Abstract
We show herein that the ability of 5 different monomeric IgEs (mIgEs) to enhance murine bone marrow-derived mast cell (BMMC) survival correlates with their ability to stimulate extracellular calcium (Ca++) entry. However, while IgE + antigen (IgE+Ag) more potently stimulates Ca++ entry, it does not enhance survival under our conditions. Exploring this further, we found that while all 5 mIgEs stimulate a less robust Ca++ entry than IgE+Ag initially, they all trigger a more prolonged Ca++ influx, generation of reactive oxygen species (ROS) and Erk phosphorylation. These prolonged signaling events correlate with their survival enhancing ability and positively feedback on each other to generate the pro-survival cytokine, IL-3. Interestingly, the prolonged Erk phosphorylation induced by IgE appears to be regulated by a MAPK phosphatase (MKP) rather than MEK. IgE-induced ROS generation, unlike that triggered by IgE+Ag, is not mediated by 5-lipoxygenase (5-LO). Moreover, ROS inhibitors, which block both IgE-induced ROS production and Ca++ influx, convert the prolonged Erk phosphorylation induced by IgE into the abbreviated phosphorylation pattern observed with IgE+Ag and prevent IL-3 generation. In support of the essential role that IgE-induced ROS plays in IgE-enhanced BMMC survival, we found the addition of H2O2 to IgE+Ag-stimulated BMMCs leads to IL-3 secretion.
Keywords: mast cells, antibodies, Fc receptors, signal transduction
Introduction
Mast cells are responsible for immediate hypersensitivity and chronic allergic reactions through the binding of extracellular IgE to their high affinity IgE receptors (FcεRIs) and the subsequent cross-linking of these IgE/FcεRI complexes by multivalent allergens. This cross-linking activates multiple signaling pathways that lead to degranulation, prostaglandin and leukotriene synthesis and production of various cytokines and chemokines (1). Within this traditional scenario it was thought until quite recently that IgE binding by itself was simply a passive pre-sensitization step that awaited receptor aggregation via multivalent antigens (Ags) to induce intracellular changes. However, there is now substantial evidence that monomeric IgE (mIgE) alone is not only capable of upregulating the cell surface expression of FcεRI (2), but of initiating cell signaling events (3). Specifically, with regard to the latter, we (4) and Kawakami’s group (5) showed that the binding of mIgE alone, in the absence of Ag, was capable of enhancing bone marrow derived mast cell (BMMC) survival while IgE followed by Ag cross-linking (IgE+Ag) was not. Moreover, we found, using SPE-7 anti-DNP IgE, that mIgE binding stimulated multiple phosphorylation events in these cells and led to a more potent production of cytokines than IgE+Ag. As well, we provided evidence that mIgE prevented the apoptosis of cytokine-deprived BMMCs, at least in part, by maintaining Bcl-XL levels and producing autocrine-acting cytokines.
A number of groups have subsequently confirmed these findings and shown that mIgE alone can also lead to enhanced degranulation, leukotriene release, histidine decarboxylase expression (6), increased adhesion to fibronectin (7), FcεRI internalization, migration and DNA synthesis (3), to varying degrees, depending on the mast cell type studied. As well, several groups have substantially increased our understanding of how mIgE enhances mast cell survival by showing that it requires the tyrosine kinase Syk (8) and the immunoreceptor tyrosine-based activation motif (ITAM) within the FcRγ chain of the FcεRI (9) (ie, the same ITAM required for IgE+Ag-induced degranulation). As well, a weak but sustained signal via this γ chain was shown to be sufficient for mast cell survival (10) and, using IL-3−/− BMMCs, that the IgE-induced autocrine production of IL-3 was responsible for mast cell survival, in part via a Jak2/Stat5-induced maintenance of Bcl-XL and Bcl-2 (11). Importantly, Kawakami’s group found that some mIgEs, defined as highly cytokinergic (HC), were far more capable of stimulating intracellular signaling and survival than others (defined as poorly cytokinergic (PC)) and this appeared to correlate with their ability to trigger FcεRI aggregation (8).
To further elucidate the mechanisms underlying the ability of some IgEs to promote BMMC survival better than others, we have compared herein the intracellular signaling of 5 different mIgEs with markedly different abilities to enhance BMMC survival. As well, we have compared the signaling of IgE with IgE+Ag to further elucidate why IgE+Ag does not enhance survival under the conditions used in our lab. Our results suggest that the ability of an IgE to promote IL-3 production, and thereby survival, depends on its ability to trigger a prolonged generation of reactive oxygen species (ROS). Interestingly, this IgE-induced ROS is not generated via 5-lipoxygenase (5-LO), distinguishing it from IgE+Ag-induced ROS, and is markedly dependent upon extracellular calcium (Ca++) entry and MEK, suggesting that positive feedback loops from these two signaling intermediates are involved.
Materials and Methods
Mast cell isolation
SHIP+/+ and −/−, Lyn+/+ and −/− and LAT +/+ and −/− bone marrow cells, aspirated from 4–8 week old C57Bl6 mice, were cultured in IMDM + 15% FCS + 150 μM monothioglycerol containing 50 ng/ml mSCF, 10 ng/ml mIL-3 and 10 ng/ml hIL-6 for 1 week and then replacing these cytokines with 30 ng/ml IL-3. By 6–8 weeks, greater than 99% of the cells were c-kit and FcεRI positive (12,13).
Preparation of mIgEs
Five distinct murine monoclonal IgEs were used in this study; clone SPE-7 anti-DNP (S) from Sigma-Aldrich Canada (Oakville, Ont), clone 91.58 anti-NP (λ, kindly provided by Dr. K. Hayglass (U. Manitoba), anti-DNP clone DNP48 (48) derived in Dr. R Siraganian’s laboratory, anti-Epo26 (E) (StemCell Technologies Inc., Vancouver, BC) and the H1 26.82 Liu anti-DNP (L) (14). mIgEs were prepared by fractionation using a Waters HPLC system with a BioSep SEC S3000 gel filtration column (300 × 7.8 mm, Phenomenex, Torrance, CA) as described previously (4) and stored at 4°C. IgE concentrations were determined using an IgE ELISA (BD PharMingen, San Diego, CA).
Survival studies
BMMCs were washed with IMDM and incubated at 5×105 cells/ml in IMDM + 10% FCS or 0.1% BSA ± IgE or IgE+Ag, as indicated, in Falcon 3047 24-well, flat bottom plates (total volume 0.2 ml/well) ± neutralizing antibodies to Stem cell factor (SCF) (R & D Systems, Minneapolis, MN) or IL-3 (15) or isotype control antibody (15). Viability was assessed by trypan blue exclusion.
BMMC stimulation and Western blotting
BMMCs were incubated without IL-3 for 4 h or overnight at 37°C in IMDM + 10% FCS + 150 μM MTG + P/S and then washed 3 times with, and resuspended in, IMDM + 0.1% BSA+ MTG +P/S or in Tyrode’s buffer ± 1.8 mM Ca++Cl2. The cells were then treated ± 25 μM U0126, SP600125, SB203580, U73122, AA861, FR122047, 1.5 mM ebselen or 2.5 mM NAC at 37°C before or after adding 5 μg/ml IgE for the indicated times. Cells were washed with 4°C PBS and solubilized by boiling for 1 min with SDS-sample buffer (using 5 × 105 BMMCs/sample for total cell lysates) and Western blotting as described (16). Polyclonal antibodies to the phospho-T202/Y204 of Erk 1/2 were from New England BioLabs, Inc. (Mississauga, Canada), to GAPDH from Upstate Cell Signaling Solutions (cat# 06-951, Charlottesville, VA) and to Bcl-XL from Transduction Laboratories (Lexington, KY). Sheep neutralizing antibodies to IL-3 were generated using chemically synthesized murine IL-3 (15) and neutralizing antibodies to SF were from R & D Systems.
Calcium measurements
Ca++ fluxes were measured as described previously (12). For stimulation with the mIgEs, BMMCs were incubated with 2 μM fura-2/AM (Molecular Probes) in Tyrode’s buffer at 23°C for 45 min, washed twice, resuspended in Tyrode’s buffer at 5×105 cells/ml in a stirring cuvette and stimulated with 10 μg/ml IgE. To stimulate with IgE+Ag, BMMCs were preloaded with 0.1μg/ml S IgE overnight in IMDM + 10% FCS + 150 μM MTG + P/S, washed 3 times to remove unbound IgE, resuspended in Tryode’s buffer + 2 μM fura-2A/M for 45 min at 23°C, washed twice, resuspended in Tyrodes at 5 × 105 cells/ml as above and stimulated with 20 ng/ml DNP-HSA (30–40 DNP/mole human serum albumin, Sigma). moles Cytosolic Ca++ was measured by monitoring fluorescence intensity at 510 nm by exciting the sample with two different wavelengths (340 and 380 nm) with a Thermo Spectronic Aminco Bowman Series 2 Luminescence Spectrometer (Urbana, IL).
ROS measurements
BMMCs were washed twice in IMDM without IL-3 (starve media) and resuspended at 0.5×106 cells/ml overnight. Cells to be stimulated with Ag were pre-loaded overnight with 0.1 μg/ml S IgE and the cells were then washed twice in starve media and resuspended at 1×106 cells/70–80μl of Tyrode’s buffer. 5-(and 6)- chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFHDA; Molecular Probes; Eugene, OR, USA) was prepared fresh each day, by dissolving 50 μg in 8 μl of DMSO and diluting immediately to 100μM in Tyrode’s buffer, and stored in the dark. 10μl of CM-DCFHDA stock was added per 106 cells 20 min prior to stimulation in a black (small well) 96-well plate at 37°C, 5%CO2 in the dark. Inhibitors (10x stocks prepared by dilution in Tyrode’s) were added 15 min prior to stimulation in a volume of 10μl if used in the assay. Cells were stimulated with 10μl of IgE or Ag prepared by dilution of stock to a 10x concentration in Tyrode’s buffer. The fluorescence of the plate was read immediately before stimulating the cells, immediately after stimulation, every 1 min for 5 min and every 5 min thereafter up to 30 min. Relative fluorescence was detected with an excitation wavelength of 485nm and an emission wavelength of 527nm in a 96 well plate fluorometer (Fluoroskan Accent FL, ThermoLabsystems; Franklin, MA, USA). Fluorescence values reported have been corrected by subtracting the background fluorescence of similarly treated but unstimulated cells.
ELISAs
IgE, IL-3 and IL-6 ELISAs (BD PharMingen, San Diego, CA) were performed according to the manufacturer’s instructions.
Results
The ability of an IgE to enhance BMMC survival correlates with its ability to trigger a prolonged calcium influx and Erk phosphorylation
To further investigate the mechanism by which IgE enhances BMMC survival, we first tested a panel of 10 different mouse monoclonal mIgEs, quantitated using an IgE ELISA, and found that they were all capable of enhancing BMMC survival to some extent (data not shown). Since the most potent IgE, i.e., SPE-7 anti-DNP (S) IgE, possessed a lambda light chain we obtained another lambda light chain IgE, ie, clone 91.58 anti-NP (λ), to see if this exceptional potency was characteristic of lambda-containing IgEs. λ IgE, however, proved to be the least potent. Based on these results we chose a subset of 5 different mIgEs with very different survival-enhancing abilities for further study. As shown in the left panel of Fig 1A, the order of survival enhancing ability of these 5 IgEs was S > the anti-DNP clone DNP48 (48) ≥ Liu anti-DNP IgE (L) > the anti-Epo26 (E) > λ. The effect of IgE+Ag was also tested (Fig 1A, right panel), using 0.01, 0.05 and 0.1 μg/ml of S IgE, followed by 20 ng/ml DNP-HSA, and was found to be incapable of enhancing BMMC survival. Thus, we could not simulate the survival enhancing ability of mIgE by simply activating a very small number of IgE receptors on BMMCs with IgE+Ag. This contrasts somewhat with a report showing that weak to moderate stimulation of BMMCs with IgE+Ag can enhance survival (17).
Fig 1.
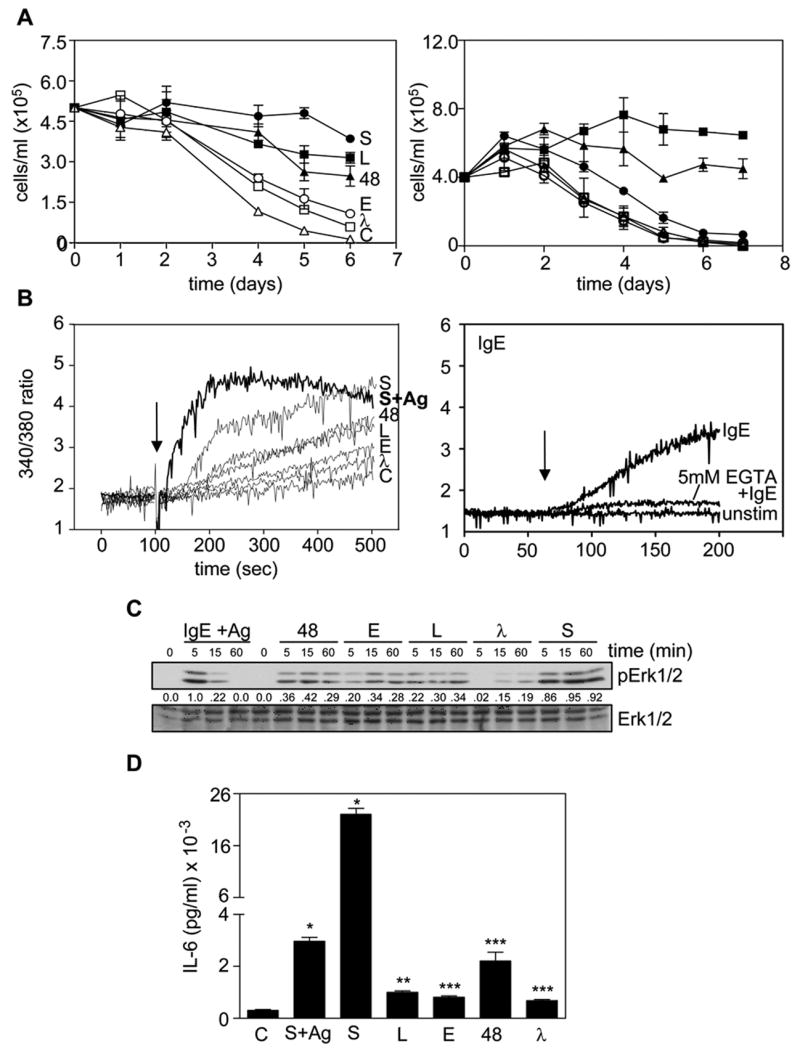
The ability of an IgE to induce survival correlates with its ability to trigger a prolonged calcium entry and Erk phosphorylation. (A) BMMCs were cultured at 37°C with 10% FCS alone (C) (△) or + 5 μg/ml S (●), L (■), 48 (▲), E (○) or λ (□) IgE (left panel) or with 10% FCS alone (C) (●) or + 5 μg/ml (■), 1μg/ml (▲) S IgE or 0.1 (○), 0.05 (□) or 0.01 (△) μg/ml S IgE overnight at 37°C, washed and 20 ng/ml DNP-HSA added (right panel), and viable cells counted on the days indicated. Values shown are the mean ± SEM of duplicate determinations. (B) BMMCs were incubated with fura-2/AM at 23°C for 45 min, washed twice and stimulated with (left panel) 10 μg/ml of S, L, 48, E or λ IgE (arrow indicates time of addition). C = unstimulated cells and S+Ag (dark line) = BMMCs pretreated with 0.1 μg/ml S overnight, washed and stimulated with 20 ng/ml DNP-HSA at the time indicated by the arrow or (right panel) 10 μg/ml S (arrow indicates time of addition) ± pretreatment for 1 min with 5mM EGTA. (C) BMMCs were treated with 5 μg/ml of the indicated IgE or with 0.1 μg/ml S overnight, washed and stimulated with 20 ng/ml DNP-HSA (IgE+Ag) at 37°C for the times shown and the SDS-solubilized total cell lysates subjected to Western analysis with phospho-specific antibodies to Erk1/2. The blot was reprobed with anti-Erk1/2 to confirm equal loading. The numbers between the two panels indicate relative densitometry values normalized to Erk1/2. (D) BMMCs were cultured with 10% FCS alone (C) or + 0.1 μg/ml S IgE overnight at 37°C, washed and exposed to 20 ng/ml DNP-HSA (S+Ag) or not pre-loaded and stimulated + 5μg/ml S, L, E, 48 or λ IgE for 3 h and the conditioned medium subjected to an IL-6 ELISA. Results are the mean ± SEM of triplicate determinations. * p< 0.0001. ** p< 0.001. *** p<0.006. Similar results were obtained in 5 (left panel of A) or 3 (right panel of A, B, C & D). For the left panel of B & D) only 2 separate experiments were carried out with IgE+Ag present.
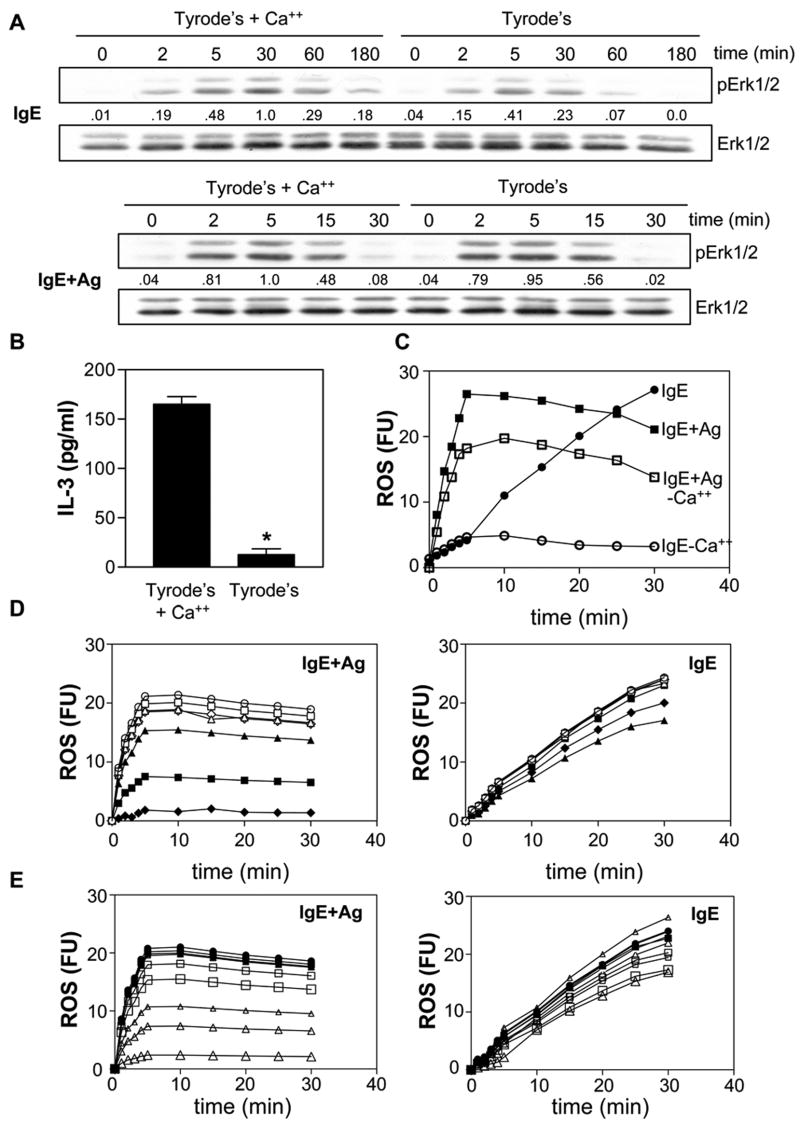
We then examined the ability of the 5 mIgEs to stimulate Ca++ fluxes and found that this correlated precisely with their ability to enhance survival (Fig 1B, left panel). The Ca++ response stimulated by IgE+Ag occurred faster than any mIgE alone, peaking at 100 sec after Ag addition and then declining. Ca++ mobilization stimulated by the 5 mIgEs, on the other hand, steadily increased over the time course of the experiment. Carrying out these studies in the presence and absence of EGTA established that the bulk of the IgE-induced Ca++ mobilization was due to extracellular Ca++ entry (Fig 1B, right panel).
We then tested the ability of the 5 mIgEs and IgE+Ag to stimulate the phosphorylation of Erk1/2 (to monitor Ras pathway activation). As can be seen in Fig 1C, IgE+Ag-induced phosphorylation of Erk peaked early and returned to baseline levels within 60 min, in keeping with earlier reports (4,10). The Erk phosphorylation induced by the 5 mIgEs, on the other hand, was prolonged, with peak levels visible out to 60 min. Importantly, the level of Erk phosphorylation correlated with the survival enhancing ability of the 5 mIgEs, with S displaying the highest and λ the lowest.
We also compared the ability of the 5 different mIgEs to stimulate IL-6 production and found that the rank order of production correlated with their survival enhancing ability (Fig 1D). However, IgE+Ag also stimulated the production of IL-6 at levels only slightly below that triggered by S IgE, in keeping with our previous findings (4) and substantially above that stimulated by the other mIgEs. Thus, IL-6 production did not correlate with BMMC survival.
IgE-induced survival of BMMCs is dependent on the production of autocrine-acting IL-3
While the above studies demonstrated that the ability of the 5 mIgEs to trigger extracellular Ca++ entry and Erk phosphorylation correlated with their survival enhancing abilities, they did not establish which signaling events were actually required for enhancing survival. In an attempt to address this we first compared the IgE-induced survival of Lyn+/+ and −/− BMMCs using the S mIgE. As shown in the left panel of Fig 2A, the IgE-mediated enhancement of BMMC survival was significantly reduced in Lyn−/− BMMCs, in keeping with results obtained by Kitaura et al (8). Similar results were obtained with these cells using the L IgE (data not shown). We also tested the ability of both S and L mIgEs to enhance the survival of LAT−/− BMMCs (18,19) and found that the absence of LAT reduced the ability of the S (Fig 2A, right panel) and L (data not shown) mIgE to enhance survival as well.
Fig 2.
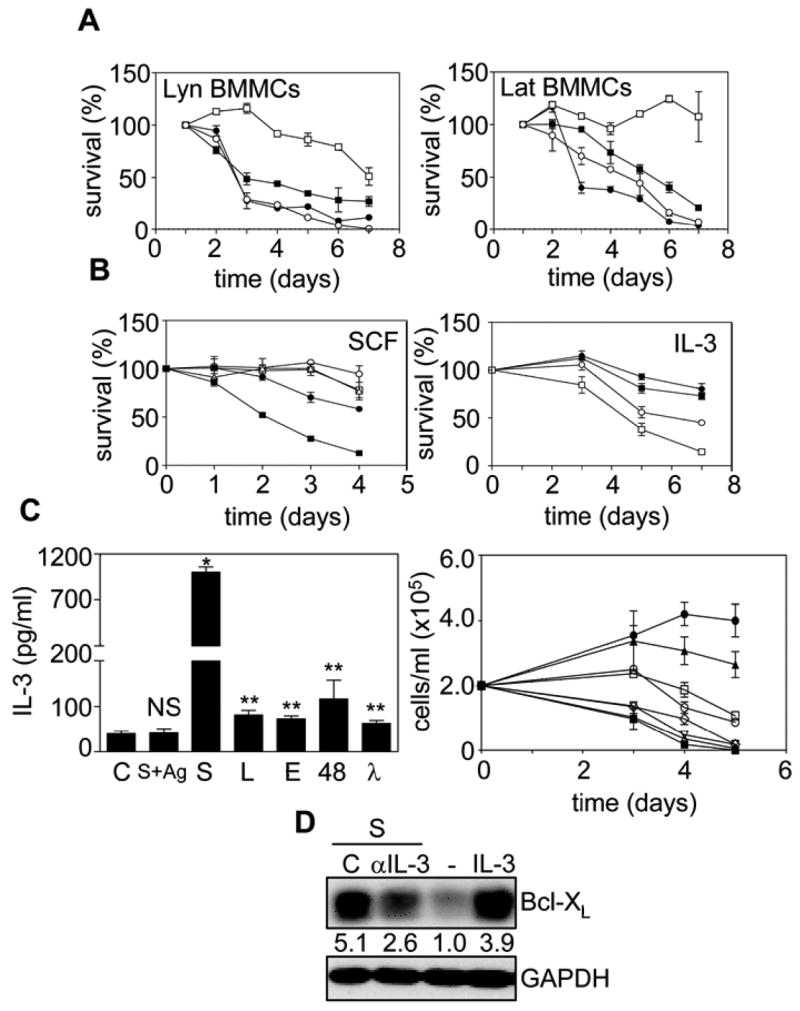
IgE-induced survival of BMMCs is dependent on the production of autocrine-acting IL-3. (A) Lyn+/+ (□, ○) and −/− (■, ●) (left panel) and LAT+/+ (□, ○) and −/− (■, ●) (right panel) BMMCs were cultured with 10% FCS alone (○, ●) or + 5 μg/ml S mIgE (□, ■). Viable cell counts were determined and the values shown are the mean ± SEM of duplicate determinations. (B) In the left panel, WT BMMCs were cultured with 10% FCS + 10 μg/ml S mIgE (△) ± neutralizing antibodies to SCF (□) or an isotype control (○) or with 10% FCS + 30 ng/ml SCF + isotype control (●) or anti-SCF (■) antibodies. In the right panel WT BMMCs were cultured with 0.1 % BSA alone (□) or + 5μg/ml S mIgE (●) ± neutralizing antibodies to IL-3 (○) or isotype control antibody (■). (C) In the left panel, WT BMMCs were cultured with 0.1 % BSA alone or + 5μg/ml S, L, 48, E or λ IgE for 3 h or + 0.1 μg/ml S overnight at 37°C, washed and given 20 ng/ml DNP-HSA for 3 hr (S+Ag) and the conditioned medium subjected to an IL-3 ELISA. NS = not significantly different. * p<0.0001, ** p<0.05. In the right panel, WT BMMCs were cultured for the indicated days at 37°C with 10% FCS (■) or with 500 (●), 250 (▲), 125 (□), 62.5 (○), 31.25 (◇), 15.6 (▽), or 7.8 (△) pg/ml IL-3 and viable cell counts determined. Results for both panels are the mean ± SEM of duplicate determinations. (D) WT BMMCs were cultured with 10% FCS alone (−) or + 30 ng/ml IL-3 or + 5μg/ml S IgE ± neutralizing antibodies to IL-3 (αIL-3) or an irrelevant antibody (C ab) for 30 h and SDS-solubilized total cell lysates subjected to Western analysis with anti-Bcl-XL. The blot was reprobed with anti-GAPDH to confirm equal loading. The numbers under the lanes indicate relative densitometry values normalized to GAPDH. Similar results were obtained in 2 (A, B & right panel of C) or 3 (left panel of C & D) separate experiments.
Since previous studies in our lab suggested that IgE was enhancing survival, at least in part, by producing autocrine-acting cytokines (4), we also attempted to identify the cytokines involved. From our earlier studies using RNAse protection assays and ELISAs, we found that S mIgE alone stimulated the production of IL-2, IL-3, IL-4, IL-6, IL-13 and TNFα and that a combination of these cytokines, at the levels produced in response to mIgE, enhanced survival almost as well as mIgE (4). We therefore tested these cytokines, as well as GM-CSF, M-CSF, IL-10 and SCF since these latter proteins have been shown to be produced by mast cells and/or affect mast cell proliferation (20–23). We found that only SCF and IL-3 supported the proliferation and survival of BMMCs (data not shown). We then used neutralizing antibodies to SCF and IL-3 to see if they could block IgE-induced BMMC survival and found that anti-SCF had no detectable effect (Fig. 2B left panel) while anti-IL-3 substantially reduced the survival of IgE-treated BMMCs (Fig 2B, right panel), in keeping with elegant studies from Saito’s group using IL-3−/− BMMCs (11). We then compared the IL-3 production from BMMCs stimulated with the 5 mIgEs. Not surprisingly, the pattern was very similar to that obtained with IgE-triggered IL-6 production (Fig 1D), with S IgE generating the most IL-3 and λ the least (Fig 2C, left panel). Importantly, however, IgE+Ag was incapable of triggering any detectable IL-3 production, in keeping with IL-3 playing a critical role in IgE-induced BMMC survival. This result, while in agreement with recent data from Saito’s lab (11), contrasts with early studies showing that IgE+Ag can trigger IL-3 production (24–26) and may reflect difference in the type of mast cells used or the nature of the Ag. To determine if the levels of IL-3 produced in response to the different mIgEs could account for the observed survival, we carried out IL-3 dose response studies and found that the level of IL-3 secreted in response to the 5 mIgEs was within the range required to maintain BMMC survival (Fig 2C, right panel), in keeping with the results of (11). Thus, autocrine-acting IL-3 alone might indeed explain the survival effects but to explore this further we asked if the IgE-induced maintenance of Bcl-XL (4,11), was dependent on IL-3 production. To test this, we examined the effect of a neutralizing anti-IL-3 antibody on IgE-induced Bcl-XL levels following 30 h of incubation, at which time there is no loss as yet of cell viability. Preliminary studies established that the level of anti-IL-3 antibody we used was sufficient to block exogenously added IL-3 (at 10 times the level of IL-3 produced in response to 5 μg/ml of S IgE) from promoting BMMC proliferation or survival (data not shown). As can be seen in Fig 2D, we found that anti-IL-3 antibody substantially, but not completely, abrogated the maintenance of Bcl-XL, while an irrelevant antibody had no effect (Fig 2D). We cannot say at this time whether the inability to completely block survival and Bcl-XL maintenance with anti-IL-3 was because other signaling pathways or cytokines play a role or because it is difficult to completely block the autocrine effects of endogenously produced cytokines with neutralizing antibodies (27).
Prolonged Erk phosphorylation is required for IL-3 production
To determine the IgE-induced signaling pathways that were required for BMMC survival, we measured IL-3 production (as a surrogate marker for survival) from BMMCs after 3 hr ± pharmacological inhibitors. This avoided toxicity concerns associated with the use of inhibitors in the longer term survival studies. Since IgE+Ag triggered only a brief activation of Erk and no IL-3 production, we asked if the prolonged Erk phosphorylation elicited by mIgE played an essential role in IL-3 production and subsequent BMMC survival. For these studies, we pretreated BMMCs for 30 min with 25 μM of the MEK inhibitor, UO126, and found it completely ablated S IgE-induced Erk phosphorylation (Fig 3A, top panel). This complete ablation was also observed when BMMCs were pretreated for either 15 min or 5 min prior to IgE treatment. This showed that UO126 rapidly enters BMMCs and inhibits MEK activity (Fig 3A, lower panel). Interestingly, however, when we added UO126 5 min after IgE exposure, in an attempt to convert the prolonged IgE-induced activation of Erk into an acute activation, (to simulate IgE+Ag stimulation), it did not inhibit Erk phosphorylation (ie, phosphorylation of Erk persisted with the same kinetics as in the absence of UO126) (Fig 3A, lower panel). This was consistent with the prolonged IgE-induced phosphorylation of Erk being due to a lack of induction or activation of one or more MAPK phosphatases (MKPs (also known as DUSPs or DSPs (28)) rather than to a prolonged activation of MEK.
Fig 3.
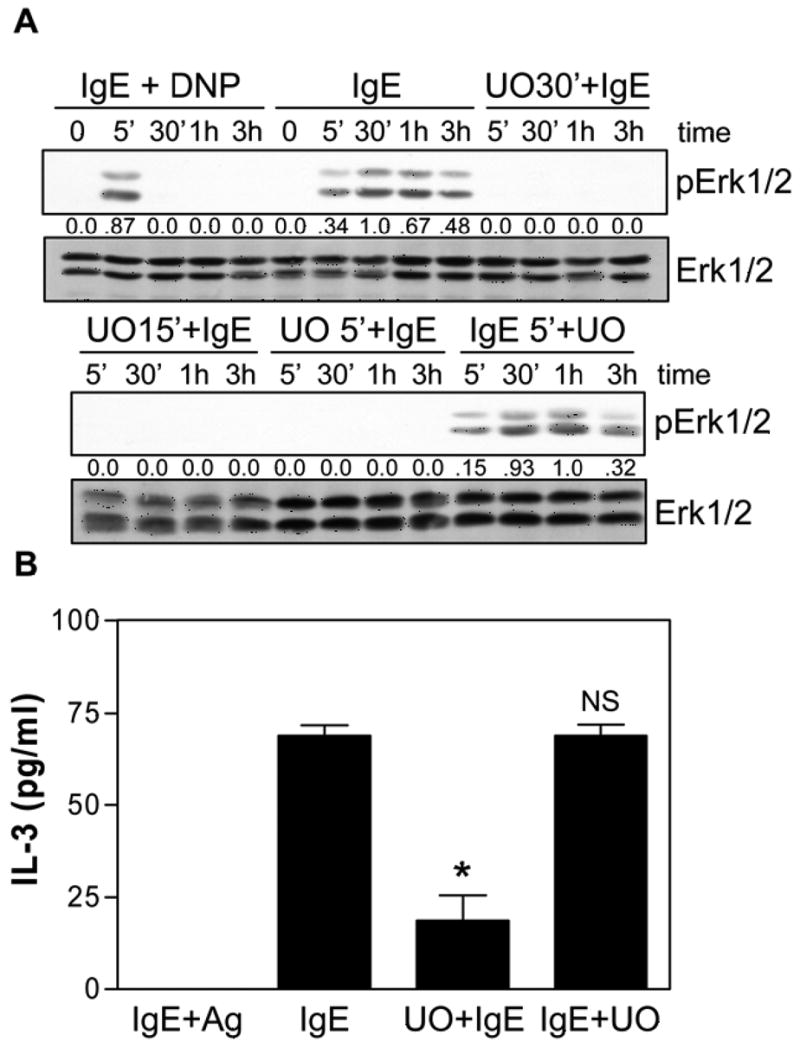
Prolonged Erk phosphorylation is required for IgE-induced IL-3 production. (A) S IgE (IgE) ± 30 min (UO30′+IgE), 15 min (UO15′+IgE) or 5 min (UO5′+IgE) with 25 μM UO126 prior to IgE stimulation or 5 min after IgE stimulation (IgE5′+UO). SDS-solubilized total cell lysates were then subjected to Western analysis with phospho-specific antibodies to Erk1/2 and with antibodies to Erk1/2. The numbers between the two panels indicate relative densitometry values normalized to Erk1/2. (B) BMMCs were stimulated with 0.1 μg/ml S overnight at 37°C, washed and given 20 ng/ml DNP-HSA for 3 hr (IgE+Ag) or stimulated with 5 μg/ml S IgE in 0.1% BSA for 3 h (IgE) in the presence of 25 μM UO126 added 5 min prior to IgE (U0+IgE) or 5 min after IgE (IgE+UO) and the conditioned medium subjected to IL-3 ELISAs. NS = not significantly different. * p<0.01. A is representative of 5 separate experiments. For B, results are the mean ± SEM of 3 independent experiments and the levels of IL-3 obtained from unstimulated cells have been subtracted.
Looking downstream at the effect of the MEK inhibitor on IL-3 production, we found that if it was added 5 min before IgE stimulation, it dramatically inhibited IL-3 production but had no effect if added 5 min after IgE (Fig 3B). Taken together, this suggested that Erk activation was important for IgE-induced IL-3 production (since IL-3 levels were markedly reduced with UO126) and that it was prolonged Erk activation that was important since IgE+Ag, which only triggers an acute Erk activation, could not stimulate IL-3 production.
Prolonged Calcium influx is required for IL-3 production
To determine why IgE results in a prolonged Erk phosphorylation while IgE+Ag does not, we asked if the delayed but sustained Ca++ influx observed with IgE alone (see Fig 1B) played a role. Specifically, we examined IgE-induced Erk phosphorylation in the presence and absence of extracellular Ca++. As shown in the top panel of Fig 4A, addition of IgE to BMMCs in the presence of Ca++ displayed a more prolonged Erk phosphorylation (peaking at 30 min) than in its absence (peaking at 5 min). However, no difference was observed with IgE+Ag-induced phosphorylation of Erk (Fig 4A, bottom panel), suggesting that IgE+Ag-induced Erk phosphorylation is not dependent on an influx of extracellular Ca++. Importantly, IgE-induced IL-3 production was dramatically reduced in the absence of extracellular Ca++ (Fig 4B), consistent with IgE inducing a prolonged Ca++ influx which leads to a prolonged Erk phosphorylation and subsequent IL-3 production.
Fig 4.
A prolonged calcium influx is required for IgE-induced IL-3 production. (A) BMMCs were stimulated for the indicated times with 5 μg/ml S IgE (top panel) or with 0.1 μg/ml S IgE overnight at 37°C followed by 20 ng/ml DNP-HSA (lower panel) in Tyrode’s buffer containing 1.8 mM CaCl2 or Tyrode’s buffer without Ca++. SDS-solubilized total cell lysates were then subjected to Western analysis with antibodies to phospho-Erk1/2 and Erk1/2. The numbers under the lanes indicate relative densitometry values normalized to Erk1/2. (B) BMMCs were stimulated for 3 h with 5 μg/ml S IgE in Tyrode’s buffer ± 1.8 mM CaCl2 and IL-3 ELISAs carried out on the conditioned medium. * p< 0.0001. Results are the mean ± SEM of 3 independent experiments. (C) BMMCs were incubated for 20 min with CM-DCFHDA as described in Materials and Methods and then stimulated with 5 μg/ml S IgE (IgE) or with 20 ng/ml DNP-HSA (after preloading with 0.1 μg/ml S IgE overnight) (IgE+Ag) ± Tyrode’s buffer ± 1.8 mM CaCl2 (no added Ca++ = - Ca++) and fluorescence levels measured. IgE (●), IgE+Ag (■), IgE - Ca++ (○), IgE+Ag -Ca++ (□). Fluorescence values shown have had the background fluorescence of unstimulated cells subtracted. (D) BMMCs were incubated for 20 min with CM-DCFHDA ± 0.1, 1 or 10 μM AA861 (or vehicle control, DMSO) or 3, 30 or 300 nM FR122047 (or DMF) and then stimulated with 20 ng/ml DNP-HSA (after preloading with 0.1 μg/ml S IgE overnight) (upper panel) or with 5 μg/ml S IgE (lower panel) and fluorescence levels measured. Fluorescence values shown have had the background fluorescence of unstimulated cells subtracted. The symbols for both the left and right panels are; stimulus alone (●), stimulus + vehicles, DMF (■), DMSO (▲) and stimulus + 0.1 △), 1 (△) and 10 (△) μM AA861 or + 3 (□), 30 (□) and 300 (□) FR122047. (E) BMMCs were incubated for 20 min with CM-DCFHDA ± 10 μM AA861 (or vehicle control, DMSO), 300 nM FR122047 (or DMF) or both and then stimulated with 20 ng/ml DNP-HSA (after preloading with 0.1 μg/ml S IgE overnight) (left panel) or with 5 μg/ml S IgE (IgE) (right panel) and fluorescence levels measured. Fluorescence values shown have had the background fluorescence of unstimulated cells subtracted. The symbols for both the left and right panels are IgE (○), IgE with the vehicles, DMF (□), DMSO (△) and both (◇) or with AA861(■), FR122047 (▲) or both (◆). Similar results were obtained in 3 separate experiments for A, B, C & D and 2 separate experiments for E.
Since a recent study suggested that B cell receptor (BCR) activation leads to both a rapid influx of Ca++ and the generation of reactive oxygen species (ROS) and that they engage in a cooperative interaction that acts in a positive feedback loop to amplify signaling (29), we asked if IgE alone generated ROS and, if so, what effect extracellular Ca++ would have on this generation. As shown in Fig 4C, we found that IgE+Ag induced a rapid generation of ROS which plateaued after 5 min of exposure to Ag. Of note, while no additional ROS is generated in response to IgE+Ag after 5–10 min, high fluorescence is still seen at 25 min because it is not degraded in this in vitro system. Interestingly, S IgE alone also induced ROS but at a much slower rate and the level continued to climb for at least 30 min (we could not follow it longer because of photobleaching of the flourescent substrate). Of note, in the absence of extracellular Ca++, IgE-induced ROS was markedly inhibited. However, this inhibition only started 5 min after IgE stimulation, consistent with IgE-induced ROS generation preceding extracellular Ca++ entry. Also worthy of note, IgE+Ag-induced ROS was also inhibited in the absence of extracellular Ca++, albeit far less than IgE-induced ROS (Fig 4C).
Since a very recent study nicely showed that IgE+Ag stimulated the production of ROS via 5-LO and, to a small extent, via COX-1 (30), we asked if these enzymes were also responsible for IgE-induced ROS. Specifically, we first tested various concentrations of the 5-LO inhibitor, AA861, and the COX-1 inhibitor, FR122047, on IgE+Ag versus IgE-induced ROS production. We found that 10 μM AA861 markedly inhibited, and 300 nM FR122047 slightly inhibited, IgE+Ag-induced ROS production (Fig 4D, left panel), consistent with the results of (30). However, while FR122047 had a similar inhibitory effect on IgE-induced ROS, AA861 had negligible effects (Fig 4D, right panel). We then retested these two inhibitors at these concentrations, alone and together, on IgE+Ag versus IgE-induced ROS production and found that while AA861 + FR122047 dramatically inhibited IgE+Ag-induced ROS (Fig 4E, left panel), they had very little effect on IgE-induced ROS (Fig 4E, right panel).
Prolonged ROS generation is required for IL-3 production
To explore the role of IgE-induced ROS further, we asked if the level of ROS generated by the 5 IgEs correlated with their ability to enhance BMMC survival. Similar to our Ca++ influx results, the 5 IgEs triggered a slow, prolonged increase in ROS, with the rate of ROS generation by each IgE correlating with survival enhancing ability (Fig 5A). We then asked if this IgE-induced ROS was important to subsequent signaling events implicated in BMMC survival. For these experiments, we first carried out preliminary dose response studies with two ROS scavengers, N-acetylcysteine (NAC), a glutathione precursor that alters the intracellular redox balance, and ebselen, which selectively scavenges H2O2 (31). We found that at 2.5 mM NAC and 1.5 mM ebselen, S IgE-induced ROS generation was completely blocked (Fig 5B). We then used these inhibitors to determine if they could block the prolonged phosphorylation of Erk obtained with IgE alone. As can be seen in the upper panel of Fig 5C, the presence of ebselen or NAC had no effect on the early phosphorylation of Erk (i.e., at 5 min) but markedly inhibited phosphorylation at later times. These two inhibitors also inhibited the more abbreviated IgE+Ag-induced Erk phosphorylation at 15 min but had less of an effect at the 5 min time point (lower panel of Fig 5C). NAC was then tested at various concentrations for its effect on IgE-induced Ca++ influx. As shown in the left panel of Fig 5D, 2 mM NAC completely blocked Ca++ entry. To determine if blocking ROS production had an impact on IgE-induced production of IL-3, we then added ebselen and NAC to IgE-stimulated BMMCs and found both inhibitors completely blocked IL-3 production (Fig 5E).
Fig 5.
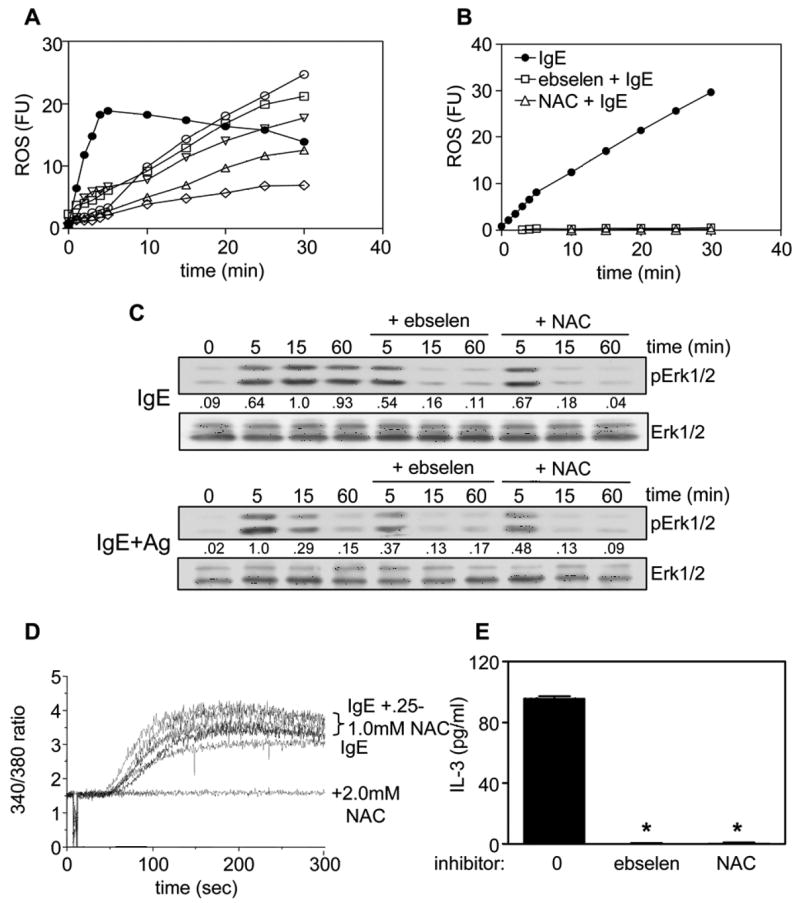
IgE-induced ROS is required for extracellular calcium influx, prolonged Erk phosphorylation and IL-3 production. (A) BMMCs were incubated for 20 min with CM-DCFHDA and then stimulated with 20 ng/ml DNP-HSA, if preloaded with 0.1 μg/ml S IgE overnight (IgE+Ag) (●), or with 5 μg/ml S IgE (○), L (□), 48 (▽), E (△) or λ (◇) IgE and fluorescence levels measured. (B) BMMCs were incubated for 20 min with CM-DCFHDA as in (A) and then stimulated with 5 μg/ml S IgE alone (●) or + 1.5 mM ebselen (□) or + 2.5 mM NAC (). Values shown in (A) and (B) have had the background fluorescence of unstimulated cells subtracted. (C) BMMCs were stimulated for the indicated times with 5 μg/ml S IgE (top panel) or with 20 ng/ml DNP-HSA after overnight pretreatment with 0.1 μg/ml S IgE (lower panel) ± 1.5 mM ebselen or 2.5 mM NAC and SDS-solubilized total cell lysates subjected to Western analysis with phospho-specific antibodies to Erk1/2 and with antibodies to Erk1/2. The numbers between the two panels indicate relative densitometry values normalized to Erk1/2. (D) BMMCs were incubated with fura-2/AM at 23°C for 45 min, washed twice and stimulated with 10 μg/ml S IgE ± increasing concentrations of NAC, added 5 min before the IgE. Cytosolic calcium levels were determined by monitoring fluorescence intensity at 510 nm following excitation at 340 and 380 nm. (E) BMMCs were stimulated with 5 μg/ml S IgE in 0.1% BSA for 3 h ± 1.5 mM ebselen or 2.5 mM NAC, added 5 min before the IgE, and the conditioned media subjected to IL-3 ELISAs. The absolute levels of IL-3 secreted by S IgE in this study were 96± 2 pg/ml (in standard buffer), 79±2 pg/ml (in the presence of 0.1% DMSO (the ebselen vehicle)) and 85±2 pg/ml (in 0.1% ethanol (the NAC vehicle). *p<0.0001. Results are the mean ± SEM of 3 independent experiments. Similar results were obtained in 4 (A) or 3 (B, C, D, E) separate experiments.
To further explore the signaling pathways upstream of IgE and IgE+Ag-induced ROS production, we tested various pathway inhibitors at the lowest concentration that totally ablates their target pathway in BMMCs (4), and unpublished). Not surprisingly, the Src family inhibitor, PP2, potently inhibited both IgE (Fig 6A, left panel) and IgE+Ag (Fig 6A, right panel) induced ROS production, consistent with Lyn initiating signaling in response to both stimuli and with our Lyn−/− BMMC results (Fig 2A). Interestingly, the PLCγ inhibitor, U73122, also markedly inhibited both IgE and IgE+Ag-induced ROS production (Fig 6A), consistent with our earlier studies (Fig 4C) showing the importance of extracellular Ca++ influx to ROS generation. On the other hand, the p38 and JNK inhibitors, SB203580 and SP600125, respectively, had little effect. Surprisingly, however, UO126 markedly inhibited both IgE and IgE+Ag-induced ROS production. Given our assumption that Erk phosphorylation was downstream of ROS generation, we wanted to confirm that UO126 was specific for MEK and was not inhibiting ROS generation via off target effects. To assess this, we first asked if IL-4 induced ROS in BMMCs and found that it did (Fig 6B). Since it is well established that IL-4 does not stimulate Erk phosphorylation in BMMCs (32), we then tested the effect of UO126 on IL-4-induced ROS production and found no inhibition (Fig 6B). This was consistent with UO126 inhibiting IgE-induced ROS generation via inhibition of MEK and not via non-specific effects on ROS generation.
Fig 6.
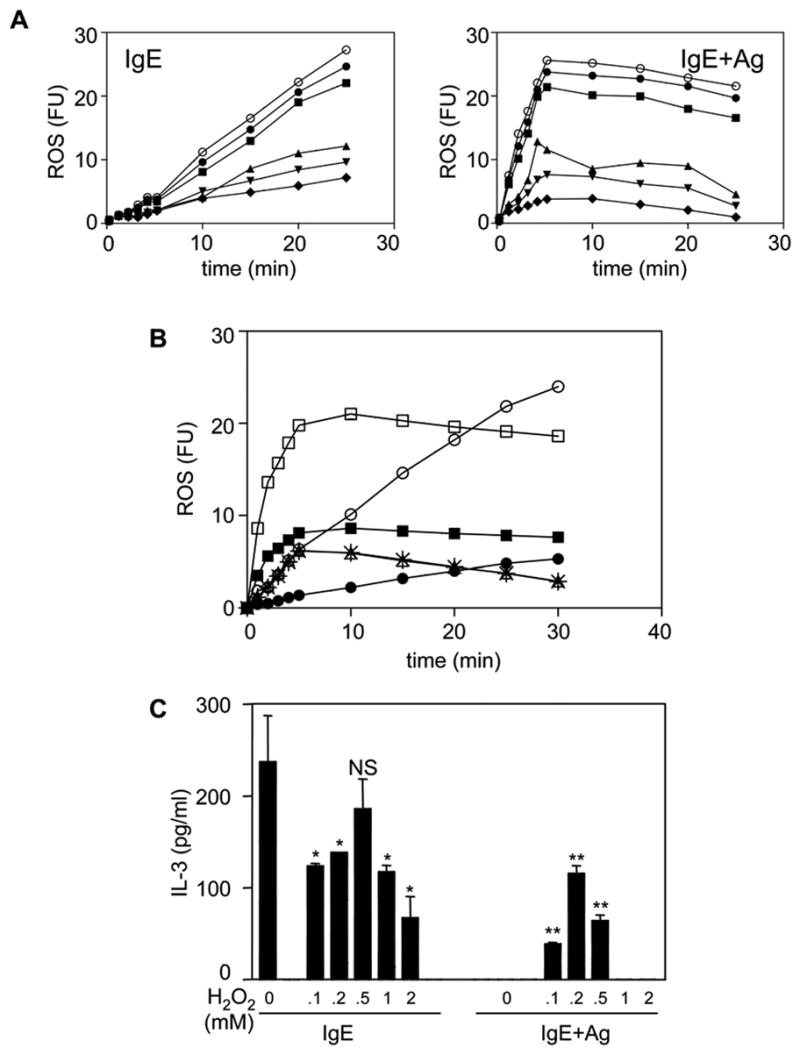
The Erk pathway stimulates ROS production and IgE+Ag-stimulated BMMCs produce IL-3 in the presence of exogenous H2O2. (A) BMMCs were incubated for 20 min with CM-DCFHDA (○) ± 1 μM SP600125 (●), 5 μM SB203580 (■), 1 μM U73122 (▲), 10 μM PP2 (▼) or 25 μM UO126 (◆) and then stimulated with 5 μg/ml S IgE (left panel) or 20 ng/ml DNP-HSA (after overnight incubation with 0.1 ug/ml S IgE) (right panel) for the indicated times and fluorescence levels measured. (B) BMMCs were incubated for 20 min with CM-DCFHDA ± 25 μM UO126 and then stimulated with 5 μg/ml S IgE (IgE) (○), (●) = UO + IgE) or with 20 ng/ml DNP-HSA (after overnight incubation with 0.1 ug/ml S IgE) (□), (■) = UO + IgE+Ag) or with 50 ng/ml IL-4 (),(* = UO + IL-4) and fluorescence levels measured. 25 μM UO126 alone yielded no detectable ROS. (C) BMMCs were stimulated for 3 h with 5 μg/ml S IgE (IgE) or with 20 ng/ml DNP-HSA (after overnight incubation with 0.1 ug/ml S IgE) (IgE+Ag) in the presence of the indicated concentrations of H2O2. These levels of H2O2 did not affect the IL-3 ELISA. The conditioned medium was then subjected to an IL-3 ELISA. NS = not significantly different from IgE alone. *p<0.01 versus IgE alone. **p<0.002 versus IgE+Ag alone. The mean ± SEM from 3 independent experiments, each assayed in duplicate.
Lastly, we asked if the inability of IgE+Ag to produce IL-3 (and thus enhance BMMC survival) might be due to the abbreviated ROS that it elicited. To test this, we added various concentrations of H2O2 to IgE+Ag-stimulated versus IgE-stimulated BMMCs. As shown in Fig 6C, addition of H2O2 slightly decreased IgE-induced IL-3 while dramatically increasing IgE+Ag-induced IL-3 production. Of note, H2O2 alone did not stimulate the production of any detectable IL-3 (data not shown). Also of note is that while H2O2 did not cause any detectable toxicity during the 3 hr incubation with BMMCs, the fact that it reduced IgE-induced IL-3 production could suggest that the level of IL-3 we obsereve when H2O2 is added to IgE+Ag-treated BMMCs is an underestimate of the level obtainable.
Discussion
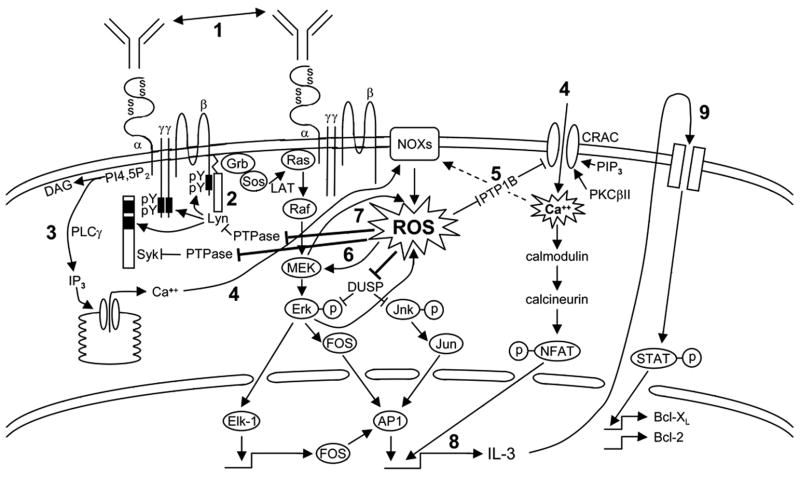
Since the initial observation that mIgE alone could enhance mast cell survival (4,5), a number of groups have made considerable progress in elucidating the intracellular pathways responsible for this phenomenon (6–8,11,33–36). Based on their studies and the results presented herein, we would like to propose a model (Fig 7) in which mIgEs trigger a slow, asynchronous FcεRI aggregation by an as yet unidentified mechanism (see (3)). Our finding that a synchronous, rapid aggregation of very few IgE/FcεRI complexes via multivalent Ag does not mimic the effects of mIgE alone (Fig 1A, right panel) suggests that a continuous, low potency activation of IgE-bound receptors, perhaps initiated by interaction with unbound IgE in solution, is required for mediating survival. This latter idea is consistent with elegant studies from Cockcroft’s group showing that constant exposure to unbound mIgE in the culture medium is required for a prolonged Ca++ influx into RBL-2H3 cells (37) and also with studies in our lab (unpublished) and Kawakami’s lab (5) showing that IgE-induced survival is abrogated if unbound IgE is washed away within 24 hr after BMMCs are bound with IgE. This slow, ongoing generation of active IgE/FcεRI aggregates, which likely localize within lipid rafts (4), leads to a low but continual activation of FcεRI β-associated Lyn which then tyrosine phosphorylates the FcεRI β (35) and γ ITAMs and this subsequently attracts Lyn and Syk (8), respectively. Although Fyn also associates with the tyrosine phosphorylated FcεRI ITAMs and activates PI3K (38), it is unlikely this plays a role in IgE-induced survival based on Fyn−/− BMMC studies carried out by Kohno et al (11). PLCγ is then recruited to tyrosine phosphorylated Syk and becomes phosphorylated/activated, generating IP3, which triggers the release of intracellular Ca++ from the endoplasmic reticulum and mitochondria. This draining of intracellular Ca++ leads to both an influx of extracellular Ca++ (39) and, we propose, an early production of ROS, perhaps via an EF hand-containing member of the Nox family of NADPH oxidases. Specifically, while all members of this family are membrane bound enzymes that transfer electrons from NADPH to O2 to produce superoxide anions (O2−) that are rapidly converted to H2O2 and other ROS, only NOX5, Duox1 and Duox2 contain EF hands and are Ca++ dependent (reviewed in (40–42)). In support of intracellular Ca++ release initiating ROS production, we found that PLCγ inhibition markedly inhibits IgE-induced ROS generation (Fig 6A) and that IgE, even in the absence of extracellular Ca++, stimulates a low level of ROS (Fig 4C). We then propose that this initial generation of ROS enhances extracellular Ca++ entry (since ROS inhibitors block this entry, Fig 5D), perhaps by inactivating PTP1B, a ROS-inhibitable tyrosine phosphatase (PTP) involved in negatively regulating extracellular Ca++ entry in HEK 293 cells (43). Similar to what has been proposed during BCR activation (29), we propose that the extracellular Ca++ influx and ROS generated by IgE engage in a cooperative interaction to amplify both upstream and downstream signaling. Specifically, we propose that the generation of ROS, which enhances upstream signaling by inactivating receptor-coupled PTPs (which typically contain a redox-regulated cysteine in their catalytic site (44), allows FcεRI β and γ ITAMs to remain phosphorylated longer and this, in turn, keeps Lyn, Syk, LAT and NTAL tyrosine phosphorylated, enabling the latter two adaptors to continue to activate the Ras pathway (36). In support of this, we have shown previously that the tyrosine phosphorylation of the FcεRI β chain remains at maximal levels 4 h after mIgE exposure whereas IgE+Ag-induced phosphorylation of this β subunit peaks at 2 min and returns to baseline by 1 h (4). Related to this, Suzuki et al have reported that IgE+Ag-induced tyrosine phosphorylation of PLCγ and LAT (which is required for extracellular Ca++ influx) is reduced by inhibiting ROS production (45).
Fig 7.
Proposed model for IgE-induced BMMC survival. A low but constant mIgE-induced activation of the FcεRI (1) leads to low level Lyn activation (2) and the subsequent phosphorylation of Syk, LAT and PLCγ. (3) PLCγ-mediated intracellular Ca++ release stimulates (4) both an initial activation of a Ca++–responsive member of the NOX family and extracellular Ca++ entry. The influx of extracellular Ca++ and the initial generation of ROS act in a cooperative loop to amplify each others signal via (5) Ca++-induced activation of the NOX family of NADPH oxidases and by ROS-induced inactivation of a negative PTPase regulator of CRAC. (6) The constant production of ROS ensures that both the PTPases which normally dephosphorylate Lyn, Syk and LAT (and perhaps the β and the γ ITAMs) and the MKPs that dephosphorylate Erk and Jnk remain inactive (allowing for higher AP1 levels). (7) Activated MEK or downstream molecules in this pathway also act in a positive feedback loop to enhance ROS production. The prolonged extracellular Ca++ influx leads to dephosphorylation of NFAT and its entry into the nucleus to enhance, together with AP1, (8) the transcription of IL-3. IL-3 then acts in an autocrine fashion to (9) increase pro-survival Bcl-2 family members.
Importantly, we also propose that ROS enhances downstream signaling by inactivating dual specificity phosphatases (DUSPs, aka DSPs and MKPs) that dephosphorylate phospho-Erk and phospho-Jnk (28,46). Related to this, we not only show that prolonged Erk phosphorylation is critical for IgE-induced IL-3 production (Fig 3B), in agreement with previous reports (10,36), but, more importantly, that ROS inhibition converts the prolonged Erk phosphorylation and subsequent IL-3 production observed with IgE to the abbreviated Erk phosphorylation and lack of IL-3 production normally observed with IgE+Ag (Fig 5C & E). This requirement for a prolonged Erk activation for survival has also been reported for other cell types (47).
In testing the effects of pharmacological inhibitors on IgE-induced ROS, we found that PP2 and the PLC inhibitor, U73122, block ROS generation, in keeping with previous studies showing that tyrosine kinase inhibitors block IgE+Ag-induced ROS (45). Unexpectedly, our studies with UO126, which dramatically suppresses IgE-induced ROS generation (Fig 6A), and appears to be specific for MEK (Fig 6B), suggest that activated MEK or downstream molecules in the Ras/Erk pathway also feedback activate ROS generation. As well, we propose that the positive feedback loop that exists between ROS, extracellular Ca++ entry and the Erk pathway results in a prolonged Ca++ influx into mast cells that is critical for the calmodulin/calcineurin-mediated dephosphorylation of NFAT and its translocation into the nucleus (37,48,49) where it stimulates, together with the Fos/Jun complex (AP1), the transcription of IL-3 (50,51) Our earlier finding that thapsigargin enhances BMMC survival (39), coupled with a very recent report that thapsigargin stimulates ROS production in mast cells (52), highlights the importance of increased Ca++ and ROS levels to BMMC survival. Support for our model comes from both our finding that exogenous H2O2 enables IgE+Ag to generate IL-3 (Fig 6C) and from recent studies by Fossi et al showing that nanomolar levels of H2O2 induce AP1 and NFAT complexes in BMMCs (53).
In contrast to IgE-induced ROS, we find that IgE+Ag-induced ROS, which has been reported previously (45,54–56) and shown to play a positive role in degranulation, leukotriene secretion and cytokine production (45,54–58); reviewed in (59) is very short lived. This may be because IgE+Ag-induces a much stronger, synchronized activation of BMMCs that triggers rapid endocytosis of the bound FcεRI and termination of signaling, perhaps in part via Lyn-mediated (3) activation of negative regulators like SHIP and SHP1 (3,60). This rapid turnoff of signaling results in an extracellular Ca++ entry that is too brief (Fig 1B) to prolong ROS generation sufficiently for prolonged Erk phosphorylation and resultant IL-3 production. Interestingly, IgE+Ag-induced ROS, like IgE-induced ROS, is inhibited, albeit to a far lesser extent, in the absence of extracellular Ca++ (Fig 4C) but, unlike IgE-induced ROS, appears to be generated primarily via 5-LO (Figs 4D & E). However, it is possible that very early production in response to IgE alone may also occur via 5-LO to some extent, i.e., note the early inhibition with AA861 in Fig 4D, right panel.
In summary, we show herein that IgE enhances BMMC survival in vitro, while IgE+Ag does not, under the conditions used in our studies. However, IgE+Ag may still trigger mast cell survival in vivo, where newly synthesized IgE produced by B cells may allow for a continuous stimulation. The studies presented herein suggest that IgEs enhance BMMC survival by inducing a low but constant extracellular Ca++ entry, ROS production and Erk pathway activation that positively reinforce each other and lead to IL-3 production.
Acknowledgments
We thank Dr. Kent Hayglass for the λ IgE, Dr Hermann Ziltener for the anti-IL-3 and control sheep antibody and Christine Kelly for typing the manuscript.
Abbreviations
- 5-LO
5-lipoxygenase
- 48
DNP48 anti-DNP IgE
- λ
clone 91.58 anti-NP IgE
- BMMCs
bone marrow derived mast cells
- COX-1
cyclooxygenase 1
- E
anti-Epo26 IgE
- FcεRI
high affinity IgE receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- HC
highly cytokinergic
- L
Liu anti-DNP IgE
- mIgE
monomeric IgE
- MKP (aka DUSP)
MAPK phosphatase
- NAC
N-acteyl cysteine
- PC
poorly cytokinergic
- S
SPE7 anti-DNP IgE
- ROS
reactive oxygen species
Footnotes
Disclosures
The authors have no financial conflict of interest.
This work was supported by the National Cancer Institute of Canada with core support from the BC Cancer Foundation and the BC Cancer Agency. The work of JR and RPS was supported by the intramural research programs of NIAMS and NICHD, NIH.
References
- 1.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu C, MacGlashan D., Jr IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett. 1996;52:129–134. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 3.Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 5.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, Liu FT, Galli SJ, Kawakami T. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S, Takasu Y, Mikura S, Satoh N, Ichikawa A. Antigen-independent induction of histamine synthesis by immunoglobulin E in mouse bone marrow-derived mast cells. J Exp Med. 2002;196:229–235. doi: 10.1084/jem.20012037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam V, Kalesnikoff J, Lee CWK, Hernandez-Hansen V, Wilson BS, Oliver JM, Krystal G. IgE alone stimulates mast cell adhesion to fibronectin via pathways similar to those used by IgE+antigen but distinct from those used by Steel factor. Blood. 2003;102:1405–1413. doi: 10.1182/blood-2002-10-3176. [DOI] [PubMed] [Google Scholar]
- 8.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, Kawakami T. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcεRI. Proc Natl Acad Sci USA. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai D, Yamasaki S, Arase K, Park SY, Arase H, Konno A, Saito T. Fcε RI γ-ITAM is differentially required for mast cell function in vivo. J Immunol. 2004;172:2374–2381. doi: 10.4049/jimmunol.172.4.2374. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki S, Ishikawa E, Kohno M, Saito T. The quantity and duration of FcRγ signals determine mast cell degranulation and survival. Blood. 2004;103:3093–3101. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- 11.Kohno M, Yamasaki S, Tybulewicz VL, Saito T. Rapid and large amount of autocrine IL-3 production is responsible for mast cell survival by IgE in the absence of antigen. Blood. 2005;105:2059–2065. doi: 10.1182/blood-2004-07-2639. [DOI] [PubMed] [Google Scholar]
- 12.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17:7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 15.Ziltener HJ, Clark-Lewis I, McDonald SL. Sandwich enzyme immunoassay for murine IL-3. Cytokine. 1989;1:56–61. doi: 10.1016/1043-4666(89)91049-1. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Damen JE, Cutler RL, Krystal G. Multiple cytokines stimulate the binding of a common 145- kilodalton protein to Shc at the Grb2 recognition site of Shc. Mol Cell Biol. 1994;14:6926–6935. doi: 10.1128/mcb.14.10.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fcε receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J Immunol. 2004;173:4317–4323. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh S, Odom S, Gomez G, Sommers CL, Young HA, Rivera J, Samelson LE. The four distal tyrosines are required for LAT-dependent signaling in FcεRI-mediated mast cell activation. J Exp Med. 2003;198:831–843. doi: 10.1084/jem.20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brambilla P, Gioria M, Brivio R, Ferrari E, Tramacere P, Colombo L, Sarto C, Mocarelli P. Granulocytic-macrophagic and macrophagic colony stimulating factors elicit colonies of mast cells in mouse bone marrow agar culture. An electron microscope study. J Submicrosc Cytol Pathol. 1993;25:239–246. [PubMed] [Google Scholar]
- 21.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 22.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 23.de Paulis A, Minopoli G, Arbustini E, de Crescenzo G, Dal Piaz F, Pucci P, Russo T, Marone G. Stem cell factor is localized in, released from, and cleaved by human mast cells. J Immunol. 1999;163:2799–2808. [PubMed] [Google Scholar]
- 24.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 25.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature. 1989;339:64–68. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 26.Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orban PC, Schrader JW. Antibodies to an autostimulatory growth factor (IL-2) or its receptor induce death of leukemogenic cells. J Immunol. 1996;156:3334–3341. [PubMed] [Google Scholar]
- 28.Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–7504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 29.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. FcεRI- and Fcγ receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J Immunol. 2007;179:7059–7071. doi: 10.4049/jimmunol.179.10.7059. [DOI] [PubMed] [Google Scholar]
- 31.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–127. [PubMed] [Google Scholar]
- 32.Welham MJ, Duronio V, Schrader JW. Interleukin-4-dependent proliferation dissociates p44erk-1, p42erk-2, and p21ras activation from cell growth. J Biol Chem. 1994;269:5865–5873. [PubMed] [Google Scholar]
- 33.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 34.Charles N, Monteiro RC, Benhamou M. p28, a novel IgE receptor-associated protein, is a sensor of receptor occupation by its ligand in mast cells. J Biol Chem. 2004;279:12312–12318. doi: 10.1074/jbc.M309456200. [DOI] [PubMed] [Google Scholar]
- 35.Nunomura S, Gon Y, Yoshimaru T, Suzuki Y, Nishimoto H, Kawakami T, Ra C. Role of the FcεRI β-chain ITAM as a signal regulator for mast cell activation with monomeric IgE. Int Immunol. 2005;17:685–694. doi: 10.1093/intimm/dxh248. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki S, Ishikawa E, Sakuma M, Kanagawa O, Cheng AM, Malissen B, Saito T. LAT and NTAL mediate immunoglobulin E-induced sustained extracellular signal-regulated kinase activation critical for mast cell survival. Mol Cell Biol. 2007;27:4406–4415. doi: 10.1128/MCB.02109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004;172:4048–4058. doi: 10.4049/jimmunol.172.7.4048. [DOI] [PubMed] [Google Scholar]
- 38.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 39.Huber M, Hughes MR, Krystal G. Thapsigargin-induced degranulation of mast cells is dependent on transient activation of phosphatidylinositol 3-kinase. J Immunol. 2000;165:124–133. doi: 10.4049/jimmunol.165.1.124. [DOI] [PubMed] [Google Scholar]
- 40.Grandvaux N, Soucy-Faulkner A, Fink K. Innate host defense: Nox and Duox on phox’s tail. Biochimie. 2007;89:1113–1122. doi: 10.1016/j.biochi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 42.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Bogeski I, Bozem M, Sternfeld L, Hofer HW, Schulz I. Inhibition of protein tyrosine phosphatase 1B by reactive oxygen species leads to maintenance of Ca2+ influx following store depletion in HEK 293 cells. Cell Calcium. 2006;40:1–10. doi: 10.1016/j.ceca.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki Y, Yoshimaru T, Matsui T, Inoue T, Niide O, Nunomura S, Ra C. FcεRI signaling of mast cells activates intracellular production of hydrogen peroxide: role in the regulation of calcium signals. J Immunol. 2003;171:6119–6127. doi: 10.4049/jimmunol.171.11.6119. [DOI] [PubMed] [Google Scholar]
- 46.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 48.Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 49.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 50.Hawwari A, Burrows J, Vadas MA, Cockerill PN. The human IL-3 locus is regulated cooperatively by two NFAT-dependent enhancers that have distinct tissue-specific activities. J Immunol. 2002;169:1876–1886. doi: 10.4049/jimmunol.169.4.1876. [DOI] [PubMed] [Google Scholar]
- 51.Macian F, Garcia-Rodriguez C, Rao A. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 2000;19:4783–4795. doi: 10.1093/emboj/19.17.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue T, Suzuki Y, Yoshimaru T, Ra C. Reactive oxygen species produced up- or downstream of calcium influx regulate proinflammatory mediator release from mast cells: Role of NADPH oxidase and mitochondria. Biochim Biophys Acta. 2008;1783:789–802. doi: 10.1016/j.bbamcr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol. 2007;178:2549–2555. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- 54.Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol Chem. 2004;279:48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki Y, Yoshimaru T, Inoue T, Niide O, Ra C. Role of oxidants in mast cell activation. Chem Immunol Allergy. 2005;87:32–42. doi: 10.1159/000087569. [DOI] [PubMed] [Google Scholar]
- 56.Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4:517–519. doi: 10.2174/1568010054526386. [DOI] [PubMed] [Google Scholar]
- 57.Matsui T, Suzuki Y, Yamashita K, Yoshimaru T, Suzuki-Karasaki M, Hayakawa S, Yamaki M, Shimizu K. Diphenyleneiodonium prevents reactive oxygen species generation, tyrosine phosphorylation, and histamine release in RBL-2H3 mast cells. Biochem Biophys Res Commun. 2000;276:742–748. doi: 10.1006/bbrc.2000.3545. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, Shimizu K. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin Exp Allergy. 2002;32:612–618. doi: 10.1046/j.0954-7894.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- 59.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 60.Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP down-regulates FCεR1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]