAGCCAGTAC -3' and antisense 5' -TAATCCCCAAACTGAGGTTCTGC -3', GenBank accession no. AB001982) [20]. Primers for Cyclophilin were used as controls. Amplification was conducted as described in the ghrelin PCR protocol (see above).
Visualized images of the gels were captured with a digital camera (Kodak), imported into a computer, and analyzed using Image Tool software (University of Texas at San Antonio) by comparing the ratio of optical densities of the bands expressing the ghrelin or the GHS-R 1a message over the expression of the control transcript (cyclophilin).
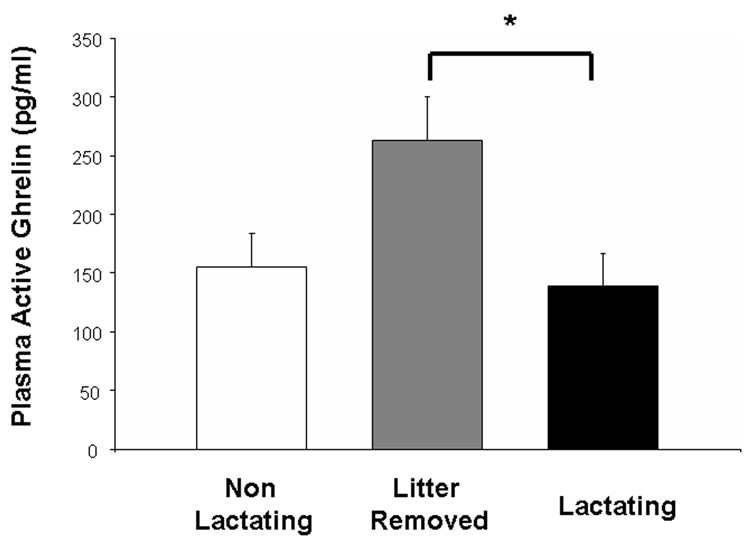
Figure 1 shows plasma ghrelin concentrations in dams in all experimental groups. A one way ANOVA showed that lactating rats allowed to keep their litters had plasma ghrelin concentrations that were not different from dams whose litters were removed on the day after parturition (P> 0.05). However, plasma ghrelin concentrations were significantly elevated in dams whose litters were removed on day 13 pp and sacrificed on day 15pp (p. < 0.05; see Figure 1).
Figure 1.
Plasma acylated ghrelin concentrations in non lactating rats (NL), litter removed (LR), and dams nursing their young until sacrifice at day 15 pp (L). Ghrelin concentrations in L did not differ from those of NL rats. Removal of the litter, however, resulted in a significant rise in ghrelin concentrations. * = significant from lactating (p<0.05).
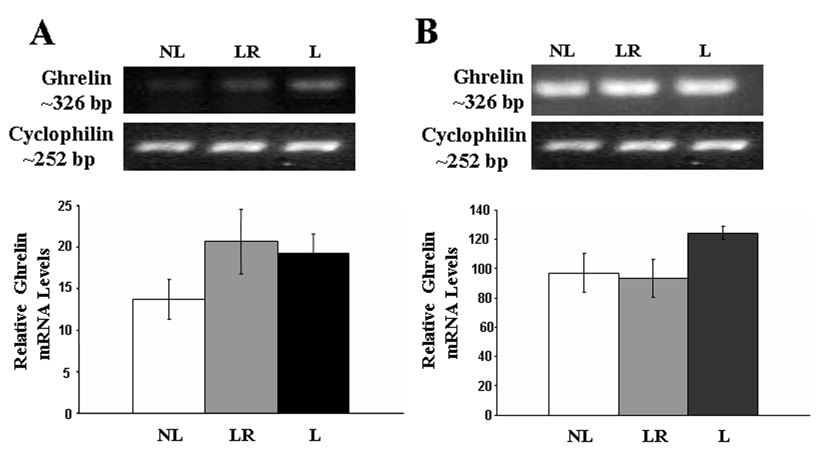
Measures of the relative transcription of the ghrelin message using RT-PCR showed that the message for the ghrelin gene was slightly elevated in both the hypothalamus and stomach of lactating animals, but these increases were not statistically significant (p.> 0.05, see Figure 2).
Figure 2.
RT-PCR analysis of Ghrelin gene expression in A) hypothalamus and B) stomach from NL dams (n=6), LR dams (n=6), and L dams (n=6), all sacrificed on day 15pp. There were no significant differences in hypothalamic nor stomach ghrelin mRNA expression between the groups (P>0.05).
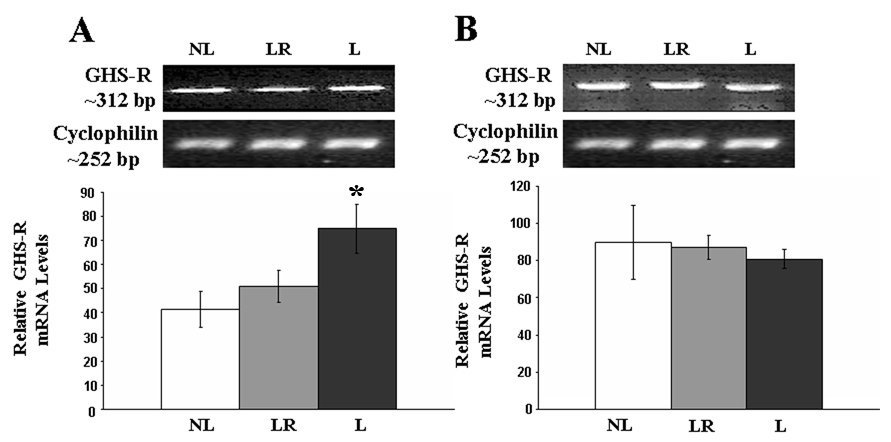
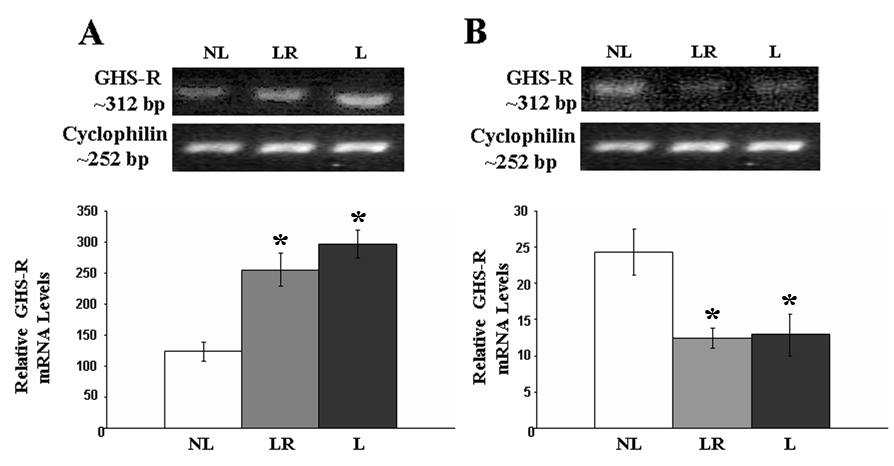
Analyses of the relative concentrations of GHS-R 1a transcript showed a significant increase in the levels of GHS-R 1a transcript in the hypothalamus of lactating rats allowed to keep their litters throughout the study (F(2,14)= 4.23, p. < 0.05) compared to those who had their litters removed soon after birth. Animals whose litters were removed two days prior to sacrifice had levels of GHS-R 1a mRNA that were not different from those that had their litters removed soon after birth (see Figure 3). Stomach levels of GHS-R 1a were not affected by the experimental manipulations (p. > 0.05; see Figure 3). The message for GHS-R in the pituitary was significantly elevated in lactating rats (F(2,14)= 14.46, p. < 0.05) compared to rats whose litter were removed soon after birth and regardless of whether they were allowed to nurse their litters throughout the experiment or if the litters were removed two days prior to sacrifice (see Figure 4). In contrast, relative GHS-R 1a mRNA levels in the mammary glands were reduced in lactating rats allowed to keep their litters for 13 or 15 pp compared to that of rats whose litters were removed after birth (F(2,14)= 6.69, p. < 0.05).
Figure 3.
RT-PCR analysis of GHS-R1a gene expression in A) hypothalamus and B)stomach from NL, LR and L dams. Lactation resulted in increased hypothalamic expression of GHS-R 1a, and the removal of the litters resulted in GHS-R 1a expression that was similar to that of non-lactating animals. Results are expressed as the mean ± SEM. * = significant from NL and D13 (p<0.05).
Figure 4.
RT-PCR analysis of GHS-R1a gene expression in A) pituitary and B)mammary gland from NL, LR, and L dams. The expression of the GHS-R1a transcript was higher in L animals and remained elevated for at least 48 hours after the removal of the pups as seen in LR animals. Mammary gland expression of GHS-R1a mRNA was significantly reduced during lactation and did not change in response to removal of the litter. Results are expressed as the mean ± SEM. * = significant from NL (p<0.05)
Discussion
The present data shows that, while lactating rats have no significant alterations in ghrelin synthesis and secretion at peak lactation (Day 15 pp), they do show changes in GHS-R 1a expression in both brain and pituitary. Moreover, our data suggest that both circulating levels of active ghrelin, and the message for the receptor in various tissues vary in response to the presence of a litter.
Lactation is an energetically expensive phase in female mammals because mothers must generate enough energy to maintain their own energy homeostasis as well as that of their young [13, 22, 39]. To do so, lactating females increase their food intake, reduce energy expenditure[23], and channel energy stored in fat towards milk synthesis[13]. This is reflected in the drop of circulating levels of plasma leptin and insulin, both indexes of adiposity levels that are seen during lactation[5, 6, 37]. It is, therefore, counterintuitive that ghrelin levels fail to increase as seen in this study and others[10], and even decrease as previously reported[25]. Nevertheless, it should be noted that ghrelin is an adipogenic hormone[31], and one that is anabolic in nature[34]. It is therefore possible that ghrelin levels are maintained at a basal level in order for the dams to continue the breakdown of fat that is required to maintain milk secretion and energy balance. In support of this contention is the fact that circulating levels of ghrelin increase following the removal of the litters, and are almost twice as high in concentration at the time of sacrifice than those of dams who are allowed to continue to nurse their litters. Presumably, the increase in circulating ghrelin concentrations seen in dams separated from their young could play a role in the rapid decrease in the lipolysis that is reported to occur following the removal of litters in lactating rats, and restore anabolic activity in adipose tissue[13].
Despite unaltered plasma ghrelin concentrations, the hypothalamus and the pituitary showed increased expression for the GHSR- 1a message, suggesting that these structures are more sensitive to ghrelin stimulation during lactation. Previous reports suggest that this is the case. For example, peripheral ghrelin injections significantly increase food intake and body weight in lactating rats[20]. This is notable considering that lactating rats already eat three times more than non-lactating rats, and have a body weight that is about 30% higher than that of control non-lactating rats. In addition, ghrelin receptor antagonists seem reduce hypothalamic neuropeptide Y (NPY) and agouti related peptide (AgRP) mRNA expression in lactating rats, but not in virgin females[10]. Similarly, lactating cows produce a greater increase in plasma glucose, cortisol, and glucagon in response to intravenous ghrelin infusions than non-lactating cows[14].
Dams whose pups were removed at birth or two days before sacrifice had lower levels of hypothalamic GHS-R1a expression than lactating rats. Similarly, pituitary GHS-R1a was also increased in lactating animals, but in contrats to hypothalamic GHS-R 1a expression, pituitary GHS-R1a message remained elevated in dams whose litter was removed on D13pp. Increases in GHS-R1a expression may result from sensory feedback generated by the suckling stimulus, which is responsible for the release of lactogenic hormones like prolactin, GH, and leptin [4, 5, 18, 28]. Changes in GHS-R1a expression in lactating rats may also be related to the large energetic drain that comes with nursing a litter [1, 37, 38]. Both of these factors may be independent and the relative contribution of each on modulating circulating ghrelin and GHS-R 1a mRNA remain to be determined. Regardless of the mechanism, it is likely that the increase in hypothalamic expression of GHS-R1a is related to enhanced sensitivity to the orexigenic effects of ghrelin[31, 33], whereas the increase in GHS-R1a expression seen in the pituitary generates increased responsiveness of this gland to the stimulatory effects of ghrelin upon the somatotropic axis in favor of milk synthesis[4, 20, 35].
Recent evidence shows that ovariectomy leads to higher feeding responses after ghrelin in female mice, an effect that is reduced after estrogen replacement treatment[8]. Considering these and other data showing that lactating rats have low circulating estrogen concentrations[29] and diminished sensitivity to exogenous estrogen[2, 26], it is possible that the expression of GHS-R 1a is mediated by estrogen, and that the lack of estrogen increases GHS-R 1a expression in the hypothalamus and pituitary of lactating rats.
Finally, levels of GHS-R 1a transcript in the mammary gland were diminished in rats that were allowed to nurse their litters for at least 13 days postpartum and removal of the litters was not a significant factor in restoring GHS-R 1a mRNA levels to those of non-lactating rats. While ghrelin has been previously found to stimulate milk secretion in rats[20], it may do so by increasing the release of GH and insulin growth factor (IgF) which then act upon mammary gland tissues to increase milk synthesis[16, 36].
A note of caution to these results is that RT-PCR is only a semiquantitative technique to evaluate differences in gene expression, and more accurate quantitative PCR (qPCR) is necessary to generate more accuracy. Nevertheless, our data suggest that while ghrelin levels do not change as a result of lactation, relative hypothalamic and pituitary sensitivity to ghrelin is enhanced in correlation with metabolic changes that are necessary for a successful lactation. Enhanced ghrelin sensitivity in the hypothalamus could increase food intake, decrease energy expenditure, and modulate glucose levels. Similar changes in the pituitary may serve to increase levels of lactogenic hormones like prolactin and growth hormone. Finally, the presence of the pups and the metabolic changes associated with milk production during lactation contribute to changes in ghrelin content and sensitivity.
Acknowledgements
Supported by the National Institute for Health SD (DK061619, SD), and by the Natural Sciences and Engineering Research Council of Canada (AA). We thank Dr. Emanuela Esposito and Marya Shanabrough for their technical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abizaid A, Kyriazis D, Woodside B. Effects of leptin administration on lactational infertility in food-restricted rats depend on milk delivery. Am J Physiol Regul Integr Comp Physiol. 2004;286:R217–R225. doi: 10.1152/ajpregu.00128.2003. [DOI] [PubMed] [Google Scholar]
- 2.Abizaid A, Service G, Woodside B. Food restriction during lactation results in prolonged hyposensitivity to the positive-feedback effects of oestradiol. J Neuroendocrinol. 2003;15:1037–1045. doi: 10.1046/j.1365-2826.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 3.Abizaid A, Walker CD, Woodside B. Changes in neuropeptide Y immunoreactivity in the arcuate nucleus during and after food restriction in lactating rats. Brain Res. 1997;761:306–312. doi: 10.1016/s0006-8993(97)00351-x. [DOI] [PubMed] [Google Scholar]
- 4.Barber MC, Clegg RA, Finley E, Vernon RG, Flint DJ. The role of growth hormone, prolactin and insulin-like growth factors in the regulation of rat mammary gland and adipose tissue metabolism during lactation. J Endocrinol. 1992;135:195–202. doi: 10.1677/joe.0.1350195. [DOI] [PubMed] [Google Scholar]
- 5.Brogan RS, Mitchell SE, Trayhurn P, Smith MS. Suppression of leptin during lactation: contribution of the suckling stimulus versus milk production. Endocrinology. 1999;140:2621–2627. doi: 10.1210/endo.140.6.6802. [DOI] [PubMed] [Google Scholar]
- 6.Burnol AF, Leturque A, Ferre P, Girard J. Glucose metabolism during lactation in the rat: quantitative and regulatory aspects. Am J Physiol. 1983;245:E351–E358. doi: 10.1152/ajpendo.1983.245.4.E351. [DOI] [PubMed] [Google Scholar]
- 7.Ciofi P, Crowley WR, Pillez A, Schmued LL, Tramu G, Mazzuca M. Plasticity in expression of immunoreactivity for neuropeptide Y, enkephalins and neurotensin in the hypothalamic tubero-infundibular dopaminergic system during lactation in mice. J Neuroendocrinol. 1993;5:599–602. doi: 10.1111/j.1365-2826.1993.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 8.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 9.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 10.Crowley WR, Ramoz G, Torto R, Keefe KA, Wang JJ, Kalra SP. Neuroendocrine actions and regulation of hypothalamic neuropeptide Y during lactation. Peptides. 2007;28:447–452. doi: 10.1016/j.peptides.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 12.Fleming AS. Control of food intake in the lactating rat: role of suckling and hormones. Physiol Behav. 1976;17:841–848. doi: 10.1016/0031-9384(76)90051-2. [DOI] [PubMed] [Google Scholar]
- 13.Flint DJ, Clegg RA, Vernon RG. Regulation of adipose-tissue metabolism during lactation [proceedings] Biochem Soc Trans. 1980;8:369–370. doi: 10.1042/bst0080369. [DOI] [PubMed] [Google Scholar]
- 14.Itoh F, Komatsu T, Kushibiki S, Hodate K. Effects of ghrelin injection on plasma concentrations of glucose, pancreatic hormones and cortisol in Holstein dairy cattle. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:97–102. doi: 10.1016/j.cbpa.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY, Lee HK. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–1320. doi: 10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 17.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 18.Lee LR, Haisenleder DJ, Marshall JC, Smith MS. The role of the suckling stimulus in regulating pituitary prolactin mRNA in the rat. Mol Cell Endocrinol. 1989;64:243–249. doi: 10.1016/0303-7207(89)90151-2. [DOI] [PubMed] [Google Scholar]
- 19.Malabu UH, Kilpatrick A, Ware M, Vernon RG, Williams G. Increased neuropeptide Y concentrations in specific hypothalamic regions of lactating rats: possible relationship to hyperphagia and adaptive changes in energy balance. Peptides. 1994;15:83–87. doi: 10.1016/0196-9781(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 20.Nakahara K, Hayashida T, Nakazato M, Kojima M, Hosoda H, Kangawa K, Murakami N. Effect of chronic treatments with ghrelin on milk secretion in lactating rats. Biochem Biophys Res Commun. 2003;303:751–755. doi: 10.1016/s0006-291x(03)00414-5. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 22.Ota K, Yokoyama A. Body weight and food consumption of lactating rats nursing various sizes of litters. J Endocrinol. 1967;38:263–268. doi: 10.1677/joe.0.0380263. [DOI] [PubMed] [Google Scholar]
- 23.Ota K, Yokoyama A. Body weight and food consumption of lactating rats: effects of ovariectomy and of arrest and resumption of suckling. J Endocrinol. 1967;38:251–261. doi: 10.1677/joe.0.0380251. [DOI] [PubMed] [Google Scholar]
- 24.Pickavance L, Dryden S, Hopkins D, Bing C, Frankish H, Wang Q, Vernon RG, Williams G. Relationships between hypothalamic neuropeptide Y and food intake in the lactating rat. Peptides. 1996;17:577–582. doi: 10.1016/0196-9781(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 25.Shibata K, Hosoda H, Kojima M, Kangawa K, Makino Y, Makino I, Kawarabayashi T, Futagami K, Gomita Y. Regulation of ghrelin secretion during pregnancy and lactation in the rat: possible involvement of hypothalamus. Peptides. 2004;25:279–287. doi: 10.1016/j.peptides.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Smith MS. Hypothalamic-pituitary responsiveness during lactation in the rat: estrogen-induced luteinizing hormone surges. Endocrinology. 1978;102:121–127. doi: 10.1210/endo-102-1-121. [DOI] [PubMed] [Google Scholar]
- 27.Smith MS. Lactation alters neuropeptide-Y and proopiomelanocortin gene expression in the arcuate nucleus of the rat. Endocrinology. 1993;133:1258–1265. doi: 10.1210/endo.133.3.8365368. [DOI] [PubMed] [Google Scholar]
- 28.Smith MS. The relative contribution of suckling and prolactin to the inhibition of gonadotropin secretion during lactation in the rat. Biol Reprod. 1978;19:77–83. doi: 10.1095/biolreprod19.1.77. [DOI] [PubMed] [Google Scholar]
- 29.Taya K, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the lactating rat. Endocrinol Jpn. 1982;29:453–459. doi: 10.1507/endocrj1954.29.453. [DOI] [PubMed] [Google Scholar]
- 30.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 31.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 32.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 33.Tung YC, Hewson AK, Carter RN, Dickson SL. Central responsiveness to a ghrelin mimetic (GHRP-6) is rapidly altered by acute changes in nutritional status in rats. J Neuroendocrinol. 2005;17:387–393. doi: 10.1111/j.1365-2826.2005.01316.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 35.Vernon RG, Piperova L, Watt PW, Finley E, Lindsay-Watt S. Mechanisms involved in the adaptations of the adipocyte adrenergic signal-transduction system and their modulation by growth hormone during the lactation cycle in the rat. Biochem J. 1993;289(Pt 3):845–851. doi: 10.1042/bj2890845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Bielanski W, Pawlik WW, Kuwahara A, Kato I. Dual age-dependent effect of ghrelin administration on serum level of insulin-like growth factor-1 and gastric growth in young rats. Eur J Pharmacol. 2006;529:145–150. doi: 10.1016/j.ejphar.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 37.Woodside B, Abizaid A, Walker C. Changes in leptin levels during lactation: implications for lactational hyperphagia and anovulation. Horm Behav. 2000;37:353–365. doi: 10.1006/hbeh.2000.1598. [DOI] [PubMed] [Google Scholar]
- 38.Woodside B, Popeski N. The contribution of changes in milk delivery to the prolongation of lactational infertility induced by food restriction or increased litter size. Physiol Behav. 1999;65:711–715. doi: 10.1016/s0031-9384(98)00210-8. [DOI] [PubMed] [Google Scholar]
- 39.Woodside B, Wilson R, Chee P, Leon M. Resource partitioning during reproduction in the Norway rat. Science. 1981;211:76–77. doi: 10.1126/science.7444451. [DOI] [PubMed] [Google Scholar]